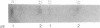Abstract
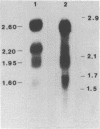
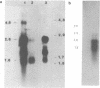
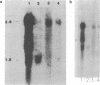
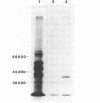
Human class I alcohol dehydrogenase (ADH) genes show developmental and tissue specific differences in expression at the polypeptide level. In these studies ADH expression was investigated at the RNA level. Northern blot analysis of total and poly (A) RNA from adult liver using pADH12 probe demonstrated multiple RNA size classes of 2.6, 2.2, 1.9 and 1.6kb. In contrast, fetal liver, and fetal intestine contained only 2.6 and 1.6kb mRNA while fetal lung showed only 2.6kb mRNA. All of these tissues showed a relative reduction in the amount of ADH mRNA present when compared to adult liver. Immunoprecipitation of in vitro translation products of adult liver RNA by polyclonal ADH antibody revealed a single polypeptide of 40,000 daltons. This result points out the homogeneity of size of class I ADH polypeptides despite mRNA size diversity. Variation in length of the 3' untranslated region probably contributes to the multiple size classes of ADH mRNA observed.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Evans R. M., Rosenfeld M. G. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984 Oct;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Duester G., Hatfield G. W., Bühler R., Hempel J., Jörnvall H., Smith M. Molecular cloning and characterization of a cDNA for the beta subunit of human alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4055–4059. doi: 10.1073/pnas.81.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Smith M., Bilanchone V., Hatfield G. W. Molecular analysis of the human class I alcohol dehydrogenase gene family and nucleotide sequence of the gene encoding the beta subunit. J Biol Chem. 1986 Feb 15;261(5):2027–2033. [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Gantt J. S., Arfin S. M. Elevated levels of asparagine synthetase activity in physiologically and genetically derepressed Chinese hamster ovary cells are due to increased rates of enzyme synthesis. J Biol Chem. 1981 Jul 25;256(14):7311–7315. [PubMed] [Google Scholar]
- Gil A., Proudfoot N. J. A sequence downstream of AAUAAA is required for rabbit beta-globin mRNA 3'-end formation. 1984 Nov 29-Dec 5Nature. 312(5993):473–474. doi: 10.1038/312473a0. [DOI] [PubMed] [Google Scholar]
- Hedén L. O., Hög J. O., Larsson K., Lake M., Lagerholm E., Holmgren A., Vallee B. L., Jörnvall H., von Bahr-Lindström H. cDNA clones coding for the beta-subunit of human liver alcohol dehydrogenase have differently sized 3'-non-coding regions. FEBS Lett. 1986 Jan 6;194(2):327–332. doi: 10.1016/0014-5793(86)80111-9. [DOI] [PubMed] [Google Scholar]
- Hempel J., Holmquist B., Fleetwood L., Kaiser R., Barros-Söderling J., Bühler R., Vallee B. L., Jörnvall H. Structural relationships among class I isozymes of human liver alcohol dehydrogenase. Biochemistry. 1985 Sep 24;24(20):5303–5307. doi: 10.1021/bi00341a005. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Goodbourn S. E., Lamb J., Clegg J. B., Weatherall D. J., Proudfoot N. J. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983 Nov 24;306(5941):398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Ikuta T., Fujiyoshi T., Kurachi K., Yoshida A. Molecular cloning of a full-length cDNA for human alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1985 May;82(9):2703–2707. doi: 10.1073/pnas.82.9.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T., Szeto S., Yoshida A. Three human alcohol dehydrogenase subunits: cDNA structure and molecular and evolutionary divergence. Proc Natl Acad Sci U S A. 1986 Feb;83(3):634–638. doi: 10.1073/pnas.83.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Giorda R., Ceccarelli A., Perlo C. mRNA stabilization controls the expression of a class of developmentally regulated genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5786–5790. doi: 10.1073/pnas.82.17.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Site-specific polyadenylation in a cell-free reaction. Cell. 1984 Mar;36(3):581–591. doi: 10.1016/0092-8674(84)90337-4. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Robinson R. R., Seidman J. G. Multiple mRNA species with distinct 3' termini are transcribed from the beta 2-microglobulin gene. 1983 Mar 31-Apr 6Nature. 302(5907):449–452. doi: 10.1038/302449a0. [DOI] [PubMed] [Google Scholar]
- Parés X., Vallee B. L. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981 Jan 15;98(1):122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sadofsky M., Alwine J. C. Sequences on the 3' side of hexanucleotide AAUAAA affect efficiency of cleavage at the polyadenylation site. Mol Cell Biol. 1984 Aug;4(8):1460–1468. doi: 10.1128/mcb.4.8.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Alcohol dehydrogenase isozymes in adult human stomach and liver: evidence for activity of the ADH 3 locus. Ann Hum Genet. 1972 Mar;35(3):243–253. doi: 10.1111/j.1469-1809.1957.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Developmental changes and polymorphism in human alcohol dehydrogenase. Ann Hum Genet. 1971 Feb;34(3):251–271. doi: 10.1111/j.1469-1809.1971.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Studies on the properties of the human alcohol dehydrogenase isozymes determined by the different loci ADH1, ADH2, ADH3. Ann Hum Genet. 1973 Jul;37(1):49–67. doi: 10.1111/j.1469-1809.1973.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Strub K., Galli G., Busslinger M., Birnstiel M. L. The cDNA sequences of the sea urchin U7 small nuclear RNA suggest specific contacts between histone mRNA precursor and U7 RNA during RNA processing. EMBO J. 1984 Dec 1;3(12):2801–2807. doi: 10.1002/j.1460-2075.1984.tb02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y., Devos R., Tavernier J., Cheroutre H., Engler G., Fiers W. Cloning and structure of the human immune interferon-gamma chromosomal gene. EMBO J. 1982;1(8):953–958. doi: 10.1002/j.1460-2075.1982.tb01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. Controlling roles for snurps. Nature. 1985 Jul 11;316(6024):105–106. doi: 10.1038/316105a0. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- li T. K. Enzymology of human alcohol metabolism. Adv Enzymol Relat Areas Mol Biol. 1977;45:427–483. doi: 10.1002/9780470122907.ch6. [DOI] [PubMed] [Google Scholar]