Abstract
Congenital melanocytic nevi (CMN) are pigmented birthmarks that affect up to 80% of the skin surface area. The increased frequency of CMN in families of severely affected individuals is suggestive of a predisposing germline genotype. We noted a high prevalence of red hair in affected families, and considered a role for MC1R in this condition. A cohort of 166 CMN subjects underwent pigmentary phenotyping, with MC1R genotyping in 113. Results were compared with a local control group of 60 unrelated children and with 300 UK children without CMN. CMN subjects had higher prevalences of red hair and a red-haired parent than local controls and had a higher rate of compound heterozygosity and homozygosity for MC1R variants. The presence of a V92M or R allele (D84E, R151C, R160W, D294H) was associated with increasing size of the CMN, implying a growth-promoting effect of these alleles. Unexpectedly, the V92M and R151C alleles were also strongly associated with birth weight in the CMN cohort, a finding confirmed in the control group. The effect of germline MC1R genotype on development and severity of CMN led us to investigate potential broader effects on growth, revealing a role for MC1R in normal fetal development.
Introduction
Congenital melanocytic nevi (CMN) are congenital moles that cover up to 80% of the body surface area and can number hundreds in a single individual. They are permanent and grow in proportion to the child, although they often lighten in color (Strauss and Newton Bishop, 2008; Kinsler and Bulstrode, 2009; Kinsler et al., 2009b). They are currently categorized by projected adult size (PAS) of the largest nevus and by total number of nevi (Ruiz-Maldonado, 2004). Neurological abnormalities such as neuromelanosis, congenital malformations, hydrocephalus, and benign tumors are the commonest complication of CMN, with the prevalence increasing with the severity of cutaneous lesions (Kadonaga and Frieden, 1991; DeDavid et al., 1996; Kinsler et al., 2008). Primary malignant melanoma of the skin or the CNS is a much rarer complication in childhood (Hale et al., 2005; Krengel et al., 2006; Kinsler et al., 2009a), although almost universally fatal, and prophylactic removal of skin lesions has not been shown to decrease the risk of malignancy (Kinsler and Bulstrode, 2009). The recent finding of characteristic facies in children with CMN has broadened the description of this condition, and the term CMN syndrome has been proposed with preliminary diagnostic criteria (Kinsler et al., Am J Med Genet, 2012 (in press)). The genetic cause of these syndromic features is likely to be a somatic change affecting the ectoderm or neural crest, but is still to be elucidated.
There is, however, evidence of a germline predisposition for the development of CMN syndrome; this includes a higher prevalence of smaller CMN in family members of affected individuals (Kinsler et al., 2009a), together with a handful of reports of large CMN in first-degree family members (Danarti et al., 2003; de Wijn et al., 2010). We hypothesized that the melanocortin-1-receptor (MC1R) gene might have a role in this predisposition, on the basis of a clinical observation of a high prevalence of red hair in families of children with CMN (for examples see Figure 1). The red hair/fair skin/freckling phenotype is strongly associated with variants of MC1R on chromosome 16q24.3 (Valverde et al., 1995; Box et al., 1997; Schioth et al., 1999; Healy et al., 2000; Bastiaens et al., 2001). MC1R is a G-protein-coupled receptor expressed on melanocytes, melanoma cells, and CMN cells (Ghanem et al., 1988; Donatien et al., 1992; Mountjoy et al., 1992; Loir et al., 1999). The gene is highly polymorphic, with over 100 variants reported to date. Variants with a stronger association with the red-hair phenotype in humans have been designated R alleles (D84E, R151C, R160W, and D294H), whereas those with a weaker association are designated r alleles (Sturm et al., 2003). This study was undertaken to examine whether the MC1R genotype influenced the development of CMN.
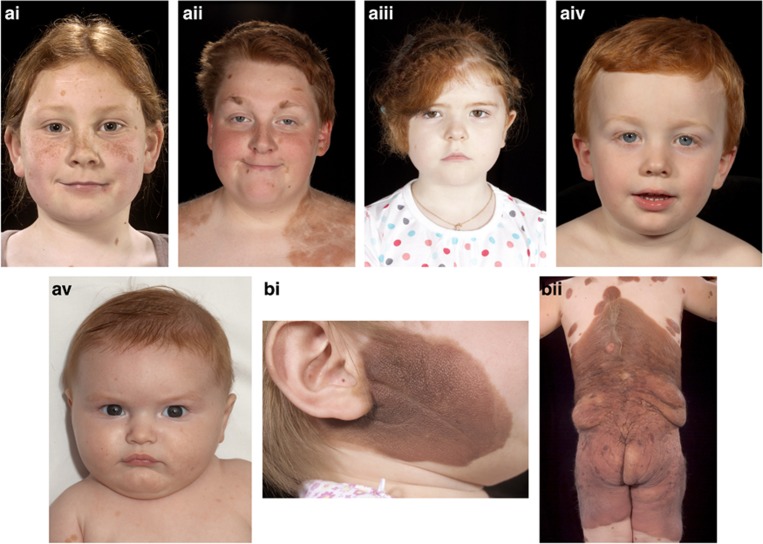
Figure 1.
