Abstract
Aspirin (acetylsalicylic acid, ASA) has been used as an analgesic, antipyretic and antiinflammatory drug for many years. A new 500 mg aspirin tablet formulation containing micronized active ingredient and an effervescent component has been developed for potential improvement in the onset of action for acute pain treatment. This paper describes the dissolution and the pharmacokinetics of the new formulation in comparison with regular aspirin tablets, aspirin granules and aspirin effervescent tablets. Micronized aspirin tablets dissolve significantly faster over a pH range from 1.2 to 6.8 compared to regular 500 mg aspirin tablets. Plasma concentration time curve comparison to regular 500 mg aspirin tablets showed a substantial improvement in the time to maximum plasma concentrations (T max) (ASA 17.5 min vs. 45 min) and an increase in maximum plasma concentration (C max) (ASA 13.8 μg/ml vs. 4.4 μg/ml) while the overall extent of exposure (AUC) remains almost unchanged. The data suggest a potential improvement for onset of action in treating acute pain with the new micronized aspirin formulation.
Keywords: Aspirin, Acetylsalicylic acid, Dissolution, Pharmacokinetic, Time to maximum plasma concentration
Introduction
A major goal of pharmaceutical formulation of analgesics is to keep improving the efficacy of the product. This can be achieved by either a faster in vivo dissolution in the stomach or a faster absorption of the active ingredient throughout the gastrointestinal (GI) tract, or both.
Acetylsalicylic acid (ASA, aspirin) is a non-steroidal anti-inflammatory drug used for the treatment of pain and fever (Hersh et al. 2000; Schrör 2009). In general, orally administrated aspirin is rapidly and completely absorbed from the GI tract. The maximal plasma levels (C max) of aspirin are usually reached within less than 30 min. During intestinal uptake and liver passage ASA is enzymatically converted in first-pass metabolism to its main active metabolite salicylic acid (SA). C max of SA is reached in a dose-dependent manner within 2–3 h (Schrör 2009).
Although aspirin was first synthesized in 1897 and sold as a tablet for many decades, new formulations have been continuously developed and marketed, e.g., dry granules, effervescent solution, and chewable tablets (Buellesbach 2007). A major challenge of pharmaceutical formulation activity is increasing the efficacy of the product by achieving faster dissolution and faster absorption of the active ingredient, which may lead to a faster onset of action in acute pain (Doyle et al. 2002). This has been proven to be extremely challenging since aspirin can exist in two forms due to its weak acidic nature (pKa = 3.5). At pH above 5, aspirin is an anion (R-COO−) that has a higher aqueous solubility and thus higher dissolution rate, while lower absorption through lipoid membranes. At pH below 2, aspirin exists as a neutral organic molecule (R-COOH) which lowers the aqueous solubility dramatically while enhancing the absorption. The molecule exists as a mixture of the ionic and neutral forms between pH 2–5. This pH dependency, combined with contradictory dissolution and absorption behaviors thus has a major impact on the pharmacokinetic profile of aspirin.
This paper describes the in vitro dissolution and the in vivo absorption of a new aspirin 500 mg tablet formulation containing micronized ASA and an effervescent component. This combination greatly enhances the in vitro dissolution and pharmacokinetics of aspirin.
Methods
Dissolution study
In vitro dissolution of micronized aspirin 500 mg tablets has been compared to conventional Aspirin® 500 tablets (Bayer HealthCare, Germany). The products were tested following the current USP/NF monograph dissolution procedure for aspirin tablets and USP general test <711> “Dissolution”. To capture the dissolution profiles of the two products, samples were taken at 1, 3, 6 and 15 min in addition to the USP required Q point at 30 min. A modified test procedure was also run by using dissolution media prepared (in accordance with the USP reagents section on the preparation of buffers) at pH 1.2 and 6.8 in addition to the standard aspirin tablet dissolution medium pH of 4.5. Calibration solutions containing ~1 mg/mL of ASA were prepared in the same pH buffer as the dissolution being performed. Prior to measurement, the isosbestic point for acetylsalicylic acid and salicylic acid was determined using 1 mg/mL solutions of each. The samples were collected and filtered through a 10 μm filter prior to measuring, at the predetermined isosbestic point, in a Cary Model 50 spectrophotometer. The calibration was current for the equipment employed for the analyses which were conducted in conformity with GMP requirements. Twelve dosage units of the products were tested and the average percent dissolution and relative standard deviation calculated.
