Abstract
Vitamin K2 (VK2) exerts cell growth inhibitory effects in various human cancer cells such as SMMC-7721 hepatocellular carcinoma (HCC) cells. BCL-2 is an antiapoptotic protein that is frequently overexpressed in numerous tumors. Modulation of multiple antiapoptotic signaling pathways involving BCL-2, which are related to growth factor-stimulated signal transduction in cell survival, is essential for enhancement of the cytotoxic effect of anticancer drugs. In this study, we tested a new strategy of gene therapy by combining BCL-2 siRNA with VK2. In SMMC-7721 HCC cells, the combined treatment significantly enhanced cytotoxicity compared with treatment with either VK2 or siBCL-2 alone. We found that combined treatment induced a significantly different level of G2 stage inhibition. Furthermore, the p53 protein was overexpressed 24 h subsequent to combination treatment, and p21 was clearly increased at 36 h as a consequence of the increased p53 activity. In conclusion, these data suggest that the antitumor effect of VK2 may be improved by silencing BCL-2 expression in SMMC-7721 HCC cells and provides support for the combined use of VK2 and siBCL-2 as a promising approach in cancer gene therapy.
Keywords: vitamin K2, RNA interference, siRNA, BCL-2, tumor therapy
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality worldwide and the prognosis for HCC patients is poor. Vitamin K2 (VK2) belongs to the vitamin K family and has been used clinically as an activator of homeostasis (1) and an inhibitor of osteoporosis (2). In addition, VK2 is able to exert cell growth inhibitory effects in various human cancer cells such as SMMC-7721 HCC cells. In particular, induction of apoptosis by VK2 is associated with p53 in SMMC-7721 HCC cells (3). p53 is a multi-faceted tumor suppressor gene capable of inducing cell cycle arrest and DNA repair, irreversible growth arrest, terminal differentiation or apoptosis (4,5). Furthermore, one important pathway through which p53 induces apoptosis may involve the transcriptional activation of proapoptotic target genes, including proapoptotic members of the BCL-2 family of proteins such as Bax and Bid, leading to intrinsic/mitochondrial apoptosis via the activation of caspase cascades (6).
BCL-2 is an antiapoptotic protein that is frequently overexpressed in numerous tumors. Modulation of multiple antiapoptotic signaling pathways involving BCL-2, which are associated with growth factor-stimulated signal transduction in cell survival, is essential for enhancement of the cytotoxic effect of anticancer drugs (7). Several exceptions to the hypothesis that BCL-2 inhibits and p53 promotes apoptosis have been encountered (8). Furthermore, BCL-2 is an antagonist to Bax and inhibits mitochondrial membrane disruption, a mechanism that likely accounts for drug resistance in BCL-2-overexpressing tumors (9).
Therefore, the aim of this study was to enhance the anticancer efficacy of VK2 through suppression of BCL-2 expression, which is known as a ‘double-targeted’ therapeutic approach. Suppression of BCL-2 expression by antisense and RNAi compounds inhibited tumor growth and enhanced apoptosis in a variety of tumor models (10,11). Silencing BCL-2 reportedly results in improvement of the antitumor effect of the chemotherapeutic agent 5-FU (12). In the present study, we examined the anticancer effect of VK2 by silencing BCL-2 expression with BCL-2 RNAi.
Materials and methods
RNA interference
siRNA duplexes were produced by Shanghai Genepharma Co. Inc. (Shanghai, China) against human BCL-2 (5′-AAG GUG UCU UCC AGA UCC UGA-3′). Scrambled fluorescent-labeled siRNA (5′-AAA UGU GUG UAC GUC UCC UCC-3′) (siNC) was also designed and used as the negative control in this study.
Cell culture
SMMC-7721 cells were cultured at 37°C under 5% CO2 in RPMI-1640 supplemented with 10% fetal bovine serum (FBS). Cells were treated with VK2 and 2% ethanol where appropriate.
Combination treatment of tumor cells with BCL-2 siRNA and VK2
SMMC-7721 cells at 70–90% confluence in 6-well plates were transfected with 100 pmol BCL-2 siRNA using a Lipofectamine 2000 reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). After 12 h, cells were treated with VK2 at a multiplicity of concentrations. Control groups included cells that were transfected with siNC.