Clinical images of children with congenital melanocytic nevi (CMN). Examples of (ai–v) red-haired children with CMN, and (b) different cutaneous phenotypes of CMN. (i) Projected adult size (PAS) 10–20 cm, (ii) PAS >60 cm with several other nevi also visible. Written, informed consent was obtained for publication in all cases.
Results
A total of 166 children with CMN were included in the study. The cutaneous phenotype was classified as in Table 1.
Table 1. Cutaneous phenotype of the CMN cohort.
| Projected adult size of largest CMN (cm) | N (%) | Site of largest CMN | N (%) |
|---|---|---|---|
| <10 | 35 (21) | Face | 10 (6) |
| 10–20 | 28 (17) | Scalp | 19 (12) |
| 20–40 | 27 (16) | Neck | 85 (51) |
| 40–60 | 24 (15) | Trunk | 25 (15) |
| >60 | 48 (29) | Scalp/neck/trunk | 9 (6) |
| No single larger lesion | 4 (2) | Face/scalp | 14 (8) |
| Missing data | 0 | No single larger lesion | 4 (2) |
| Missing data | |||
| Total | 166 (100) | Total | 166 (100) |
|
Total number of other nevi at birth |
N (%) |
Total number of other nevi at enrollment |
N (%) |
| 0 | 28 (17) | 0 | 28 (17) |
| 1–10 | 37 (22) | 1–10 | 25 (15) |
| 11–20 | 25 (15) | 11–20 | 20 (12) |
| 21–50 | 26 (16) | 21–50 | 19 (11) |
| 51–100 | 17 (10) | 51–100 | 22 (13) |
| 101–200 | 8 (5) | 101–200 | 28 (17) |
| >200 | 3 (2) | >200 | 18 (11) |
| Missing data | 22 (13) | Missing data | 6 (4) |
| Total | 166 (100) | Total | 166 (100) |
Abbreviation: CMN, congenital melanocytic nevi.
Pigmentary phenotype data
Comparisons between affected and unaffected subjects were restricted to Caucasian subjects of Northern European ancestry (i.e., white-skinned individuals with four Northern European grandparents) who constituted the majority of the study group. Two control groups were ascertained; these were designated as control groups A (60 unrelated children from the same school, street, or locality as the affected individuals) and B (300 UK children without CMN from the Avon Longitudinal Study of Parents and Children (ALSPAC)) (Jones et al., 2000; Golding et al., 2001). Comparison of the pigmentary phenotype of the Northern European white children showed no statistical differences in phenotype between control groups A and B, although as the numbers in group A are relatively small we cannot exclude the possibility that these groups were different in some respect, and as a result we have used control group B for genotype data. There were significantly fewer blonde/light brown–haired individuals in the CMN cohort than in control group A and separately in control group B (P=0.0215 and P=0.0275 respectively; Table 2). In addition, fewer dark brown/black-haired individuals were present in the CMN cohort than in control group A (P=0.0293), whereas this was similar in the CMN cohort and control group B. Conversely, CMN children were more likely to have red hair than the larger control group B (P=0.0085), whereas this was not significantly different from the number of red-haired children in control group A. However, CMN children were more likely to have at least one red-haired parent (32 of 107 (30%)) than control group A (1 of 47 (2%)), respectively (P<0.0001), evenly divided between mothers and fathers. Parental pigmentary phenotype was not available for control group B. The pigmentary phenotype of the Northern European white children is shown in Table 2.
Table 2. Pigmentary phenotype of the CMN cohort and control groups.
| CMN cohort (N=129) | Control group A (N=55) | Control group B (N=287) | |
|---|---|---|---|
| Predominant hair color | |||
| Blonde/light brown | 83/119 (69.7) | 46/53 (86.8) | 230/287 (80.1) |
| Dark brown/black | 25/119 (21.0) | 4/53 (7.5) | 49/287 (17.1) |
| Red | 11/119 (9.3) | 3/53 (5.7) | 8/287 (2.8) |
| Peripheral predominant iris color | |||
| Blue/gray | 69/107 (64.5) | 34/54 (63.0) | 164/287 (57.2) |
| Green/hazel | 12/107 (11.2) | 10/54 (18.5) | 69/287 (24.0) |
| Dark brown | 26/107 (24.3) | 10/54 (18.5) | 54/287 (18.8) |
| Freckles | 24/110 (21.8) | 19/54 (35.2) | 77/287 (26.8) |
Abbreviation: CMN, congenital melanocytic nevi.
Numbers shown are absolute values with percentages in brackets.