Pharmacokinetic studies
Two identically designed phase 1 pharmacokinetic studies were conducted. Both were randomized, open-label, cross-over, single-dose, single-center studies conducted in healthy adult male and female subjects. Study treatments in study 1 included two different development candidates of 500 mg micronized aspirin tablets, 500 mg aspirin tablets (Aspirin®) and 500 mg aspirin dry granules (Aspirin® Effect). Study 2 investigated the final 500 mg micronized aspirin tablet formulation in comparison to 3 different 500 mg aspirin effervescent tablet formulations (Aspirin® Migraine, Alka Seltzer® Extra Strength, Aspro®). Institutional Review Board/Ethics Committee approval of these studies was obtained. The studies were conducted under Good Clinical Practice and the Declaration of Helsinki. Subjects gave written informed consent prior to randomization.
Blood samples for determination of plasma drug concentration of ASA and SA were collected in both studies prior to dosing (time 0) and at 5, 7.5, 10, 12.5, 15, 17.5, 20, 22.5, 25, 30, 35, 40, 45, 50, 55 min and at 1, 1.25, 1.5, 2, 3, 4, 5, 7, 9, 12 and 24 h post-dose. There was a 7-day wash-out period between dosing on day 1 and each subsequent treatment period. Acetylsalicylic acid and SA plasma concentrations were determined, and area under plasma concentration–time curve from time zero to the time of the last measurable concentration (AUC0–t), AUC from time zero extrapolated to infinity (AUC0–inf), maximum plasma concentration (C max), time to reach maximum plasma concentration (t max) and half-life (t 1/2) were calculated.
Statistical comparisons were performed after log transformation (natural log) of the data and tested for bioequivalence. Bioequivalence was based on C max and AUC0–t and AUC0–inf using 90% confidence intervals (CI) and equivalence was concluded if the CI for the ratios is contained within 80–125%.
Results
Dissolution study
Table 1 shows the average percent of dissolved aspirin of the final 500 mg micronized aspirin tablet formulation and the 500 mg aspirin standard tablet at pH of 1.2, 4.5 and 6.8, respectively, at time points measured. The micronized aspirin tablet formulation was dissolved to an extent of 92.5, 98.8 and 100.9%, respectively, at 15 min, while at the same time the aspirin standard tablet was dissolved to an extent of 47.6, 78.9 and 82.8%, at the three pH values, respectively. The mean percent dissolution versus time curves is shown in Fig. 1. The in vitro dissolution data show that the micronized aspirin tablet enters solution faster than the aspirin standard tablet at pH 1.2, 4.5 and 6.8. The rate of dissolution for both products appears dependent upon the pH of the dissolution medium, with a lower pH producing a slower rate of dissolution.
Table 1.
Percentage of dissolved active ingredient from micronized aspirin 500 mg tablet and aspirin 500 mg regular tablet (average% (RSD%))
| Time | Micronized aspirin 500 mg tablet | Aspirn® 500 mg tablet | ||||
|---|---|---|---|---|---|---|
| pH 1.2 | pH 4.5 | pH 6.8 | pH 1.2 | pH 4.5 | pH 6.8 | |
| 1 min | 28.8 (29.6) | 32.4 (15.3) | 30.3 (24.2) | 3.4 (23.8) | 5.6 (27.1) | 6.1 (20.7) |
| 3 min | 64.6 (6.1) | 87.4 (2.9) | 86.5 (3.6) | 8.4 (16.3) | 16.0 (9.6) | 16.5 (9.9) |
| 6 min | 80.6 (1.2) | 95.8 (0.8) | 98.1 (0.8) | 18.0 (6.2) | 34.9 (9.1) | 36.4 (5.5) |
| 15 min | 92.5 (3.1) | 98.8 (0.8) | 100.9 (0.7) | 47.6 (5.9) | 78.9 (9.1) | 82.8 (3.7) |
| 30 min | 101.7 (1.2) | 98.9 (0.7) | 101.0 (0.7) | 84.1 (3.6) | 99.3 (1.5) | 101.2 (1.6) |
Only the final development candidate is shown
Fig. 1.