RNA extraction and reverse transcription-PCR analysis
Total RNA was isolated from treated cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse-transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen). The cDNA was used to amplify the BCL-2 fragments. For normalization of RNA, the housekeeping gene GAPDH was also amplified from each sample. The primer sequences used were: BCL-2 forward: 5′-ATGTGTGTGGAGAGCGTCAA-3′ and reverse: 5′-CAGGAGAAATCAAACAGAGGC-3′, 173 bp; and GAPDH forward: 5′-GGATTTGGTGGTATTGGG-3′ and reverse: 5′-GGAAGATGGTGATGGGATT-3′, 205 bp.
Quantitative real-time RT-PCR amplification was carried out using Real-Time MIX (SYBR Premix Ex Taq™, Takara Bio, Inc., Shiga, Japan). Specifically, total RNA was extracted by TRIzol reagent (Invitrogen) as described and cDNA was synthesized with RNA reverse transcriptase. The CT (threshold cycle) value of BCL-2 and GAPDH was quantitated by Q-PCR in triplicate using an ABI Prism 7500 HT sequence detector (AB Applied Biosciences, Foster City, CA, USA) according to the manufacturer’s instructions, and was normalized over the CT of the GAPDH control.
Western blot analysis
Cells were harvested at the indicated times, and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis in 10% SDS-polyacrylamide Tris-glycine gels for protein expression. The immunoblotting was performed with BCL-2 (Cell Signaling Technology, Inc., Danvers, MA, USA), p53 (Cell Signaling), p21 (Proteintech, Chicago, IL, USA) and mouse β-actin (Sigma, St. Louis, MO, USA), followed by detection with a horseradish peroxidase-conjugated secondary antibody.
MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed to assess the effect of VK2 in combination with RNA interference on cell proliferation. SMMC-7721 cells were seeded in 96-well plates at a concentration of 1×105 cells/well. At the end of the incubation period, 20 μl of 5 mg/ml MTT (Sigma) in PBS was added to each well. Each experiment was repeated three times. Absorbance was measured following incubation for a further 4 h at 37°C with a solution of MTT (0.2 mg/ml) that contained 12.5 μM dimethyl sulfoxide (DMSO). The absorbance was measured on a spectrophotometer microplate reader at a wavelength of 490 nm.
Cell cycle
Cells were seeded at 1×106 cells per well in flat-bottomed 6-well plates. Cells were harvested at 6, 12, 24 and 36 h following treatment with 40 μM VK2 and 18, 24, 36 and 48 h following transfection with siBCL-2. Cells were washed twice with PBS and stained with 10 μg/ml PI. Cell cycle distribution was determined by flow cytometry. Cell cycle analysis was performed using FACS Calibur (Becton-Dickinson and company, Franklin Lakes, NJ, USA). Software were obtained from CELL Quest software.
Statistical analysis
All experiments were performed in triplicate, and the data were expressed as mean ± SD. The comparative Ct method was applied in the quantitative real-time RT-PCR assay according to the delta-delta Ct method. The data were analyzed with Student’s t-test or by one-way analysis of variance, and results were considered statistically significant at p≤0.05.
Results
Suppression of BCL-2 by siRNA in SMMC-7721 cells
The suppression of BCL-2 was examined using its siRNA in tumor cells. siBCL-2 and control siNC were used to transfect SMMC-7721 cells, respectively, and the efficiency of siRNA on SMMC-7721 expression was examined by real-time RT-PCR and western blot analysis.
As expected, no change occurred in the level of BCL-2 mRNA in the VK2 group. However, when compared with the siNC control, the level of BCL-2 mRNA decreased in the siBCL-2 groups, used alone or in combination with VK2 (Fig. 1A). The same result was found at the protein level by western blot analysis (Fig. 1B).
Figure 1.