MC1R variants, particularly the R alleles, are known to be causally associated with red hair (Valverde et al., 1995; Box et al., 1997; Smith et al., 1998; Sulem et al., 2007), and there is some evidence that the MC1R genotype may be associated with blonde/light brown hair (Box et al., 1997; Smith et al., 1998). On the basis of these phenotypic observations between affected and unaffected groups, we hypothesized that the MC1R genotype may influence the development of CMN.
MC1R genotype data
According to the phenotyping described above, comparison of the MC1R gene between cohorts was restricted to white-skinned children of Northern European ancestry. To ensure sufficient numbers for statistical analysis of prevalence of MC1R polymorphisms, members of the affected group and control group B were sequenced for MC1R. Individuals with CMN were more likely to have two MC1R variant alleles than controls (P=0.0052, Table 3). More detailed analysis indicated that this was not due to a difference in the frequency of a particular MC1R variant between these two groups (see Supplementary table S1 online). The MC1R genotype in three pairs of affected and unaffected skin samples was identical with the germline genotypes, suggesting that somatic MC1R mosaicism was not the causative feature of affected versus unaffected skin.
Table 3. Comparison of prevalence of carriage of non-synonymous MC1R variants found in the white Northern European Caucasian children of the CMN cohort and control group B.
| CMN cohort, N (%) | Control group B, N (%) | Fisher's exact P-value | |
|---|---|---|---|
| Wild type | 17/84 (20.2) | 82/267 (30.7) | 0.0711 |
| Heterozygous | 40/84 (47.6) | 139/267 (52.1) | 0.5320 |
| Compound heterozygous | 20/84 (23.8) | 39/267 (14.6) | 0.0647 |
| Homozygous | 7/84 (8.3) | 7/267 (2.6) | 0.0478* |
| Compound heterozygous or homozygous | 27/84 (32.1) | 46/267 (17.2) | 0.0052** |
| Total | 84/84 (100) | 267/267 (100) |
Abbreviation: CMN, congenital melanocytic nevi.
Significance at 0.05 level* and **0.01 level.
In addition to coding region variants, a known SNP in the 3′ UTR (rs3212369) was found at a high frequency in both populations, and found to cosegregate strongly but not exclusively with the V92M and T314T alleles (P<0.001 for Pearson's correlation coefficients on all combinations of two variables). However, as an extension to previous reports (Harding et al., 2000), the pattern of segregation suggested that this SNP arose independently of the T314T variant. This implies that either the V92M variant arose twice independently, on the 3′ UTR (rs3212369) and T314T alleles, or that there was intragenic recombination within MC1R.
Association between the MC1R genotype and CMN phenotype
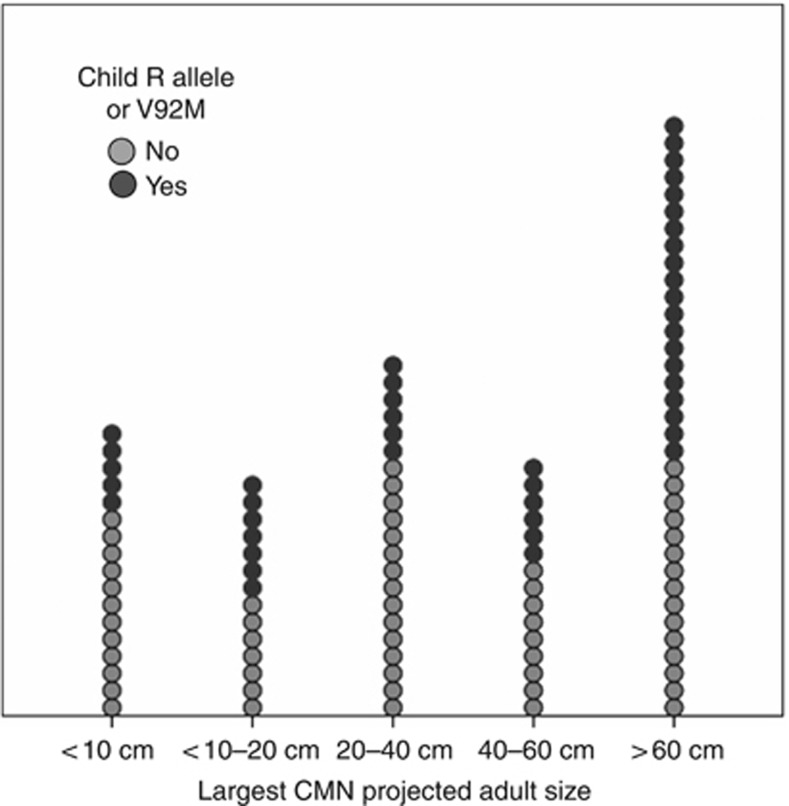
In the CMN cohort, the presence of a V92M or an R allele (D84E, R151C, R160W, D294H) was found to be significantly associated with increasing projected adult size (PAS) of the largest CMN (P=0.01) (Figure 2). We examined this effect in more detail using >60 cm PAS as a cutoff, as this has previously been noted to be a significant predictor for increased incidence of malignant melanoma (Demenais et al., 2010). The odds ratio for having a CMN of PAS >60 cm compared with those of <60 cm if carrying V92M or an R allele was 2.867 (95% CI 1.1257–6.537, P=0.012).