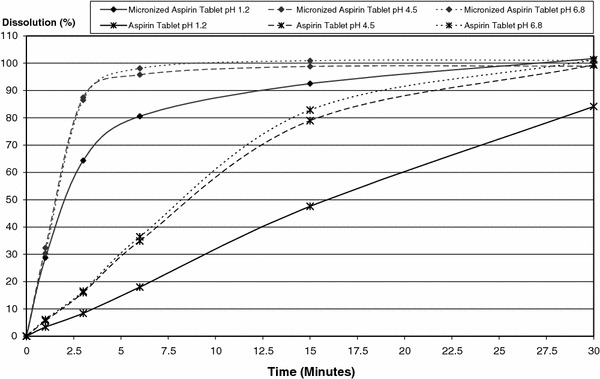
In vitro dissolution versus time curve of micronized aspirin 500 mg tablet* and aspirin 500 mg standard tablet at pH 1.2, 4.5 and 6.8 (*only the final development candidate is shown)
Pharmacokinetic studies
Study 1: micronized aspirin vs. aspirin tablet vs. aspirin dry granules
Thirty subjects were randomized (17 males, 13 females). Four subjects discontinued prematurely and 26 subjects completed the study. The mean (SD) age of randomized subjects was 33.5 (8.58) years, range 24–54. Mean weight of subjects was 72.4 kg (SD = 13.6), mean height 166.6 cm (SD = 10.1) and body mass index 25.9 (SD = 3.3).
The average maximum plasma concentration levels (C max) for ASA and SA were higher for micronized aspirin compared to aspirin plain tablets and aspirin dry granules (ASA: 13.8 μg/ml vs. 4.4 and 6.0 μg/ml, respectively, and SA: 35.1 μg/ml vs. 27.0 and 29.8 μg/ml, respectively) (Table 2, only the data of the final micronized aspirin development candidate are shown). With respect to total extent of absorption AUC0–t and AUC0–inf, all formulations were equivalent. Acetylsalicylic acid AUCs are between 6.2 and 7.0, SA AUCs are between 177 and 205 (Table 2). Confidence intervals of the ratios were within the equivalence acceptance range of 80-125% (Table 3).
Table 2.
Pharmacokinetic data of aspirin formulations in plasma (mean (SD) for C max, AUCs and t 1/2 and median (min, max) for t max)
| Analyte | Parameter (unit) | Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Micronized aspirin tableta | Aspirin® tablet | Aspirin® dry granules | Micronized aspirin tabletb | Aspirin Migraine® effervescent tablet | Alka Seltzer® effervescent tablet | Aspro® effervescent tablet | ||
| ASA | C max (μg/mL) | 13.8 (2.88) | 4.4 (1.54) | 6.0 (1.64) | 13.1 (3.09) | 11.7 (3.27) | 9.4 (2.45) | 11.5 (3.01) |
| AUC0–t (μg h/mL) | 6.2 (1.12) | 6.4 (1.53) | 6.8 (1.33) | 6.6 (1.43) | 5.4 (1.36) | 4.8 (1.29) | 5.8 (1.27) | |
| AUC0–inf (μg h/mL) | 6.2 (1.12) | 6.5 (1.55) | 7.0 (1.33) | 6.7 (1.42) | 5.5 (1.37) | 4.9 (1.30) | 5.9 (1.28) | |
| t max (min) | 17.5 (15.0, 25.0) | 45.0 (22.5, 240.0) | 25.0 (15.0, 75.0) | 19.8 (12.6, 30.0) | 17.4 (12.6, 49.8) | 19.8 (10.2, 55.2) | 19.8 (10.2, 40.2) | |
| t 1/2 (h) | 0.35 (0.06) | 0.54 (0.17) | 0.46 (0.19) | 0.36 (0.06) | 0.32 (0.05) | 0.31 (0.06) | 0.33 (0.07) | |
| SA | C max (μg/mL) | 35.1 (6.68) | 27.0 (4.86) | 29.8 (6.57) | 31.9 (6.51) | 29.2 (4.95) | 27.0 (5.27) | 27.8 (5.47) |
| AUC0–t (μg h/mL) | 190.0 (52.03) | 177.1 (46.08) | 194.6 (51.76) | 175.2 (51.87) | 143.1 (35.13) | 129.0 (39.90) | 142.9 (43.45) | |
| AUC0–inf (μg h/mL) | 198.4 (54.12) | 189.4 (50.59) | 205.2 (54.29) | 183.9 (53.99) | 148.9 (36.94) | 133.5 (40.73) | 147.8 (44.22) | |
| t max (min) | 45.0 (20.0, 90.0) | 180.0 (90.0, 300.0) | 120.0 (75.0, 300.0) | 49.8 (17.4, 90.0) | 45.0 (17.4, 90.0) | 45.0 (17.4, 90.0) | 49.8 (19.8, 121.2) | |
| t 1/2 (h) | 2.55 (0.59) | 2.79 (0.55) | 2.70 (0.55) | 2.68 (0.65) | 2.50 (0.59) | 2.46 (0.64) | 2.49 (0.55) | |
aOnly the final developmental candidate is shown
bFinal development candidate
Table 3.