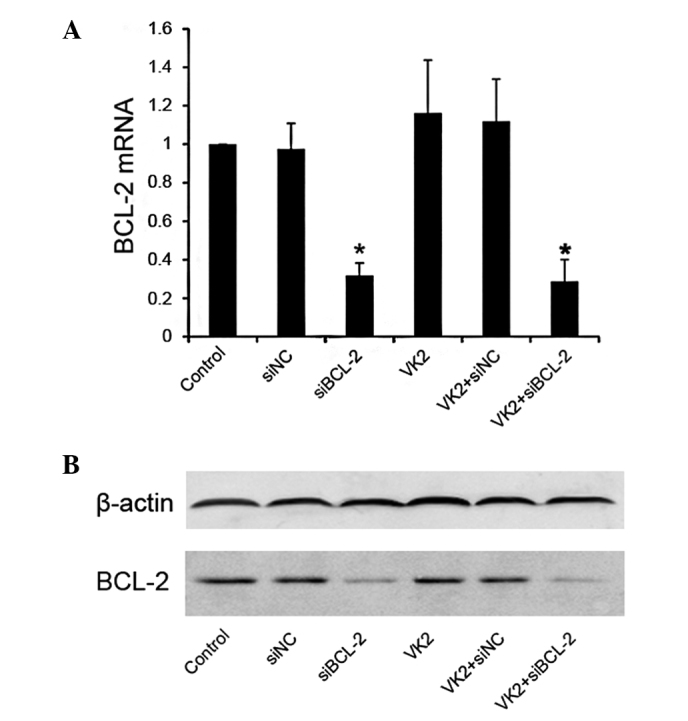
BCL-2 gene knockdown by siRNA. (A) Quantitative real-time RT-PCR analysis of BCL-2 gene transcripts in SMMC-7721 cells. The experiment was performed 24 h following siBCL-2 (100 nmol/l) transfection with or without vitamin K2 (40 μM) infection. GAPDH was used as the internal control in the calculation. *P<0.05 as compared with the negative control. (B) Western blot analysis of BCL-2 protein in SMMC-7721 cells. The experiment was performed 48 h following siBCL-2 (100 nmol/l) transfection with or without VK2 (40 μM). Western bands were scanned and normalized over the internal control β-actin.
Enhanced cytotoxicity by the combined treatment with siBCL-2 and VK2
Subsequent to establishing BCL-2-silencing cells, we determined the proliferation of cells treated with VK2. Compared with the controls, there was a significant reduction in cell survival in cells treated with BCL-2 siRNA (siBCL-2) plus VK2. In particular, at the concentration of 40 μM, the combination group demonstrated a significant degree of proliferative inhibition after 24 h compared with the negative control and PBS groupσ, indicating enhanced growth inhibition (Fig. 2).
Figure 2.
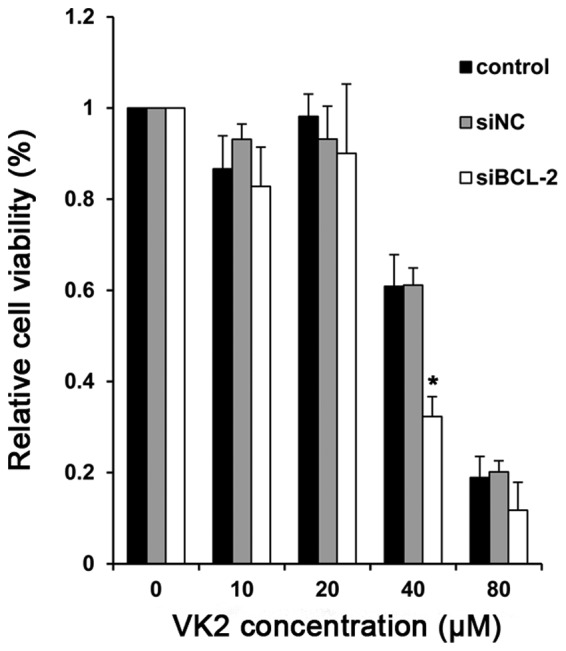
Cell proliferation of SMMC-7721 cells following combined treatment with siBCL-2 and VK2. Cell proliferation was measured by MTT assays 36 h following co-treatment with siBCL-2 (100 nmol/l) and 24 h following 0, 10, 20, 40 and 80 μM of VK2. Data are shown as the means ± SD for three independent experiments. *P<0.05, as compared with the negative control.