Figure 2.
Association between MC1R genotype and severity of cutaneous phenotype in congenital melanocytic nevi (CMN). Dot plot of proportions of V92M- or R allele–positive individuals with increasing projected adult size of largest CMN.
Association between the MC1R genotype and family history of CMN
Thirty-four percent of the children with CMN had a first- or second-degree family history of CMN. If the MC1R genotype were one of the predisposing factors for development of CMN, a difference in MC1R genotype between those with and without a positive family history of CMN would be expected. This was indeed the case, with a significantly higher prevalence of at least one MC1R variant in those with a family history of CMN versus those in whom no first- or second-degree relative had a CMN (91 vs. 59% respectively, P=0.0062). When confined to the V92M and/or R alleles, a similar preponderance of variants was noted in CMN children with a family history versus those without a family history of CMN (65 vs. 30%, P=0.018).
Association of the MC1R genotype and birth weight
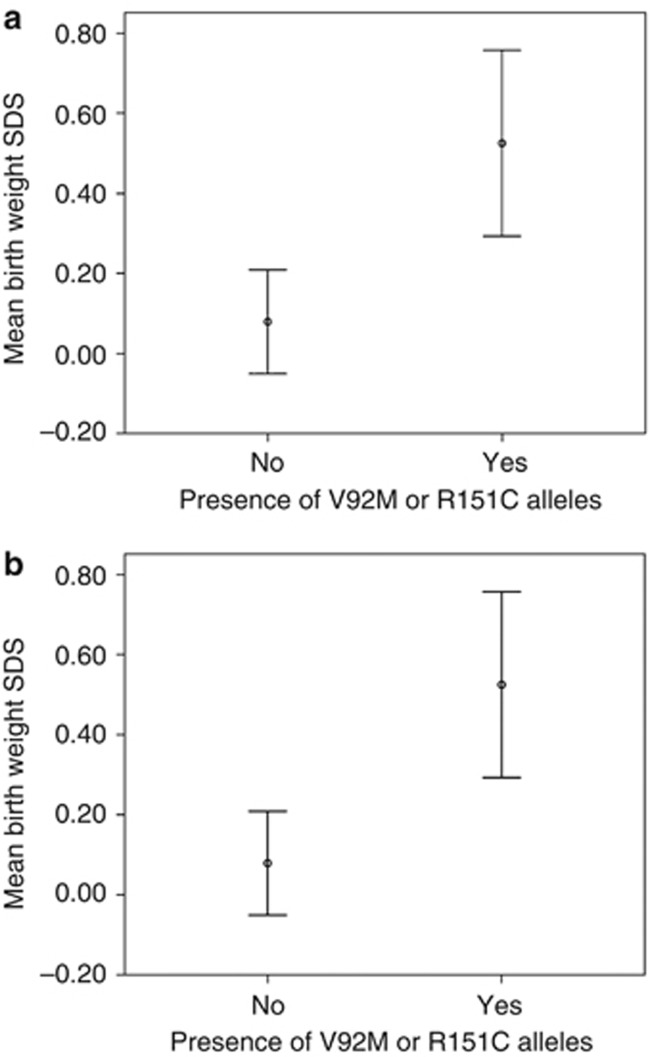
The above results, in which MC1R variants were associated with larger CMN and more nevi, indicate that the MC1R genotype influences the development of CMN. However, an additional possibility is the existence of a broader role for MC1R genotype in fetal growth and development, especially that MC1R variants function in promoting growth of the fetus. Therefore, we examined for associations between V92M and R alleles and birth weight in the CMN cohort and control group B. The presence of a V92M or R151C was significantly associated with increased birth weight in the CMN cohort (unstandardized coefficient +697 g, SE 216 g, P=0.002), independent of gender and maternal smoking (Figure 3a). A similar significant effect of the same MC1R alleles was observed on birth weight (+187 g, SE 59 g, P=0.002) in the ALSPAC cohort, control group B (Figure 3b). This effect was independent of sex, gestation, maternal smoking, maternal weight pre-pregnancy, and maternal hypertension. Thus, the magnitude of this genotype effect is comparable to the effect of gender in this study. There was no significant association between R alleles as a group and birth weight in either cohort. No association between postnatal weight at the age of 49 months and MC1R genotype was found in control group B.
Figure 3.
Association between MC1R genotype and birth weight. Effect of the presence of V92M or R151C alleles on birth-weight standard deviation score (SDS) in (a) the congenital melanocytic nevi (CMN) cohort, and (b) control group B. SDS includes the effects of gestation and sex, and a score of +1 is equivalent to an increase in one SD from the mean of the population. Error bars are 95% confidence intervals.