Ratios of geometric means and 90% confidence intervals of C max and AUC
| Analyte | Parameter (unit) | Study 1 | Study 2 | |||
|---|---|---|---|---|---|---|
| Micronized aspirin tableta/Aspirin® tablet | Micronized aspirin tableta/Aspirin® dry granules | Micronized aspirin tabletb/Aspirin Migraine® effervescent tablet | Micronized aspirin tabletb/Alka Seltzer® effervescent tablet | Micronized aspirin tabletb/Aspro® effervescent tablet | ||
| ASA | C max (μg/mL) | 3.29 (292–370) | 2.33 (207–262) | 1.15 (106–125) | 1.39 (128–151) | 1.14 (105–124) |
| AUC0–t (μg h/mL) | 0.96 (92–100) | 0.92 (88–96) | 1.22 (118–128) | 1.37 (132–143) | 1.14 (109–118) | |
| AUC0–inf (μg h/mL) | 0.95 (91–99) | 0.91 (87–95) | 1.23 (118–128) | 1.37 (132–143) | 1.13 (109–118) | |
| SA | Cmax (μg/mL) | 1.28 (123–133) | 1.18 (113–122) | 1.10 (106–113) | 1.19 (116–123) | 1.16 (113–119) |
| AUC0–t (μg h/mL) | 1.07 (104–110) | 0.99 (96–102) | 1.21 (118–125) | 1.40 (136–144) | 1.25 (122–129) | |
| AUC0–inf (μg h/mL) | 1.04 (101–107) | 0.98 (95–101) | 1.23 (119–126) | 1.42 (137–146) | 1.27 (123–130) | |
aOnly the final developmental candidate is shown
bFinal development candidate
Plasma concentration versus time curves for ASA and SA display the increase in C max for the micronized aspirin formulation (Fig. 2, only the data of the final micronized aspirin development candidate are shown). A decrease in t max for ASA and SA of the micronized aspirin formulation compared to both comparisons also is shown. Median time to t max for ASA and SA was 17.5 and 45.0 min for micronized aspirin compared to 45.0 and 180 min for the plain tablet and 25.0 and 120 min for dry granules (Table 2). Mean half-life (t 1/2) of plasma ASA was relatively short for all treatments ranging from 0.35 to 0.54 h. The respective results for plasma SA were longer, ranging from 2.5 to 2.8 h (Table 2).
Fig. 2.
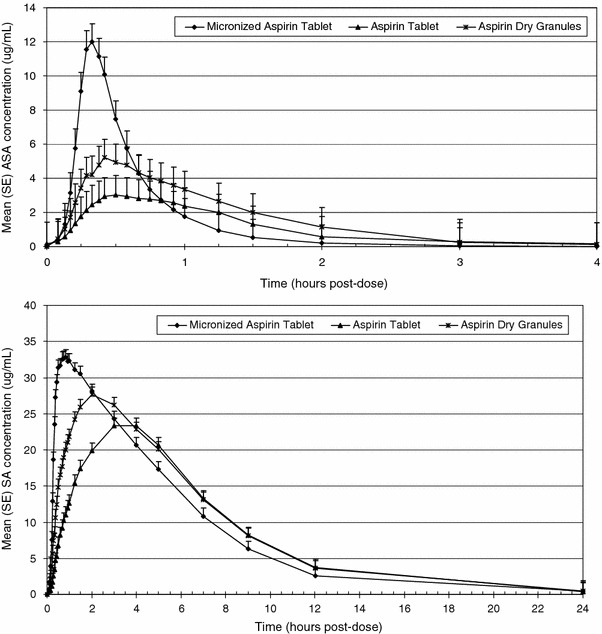
Plasma concentration versus time curves of acetylsalicylic acid (ASA) and salicylic acid (SA) for micronized aspirin tablet*, aspirin regular tablet and aspirin dry granules (*only the final development candidate is shown)
Study 2: micronized aspirin vs. aspirin effervescent tablets
Thirty-two subjects were randomized (20 males, 12 females). Six subjects discontinued prematurely and 26 subjects completed the study. The mean (SD) age of randomized subjects was 34.0 (8.98) years, range 20–52. Mean weight of subjects was 76.1 kg (SD = 11.9), mean height 168.8 cm (SD = 9.0) and body mass index 26.6 (SD = 2.8).