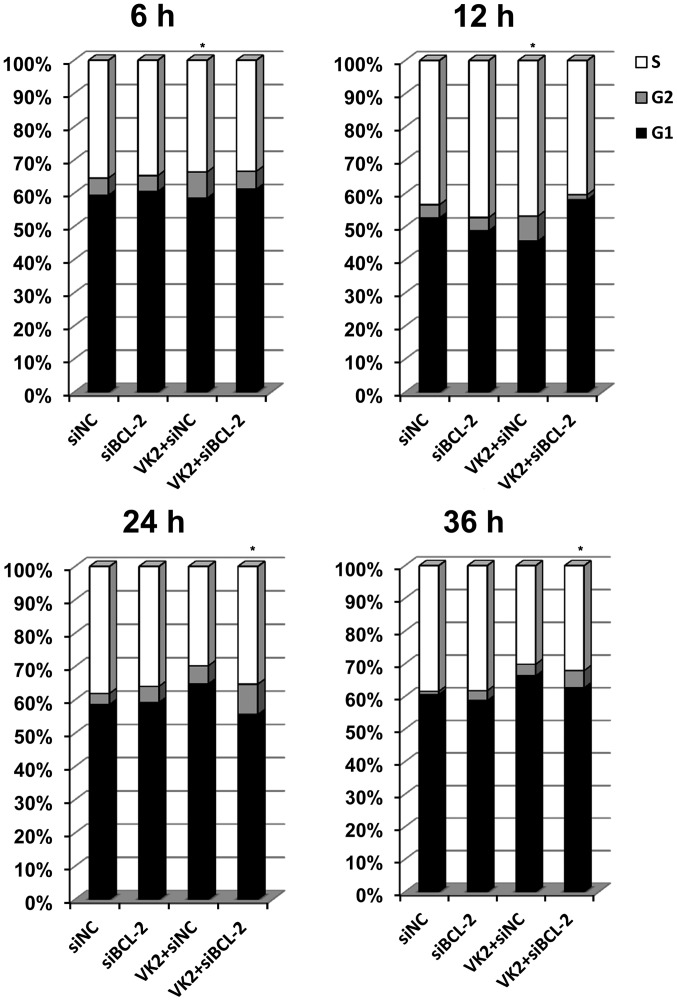
Combined treatment induced changes in the cell cycle
The cell cycle was evaluated to investigate the possible mechanism of proliferative inhibition. A significant arrest was observed in the G2 phase following transfection of the cells with siBCL-2 for 18 h and treatment with VK2 for 6 h (Fig. 3). The same result was observed in cells transfected with siBCL-2 for 24 h and treated with VK2 for 12 h. However, when the cells were transfected with siBCL-2 for 36 h and treated with VK2 for 24 h, the combination treatment group demonstrated a different level of G2 stage inhibition in comparison to the VK2 treatment, siBCL-2 treatment and the control groups, and the difference was statistically significant. In addition, when the cells were transfected with siBCL-2 for 48 h and treated with VK2 for 36 h, the same result was observed.
Figure 3.
Combined treatment with siBCL-2 and VK2 induces cell cycle arrest in SMMC-7721 cancer cell lines. SMMC-7721 cells were transfected with siBCL-2 (18, 24, 36 and 48 h) and treated with 40 μM of VK2 (6, 12, 24 and 36 h). *P<0.05, as compared with the negative control.
Expression of phosphorylation of p53 and p21 following combined treatment with siBCL-2 and VK2
In the nucleus, p53 induces cell cycle arrest and has an ‘extranuclear’ proapoptotic function as it combines with BCL-2 (13,14). Thus, we determined the protein level of p53. The protein level of phosphorylation of p53 (Ser 20 phosphorylation) was significantly increased in cells transfected with siBCL-2 for 36 h and treated with VK2 for 24 h, compared with other groups (Fig. 4). We then tested the protein level of p21, a transcriptional target protein of p53. The data revealed that following transfection with siBCL-2 for 36 and 48 h and treatment with VK2 for 24 and 36 h, there was an increase in the protein level of p21 in the combination groups compared with other groups (Fig. 5).