Discussion
Previous studies of the effects of MC1R genotype in postnatal life have documented fewer acquired melanocytic nevi in red-haired children and adults (Duffy et al., 2004; Dellavalle et al., 2005), and in individuals homozygous or compound heterozygous for R alleles at the MC1R locus (Duffy et al., 2004). In addition, genome-wide association studies have not found any association between MC1R genotype and numbers of acquired melanocytic nevi (Falchi et al., 2006; Yeh and Bastian, 2009), and no association between MC1R variants and higher numbers of acquired nevi in families with inherited melanoma (Demenais et al., 2010). However, in the current study, we have identified associations between families with CMN-affected children and the red hair pigmentary phenotype, and associations between MC1R variants and the presence of CMN, as well with more extensive CMN. Taken in the context of the effect on birth weight, this difference between congenital and acquired nevi may be due to the differential effects of MC1R during pre- and postnatal life, although other inherent genetic differences between congenital and acquired nevi could also be significant factors.
Our findings indicate that certain MC1R variants function to promote the growth of CMN during embryogenesis, and possibly the growth of the fetus as a whole. The exact mechanism whereby MC1R variants alter fetal growth and development are unknown, but might involve direct effects on the fetus and/or indirect effects through the presence of MC1R on trophoblast cells of the placenta (Thornwall et al., 1997). MC1R is expressed on a wide range of adult human cell types including adipocytes (Hoch et al., 2007; www.genecards.com; Rebhan et al., 1997), supporting the increasing body of evidence that it has significant non-pigmentary roles (Robinson and Healy, 2002; Mogil et al., 2005; Robinson et al., 2010); alpha-MSH is involved in metabolic pathways, but this is currently understood to be via the other melanocortin receptors. Melanocortin-1-receptor (MC1R) genotype is known to interact with pigmentary and non-pigmentary pathways (Robinson and Healy, 2002; Cooper et al., 2005; Robinson et al., 2010), for example, altering the penetrance of other genes in melanoma such as p53 (Patton et al., 2005; Stefanaki et al., 2007; Nan et al., 2008) and CDKN2A (Box et al., 2001; Debniak et al., 2006). The latter effects are dependent not only on particular alleles but also on the number of variant alleles present (Demenais et al., 2010). A similar interactive effect may be operating in individuals with CMN, with MC1R variants exerting a phenotype-exacerbating effect on a different somatic, or possibly germline, genetic alteration that affects CMN development. The effect of MC1R genotype on birth weight in our study was a generalized phenomenon that was found in two separate cohorts (CMN and ALSPAC), and in the MC1R-pigmentary context the previously reported interactive effects of MC1R genotype on oculocutaneous albinism (King et al., 2003) was a generalized, rather than localized, effect on pigmentation. The fact that in the ALSPAC cohort of healthy children the larger birth weight in subjects with MC1R variants was not associated with any other dysmorphic features suggests that in the normal situation MC1R genotype may influence fetal development to affect body size in a proportionate manner. Interactive effects of MC1R with other genes affecting birth weight have not yet been investigated, and further work on this topic will be required in the future.
MC1R variants are a risk factor for melanoma development in the general population and are associated with increased risk of melanoma in CDKN2A mutation carriers (Box et al., 2001; Demenais et al., 2010). One important clinical consideration in our patient cohort is whether the pattern of germline MC1R genotype is responsible for the excess risk of malignant melanoma in individuals with CMN. The lifetime risk for melanoma is around 1–2% when all CMN are considered together as a group (Krengel et al., 2006; Kinsler et al., 2009a), which is similar in magnitude to the excess risk associated with MC1R variants in the normal population. The numbers of melanomas in our CMN cohort are too few to allow detailed investigations on the risk of melanoma attributable to MC1R variants, but it is likely that this genotype is at least contributing to melanoma risk. In a recent family-based melanoma study, the odds ratio for melanoma in CDKN2A mutation carriers was 5.67 (95% CI 2.1–15.29) for subjects with MC1R variants (Demenais et al., 2010); thus, it is possible that the MC1R genotype may similarly alter the risk of melanoma in people with larger CMNs.
In conclusion, we have identified associations between CMN and red-hair/MC1R variants. Furthermore, the presence of a V92M or R allele was associated with larger CMN size and with larger birth weight in the CMN, and separately in the ALSPAC, groups consistent with a growth-promoting effect of these alleles during embryogenesis.
Materials and Methods
This study was approved by the research ethics committee of Great Ormond Street Hospital for Children/Institute of Child Health, London. All aspects of this study comply with the Declaration of Helsinki Principles. Written, informed consent was given by parents in all cases, and by subjects, where appropriate.