The average maximum plasma concentration levels (C max) ASA and SA were slightly higher for micronized aspirin compared to aspirin effervescent tablets (ASA: 13.1 μg/ml vs. 11.7, 9.4 and 11.5 μg/ml, respectively and SA: 31.9 μg/ml vs. 29.2, 27.0 and 27.8 μg/ml, respectively) (Table 2), but 90% confidence intervals of the ratios were still within the equivalence acceptance range of 80–125% for the comparisons with Aspirin Migraine® and Aspro®, but not with ASA Alka Seltzer® (Table 3). Measures of the total extent of absorption (AUC0–t and AUC0–inf) were also slightly higher (ASA: 6.6 and 6.7 for micronized aspirin and 4.8–5.8 and 4.9–5.9 for effervescent aspirin formulations; SA: 175.2 and 183.9 for micronized aspirin and 129.0–143.1 and 133.5–148.9 for effervescent aspirin formulation).
Plasma concentration versus time curves for ASA and SA demonstrated an increase in C max for the micronized aspirin formulation (curves not shown). A slightly greater t max for ASA of the micronized aspirin formulation compared to Aspirin Migraine® effervescent tablet, but identical to Alka Seltzer® and Aspro® effervescent tablets was observed (19.8 min vs. 17.4, 19.8 and 19.8 min, respectively) (Table 2). With respect to SA data were 49.8 min for micronized aspirin tablet compared to 45.0, 45.0 and 49.8 min, respectively, for Aspirin Migraine®, Alka Seltzer® and Aspro® effervescent tablets (Table 2). Mean half-life (t 1/2) of plasma ASA was relatively short for all treatments (0.31 to 0.36 h). The respective results for plasma SA were longer (2.46 and 2.68 h) (Table 2).
Discussion
Solid material dissolution can be described by the Nernst–Brunner/Noyes–Whitney equation, which shows that the dissolution rate is proportional to the surface area available for dissolution, provided the amount dissolved is less than the saturation solubility of the material (Neeti et al. 2011). The published solubility data of aspirin, although not always consistent, suggest a solubility of more than 4 mg/mL at 37o C in 0.1 N HCl (Al-Maaieh and Aburub 2005). The solubility increases with increasing environmental pH and the solubility of aspirin at starting pH above 3.5 is limited by the solution buffer capacity. It has been suggested that aspirin solubility can exceed 200 mg/ml (i.e., 100 g/500 ml) if the solution ending pH can be kept above 5 (Fahmy et al. 2001). It is clear, therefore, that 500 mg aspirin should be soluble in 500 mL of the dissolution media at pH 1.2, where the molecule exists at its neutral form. The incomplete dissolution of the regular aspirin tablet at pH 1.2 is then not due to saturation solubility but rather due to the dissolution kinetics.
The pKa of acetylsalicylic acid is 3.5; therefore, at a pH of 1.2 it will be mostly in the protonated state and have relatively lower solubility. At a pH of 4.5, it will be mostly in the ionized state and its solubility will be significantly greater. At a pH of 6.8, it will be nearly completely ionized and maximum solubility will be approached. This can be observed in Fig. 1 where the 1.2 pH dissolution medium results in both a much lower solubility and lower percent dissolution profile compared to the pH 6.8 medium and pH 4.5 medium. However, the micronized aspirin tablet reached >80% dissolution within 6 min and >90% within 15 min at all measured pH. An increase in the dissolution rate of the new formulation compared to the standard aspirin tablet was observed at every pH.