CMN cohort
In all, 166 families with children with CMN were recruited for pigmentary phenotyping during clinical consultations, and offered MC1R genotyping. CMN phenotype was classified as previously published (Ruiz-Maldonado, 2004; Kinsler et al., 2008, 2009a, 2009b) (Table 1; for examples see Figure 1). The total number of nevi at birth was ascertained by history, direct examination, or photographs. Mean and median ages at enrollment to the study were 5.02 years (SEM 0.53) and 2.54 years (range 0.03–43.12), respectively. Follow-up during the study period was for a mean of 3.09 years and median of 3.12 years. Pigmentary phenotyping was performed by a single observer (VK) and was assessed after 1 year of age because of the changes frequently seen in hair and eye color in the first year. Hair color was classified using a chart of 12 colors; eye color was grouped into six standard colors, with peri-pupillary and predominant background iris color considered separately; freckling was recorded by grouped number and distribution; and skin type was assessed using the Fitzpatrick score. Pigmentary phenotype was obtained for 148 children, 143 mothers, and 130 fathers. Ancestry, up to the four grandparents, was also recorded; patients were classified as white Northern European Caucasians if their skin color was white and if all four of their grandparents were born in Northern Europe. Consent was obtained for MC1R genotyping in 113 families. Adequate blood samples for DNA extraction were obtained from 104 children, 78 mothers, and 58 fathers. With further written consent, three children had skin biopsies taken from affected and unaffected skin, whereas they were undergoing general anesthesia for other procedures.
Control group A
With the assistance of the CMN families, we recruited a geographically matched and sex-matched control population of individuals without CMN. Sixty families completed the same pigmentary phenotyping questionnaires as the CMN group, with telephone guidance from the same researcher. Fifty-five were white Northern European Caucasians, i.e., four grandparents born in Northern Europe; the mean and median age of these 55 children was 7.2 years (SEM 1.1) and 5.6 years (range 0.7–37.6), respectively. To check whether self-reported phenotypes were accurate, 25 CMN families (66 individuals) completed the pigmentary phenotype questionnaire before undergoing face-to-face phenotyping. Pearson's correlations for hair color and eye color were 0.509 and 0.855, respectively, significant at <0.05 level, supporting the use of self-reported questionnaires in this context.
Control group B
Ethical approval for this part of the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees. The children in this group born in 1992 had face-to-face pigmentary phenotyping by trained observers at the age of 49 months, and the presence of CMN was noted. The 300 samples were chosen randomly from those without CMN. Pigmentary phenotyping in the ALSPAC study was performed by trained research nurses as follows: hair color was compared with 11 real-hair swatches of measured luminosities, eye color was compared with six standard iris photographs, and skin reflectance was measured by spectrophotometer. MC1R genotyping was performed for all 300 samples, with 270 reliable results for Northern European Caucasian white children.
MC1R genotyping
DNA was extracted directly from all samples using standard extraction procedures. The 951 coding base pairs of the single exon of MC1R, as well as a further 416 base pairs of the 3′ UTR, were amplified by PCR as a single product and sequenced using four overlapping primer sets (5′-TCCAGCCAGGGAGAGGGTGTGA-3′ and 5′-AGCTGCAGGTGATCACGTCAAT-3′ 5′-CTCACCCATGTACTGCTTCA-3′ and 5′-GAGTGTGAGATGCAGGAAGAAG-3′ 5′-CTCATGGCCGTGCTGTACGT-3′ and 5′-CTTTGACAAACGGGGACCAG-3′ 5′-ACGCTCAAGGAGGTGCTGACAT-3′ and 5′-CCACCCAGAGACTTCACAT-3′).
Birth-weight analysis
In the CMN cohort, data on birth weight and maternal smoking were available for 128 children. Pre-term children were excluded on the basis that there might have been other abnormalities during the pregnancy that could confound any genotype effect. In control population B, data were available for birth weight, gestation, maternal smoking, maternal weight before pregnancy, and maternal hypertension for all children.
Statistical analysis
Comparisons of prevalence between groups (pigmentary characteristics and MC1R genotype) were made by Fisher's exact test. Analysis of the statistical effect of genotype on CMN phenotype and on birth weight was by ordinal and linear logistic regression analysis, respectively. For the MC1R genotype associations, we tested the following four variables initially against projected adult size of the main nevus: V92M, V60L, R163Q, and R alleles (grouped together, as they are known to be a clinically and physiologically cohesive group, but some are rare alleles); other alleles were excluded from this analysis as they were found in <5% of our population. To improve our model fit, we then combined the V92M and R allele groups to make the variable V92M or R allele. A Bonferroni correction was applied, reducing the level of significance from 0.05 to less than 0.0102062 (i.e., for a total of five statistical tests). We then used this combined variable, V92M or R allele, to test against birth weight in the CMN cohort, with a further refinement made to the model by using V92M combined with R151C (the commonest of the R alleles), to make the variable V92M or R151C. Again, with a Bonferroni correction (for two tests), this reduced the level of significance (P-value) from 0.05 to 0.0253206. For genotype testing against birth weight in the control group, we tested the single variable V92M or R151C.
Acknowledgments
This research was funded by the Wellcome Trust, and the Caring Matters Now charity. For the CMN cohort, we are extremely grateful to all the families who took part in the study. For the ALSPAC cohort, we are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council (grant ref: 74882), the Wellcome Trust (grant ref: 076467), and the University of Bristol provide core support for ALSPAC.
Glossary
- ALSPAC
Avon Longitudinal Study of Parents and Children
- CMN
congenital melanocytic nevus or nevi
- MC1R
melanocortin-1-receptor
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Bastiaens M, ter Huurne J, Gruis N, et al. The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet. 2001;10:1701–1708. doi: 10.1093/hmg/10.16.1701. [DOI] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Chen W, et al. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet. 2001;69:765–773. doi: 10.1086/323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O'Gorman LE, et al. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6:1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- Cooper A, Robinson SJ, Pickard C, et al. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. 2005;175:4806–4813. doi: 10.4049/jimmunol.175.7.4806. [DOI] [PubMed] [Google Scholar]
- Danarti R, Konig A, Happle R. Large congenital melanocytic nevi may reflect paradominant inheritance implying allelic loss. Eur J Dermatol. 2003;13:430–432. [PubMed] [Google Scholar]
- Debniak T, Scott R, Masojc B, et al. MC1R common variants, CDKN2A and their association with melanoma and breast cancer risk. Int J Cancer. 2006;119:2597–2602. doi: 10.1002/ijc.22210. [DOI] [PubMed] [Google Scholar]
- DeDavid M, Orlow SJ, Provost N, et al. Neurocutaneous melanosis: clinical features of large congenital melanocytic nevi in patients with manifest central nervous system melanosis. J Am Acad Dermatol. 1996;35:529–538. doi: 10.1016/s0190-9622(96)90674-x. [DOI] [PubMed] [Google Scholar]
- Dellavalle RP, Johnson KR, Hester EJ, et al. Children with red hair have more freckles but fewer melanocytic nevi: results from a cohort study of 280 three-year-olds. Arch Dermatol. 2005;141:1042–1043. doi: 10.1001/archderm.141.8.1042. [DOI] [PubMed] [Google Scholar]
- Demenais F, Mohamdi H, Chaudru V, et al. Association of MC1R variants and host phenotypes with melanoma risk in CDKN2A mutation carriers: a GenoMEL study. J Natl Cancer Inst. 2010;102:1568–1583. doi: 10.1093/jnci/djq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wijn RS, Zaal LH, Hennekam RC, et al. Familial clustering of giant congenital melanocytic nevi. J Plast Reconstr Aesthet Surg. 2010;63:906–913. doi: 10.1016/j.bjps.2009.02.090. [DOI] [PubMed] [Google Scholar]
- Donatien PD, Hunt G, Pieron C, et al. The expression of functional MSH receptors on cultured human melanocytes. Arch Dermatol Res. 1992;284:424–426. doi: 10.1007/BF00372074. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, et al. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13:447–461. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- Falchi M, Spector TD, Perks U, et al. Genome-wide search for nevus density shows linkage to two melanoma loci on chromosome 9 and identifies a new QTL on 5q31 in an adult twin cohort. Hum Mol Genet. 2006;15:2975–2979. doi: 10.1093/hmg/ddl227. [DOI] [PubMed] [Google Scholar]
- Ghanem GE, Comunale G, Libert A, et al. Evidence for alpha-melanocyte-stimulating hormone (alpha-MSH) receptors on human malignant melanoma cells. Int J Cancer. 1988;41:248–255. doi: 10.1002/ijc.2910410216. [DOI] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Hale EK, Stein J, Ben-Porat L, et al. Association of melanoma and neurocutaneous melanocytosis with large congenital melanocytic naevi—results from the NYU-LCMN registry. Br J Dermatol. 2005;152:512–517. doi: 10.1111/j.1365-2133.2005.06316.x. [DOI] [PubMed] [Google Scholar]
- Harding RM, Healy E, Ray AJ, et al. Evidence for variable selective pressures at MC1R. Am J Hum Genet. 2000;66:1351–1361. doi: 10.1086/302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy E, Flannagan N, Ray A, et al. Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet. 2000;355:1072–1073. doi: 10.1016/S0140-6736(00)02042-0. [DOI] [PubMed] [Google Scholar]
- Hoch M, Eberle AN, Wagner U, et al. Expression and localization of melanocortin-1 receptor in human adipose tissues of severely obese patients. Obesity. 2007;15:40–49. doi: 10.1038/oby.2007.525. [DOI] [PubMed] [Google Scholar]
- Jones RW, Ring S, Tyfield L, et al. A new human genetic resource: a DNA bank established as part of the Avon longitudinal study of pregnancy and childhood (ALSPAC) Eur J Hum Genet. 2000;8:653–660. doi: 10.1038/sj.ejhg.5200502. [DOI] [PubMed] [Google Scholar]
- Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991;24:747–755. doi: 10.1016/0190-9622(91)70115-i. [DOI] [PubMed] [Google Scholar]