The European Medicines Agency on the Investigation of Bioequivalence has defined that for an immediate release drug product very rapid dissolution means the in vitro dissolution is >85% in less than 15 min (European Medicines Agency 2010). The new micronized aspirin 500 mg tablet is much faster dissolving than the regular aspirin 500 mg tablet and reaches more than 90% dissolution within 15 min for the pH range from 1.2 to 6.8. The interesting feature is that even at acidic conditions, representative of the stomach fluid, the new product is highly dissolved although the non-ionized form of aspirin, mainly existing in the stomach, is of very poor solubility (Schrör 2009). In addition to the fast dissolution, the new aspirin formulation has a significantly shorter t max compared to marketed Aspirin® formulations tablet and dry granules. A reduction of 30% (7.5 min) and 61% (27.5 min), respectively, for ASA and a reduction of 62% (75 min) and 75% (135 min), respectively, for SA compared to the aspirin dry granules and aspirin tablets was observed. It is quite reasonable to anticipate that this may lead to an improvement in the onset of analgesia. On the other hand, total drug exposure is almost identical to existing formulations. AUC ratio of the micronized aspirin tablet and aspirin regular tablet showed equivalence (90% confidence intervals of the ratios are within 80–125%) of total exposure of the two formulations. With comparable outcomes relative to the effervescent formulations Aspirin Migraine®, Alka Seltzer® and Aspro®, it could be considered that the overall efficacy and safety profile of aspirin has not changed. With respect to C max the new formulation shows an increase compared to the tablets (as a consequence of the faster t max), but still shows equivalence with pre-dissolved effervescent formulations Aspirin Migraine® and Aspro®.
The observed pharmacokinetic behavior can be explained by a pH-dependent ionization model of acetylsalicylic acid. The cell membranes are lipoid and allow lipophilic drugs to diffuse most rapidly. Unionized forms of a drug substance are more lipophilic and diffuse readily across the membrane. The improvement in absorption for the micronized aspirin tablet is predicted by the degree of ionization of the drug. The distribution of acetylsalicylic acid between its non-ionic, lipophilic form and its ionic, negatively charged hydrophilic form is strongly dependent on pH. Although the lipophilic form can be absorbed easily, the ionic form is less dissolvable at an acidic pH. An in vitro simulation of local stomach pH of different aspirin products after dosing with water showed that the micronized aspirin tablet did not raise the pH of the stomach significantly as compared to other acetylsalicylic acid formulations. As the pKa of acetylsalicylic acid is 3.5, the micronized aspirin tablet would have a very low degree of ionization, while other products would be mostly ionized, leading to more rapid absorption of the drug for the micronized aspirin tablet. Given the rapid dissolution of the micronized tablet even in low pH environments and low ionization of the drug, it is reasonable that the rate of absorption (C max) is almost identical to pre-solubilized effervescent tablets.
Whether the improved dissolution and pharmacokinetic data will result in a improvement of onset of action in treating acute pain needs to be proven in a clinical study.
Conclusions
The new micronized aspirin tablet is a very rapid dissolving aspirin formulation showing a substantial improvement of time to maximum plasma concentration compared to regular aspirin tablets. This suggests that this new formulation has a faster onset of analgesic effect. Total extent of exposure is almost identical with the regular tablet and C max values are comparable to effervescent solutions of aspirin suggesting no major changes in overall tolerability of the drug.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Al-Maaieh A, Aburub A (2005) Salt effect on a neutral species reaction: acidic hydrolysis of aspirin. AAPS J 7(S2)
- Buellesbach R. Aspirin®: a sucessful example of formulation technology. In: Bröckel U, Meier W, Wagner G, editors. Product design and engineering: best practices. Weinheim: Wiley-VCH; 2007. pp. 569–582. [Google Scholar]
- Doyle G, Jayawardena S, Ashraf E, Cooper SA. Efficacy and tolerability of non-prescription ibuprofen versus celecoxib for dental pain. J Clin Pharmacol. 2002;42:912–919. doi: 10.1177/009127002401102830. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2010) Committee for Medicinal Products for Human Use (CHMP). Guideline on the Investigation of Bioequivalence. CPMP/QWP/EWP/1401/98 Rev. 1, London, 20 January 2010
- Fahmy R, Marnane W, Bensley D, Hollenbeck RG. Dissolution testing of veterinary products. Dissolution testing of aspirin boluses. Dissolution Technol. 2001;8(1):8–14. [Google Scholar]
- Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a criticalassessment. Clin Ther. 2000;22:500–548. doi: 10.1016/S0149-2918(00)80043-0. [DOI] [PubMed] [Google Scholar]
- Neeti R, Murugesan SK, Nanjaian M. Solubility: particle size reduction is a promising approach to improve the bioavailability of lipophilic drugs. Int J Recent Adv Pharm Res. 2011;1:8–18. [Google Scholar]
- Schrör K. Acetylsalicylic acid. Weinheim: Wiley-VCH; 2009. [Google Scholar]