- King RA, Willaert RK, Schmidt RM, et al. MC1R mutations modify the classic phenotype of oculocutaneous albinism type 2 (OCA2) Am J Hum Genet. 2003;73:638–645. doi: 10.1086/377569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsler V, Bulstrode N. The role of surgery in the management of congenital melanocytic naevi in children: a perspective from Great Ormond Street Hospital. J Plast Reconstr Aesthet Surg. 2009;62:595–601. doi: 10.1016/j.bjps.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Kinsler VA, Birley J, Atherton DJ. Great Ormond street hospital for children registry for congenital melanocytic naevi: prospective study 1988–2007. Part 1-epidemiology, phenotype and outcomes. Br J Dermatol. 2009a;160:143–150. doi: 10.1111/j.1365-2133.2008.08849.x. [DOI] [PubMed] [Google Scholar]
- Kinsler VA, Birley J, Atherton DJ. Great Ormond street hospital for children registry for congenital melanocytic naevi: prospective study 1988–2007. Part 2—evaluation of treatments. Br J Dermatol. 2009b;160:387–392. doi: 10.1111/j.1365-2133.2008.08901.x. [DOI] [PubMed] [Google Scholar]
- Kinsler VA, Chong WK, Aylett SE, et al. Complications of congenital melanocytic naevi in children: analysis of 16 years' experience and clinical practice. Br J Dermatol. 2008;159:907–914. doi: 10.1111/j.1365-2133.2008.08775.x. [DOI] [PubMed] [Google Scholar]
- Krengel S, Hauschild A, Schafer T. Melanoma risk in congenital melanocytic naevi: a systematic review. Br J Dermatol. 2006;155:1–8. doi: 10.1111/j.1365-2133.2006.07218.x. [DOI] [PubMed] [Google Scholar]
- Loir B, Perez SC, Ghanem G, et al. Expression of the MC1 receptor gene in normal and malignant human melanocytes. A semiquantitative RT-PCR study. Cell Mol Biol (Noisy-le-grand) 1999;45:1083–1092. [PubMed] [Google Scholar]
- Mogil JS, Ritchie J, Smith SB, et al. Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet. 2005;42:583–587. doi: 10.1136/jmg.2004.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, et al. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Nan H, Qureshi AA, Hunter DJ, et al. Interaction between p53 codon 72 polymorphism and melanocortin 1 receptor variants on suntan response and cutaneous melanoma risk. Br J Dermatol. 2008;159:314–321. doi: 10.1111/j.1365-2133.2008.08624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Widlund HR, Kutok JL, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Rebhan M, Chalifa-Caspi V, Prilusky J, et al. GeneCards: integrating information about genes, proteins and diseases. Trends in Genetics. 1997;13:163. doi: 10.1016/s0168-9525(97)01103-7. [DOI] [PubMed] [Google Scholar]
- Robinson S, Dixon S, August S, et al. Protection against UVR Involves MC1R-mediated non-pigmentary and pigmentary mechanisms in vivo. J Invest Dermatol. 2010;130:1904–1913. doi: 10.1038/jid.2010.48. [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Healy E. Human melanocortin 1 receptor (MC1R) gene variants alter melanoma cell growth and adhesion to extracellular matrix. Oncogene. 2002;21:8037–8046. doi: 10.1038/sj.onc.1205913. [DOI] [PubMed] [Google Scholar]
- Ruiz-Maldonado R. Measuring congenital melanocytic nevi. Pediatr Dermatol. 2004;21:178–179. doi: 10.1111/j.0736-8046.2004.21222.x. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Phillips SR, Rudzish R, et al. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun. 1999;260:488–491. doi: 10.1006/bbrc.1999.0935. [DOI] [PubMed] [Google Scholar]
- Smith R, Healy E, Siddiqui S, et al. Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol. 1998;111:119–122. doi: 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- Stefanaki I, Stratigos AJ, Dimisianos G, et al. p53 codon 72 Pro homozygosity increases the risk of cutaneous melanoma in individuals with dark skin complexion and among noncarriers of melanocortin 1 receptor red hair variants. Br J Dermatol. 2007;156:357–362. doi: 10.1111/j.1365-2133.2006.07645.x. [DOI] [PubMed] [Google Scholar]
- Strauss RM, Newton Bishop JA. Spontaneous involution of congenital melanocytic nevi of the scalp. J Am Acad Dermatol. 2008;58:508–511. doi: 10.1016/j.jaad.2006.05.076. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, et al. The role of melanocortin-1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Res. 2003;16:266–272. doi: 10.1034/j.1600-0749.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- Thornwall M, Dimitriou A, Xu X, et al. Immunohistochemical detection of the melanocortin 1 receptor in human testis, ovary and placenta using specific monoclonal antibody. Horm Res. 1997;48:215–218. doi: 10.1159/000185518. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, et al. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Yeh I, Bastian BC. Genome-wide associations studies for melanoma and nevi. Pigment Cell Melanoma Res. 2009;22:527–528. doi: 10.1111/j.1755-148X.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.