Figure 4.
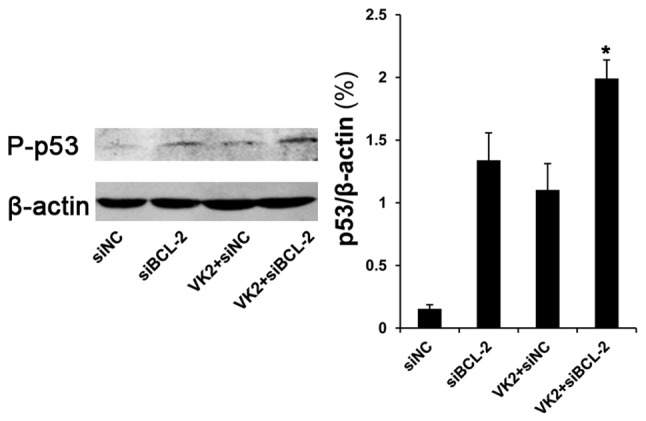
Phosphorylation of p53 expression in SMMC-7721 tumor cells. Western blot analysis of P-p53 (Ser 20) protein expression at 24 h following co-treatment. Bar: average band density of quantified P-p53 protein following normalization by the internal control β-actin. *P<0.05, as compared with VK2 and the siBCL-2 group.
Figure 5.
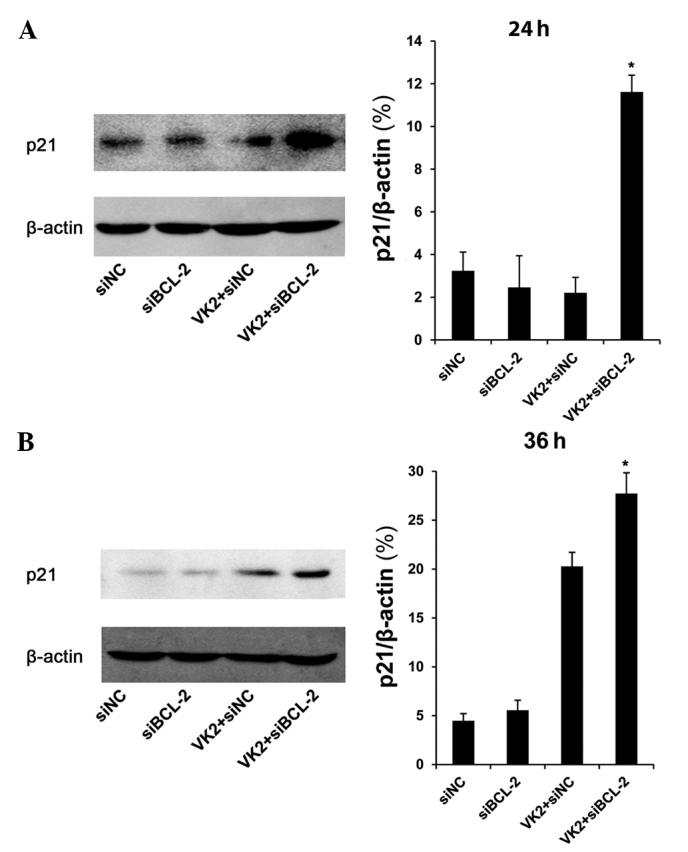
p21 expression in SMMC-7721 tumor cells. Cells were transfected with siBCL-2 for 36 and 48 h and treated with VK2 for 24 and 36 h, and total protein was analyzed by Western blot analysis with p21 antibodies. Bar, average band density of quantified p21 protein following normalization by the internal control β-actin. *P<0.05, as compared with VK2 and siBCL-2 group.
Discussion
The anti-proliferative action of VK2 has been reported in a variety of cancer cells including lung carcinomas (8), human ovarian cancer cells (6) and acute myeloid leukemia cells (4). In leukemia cells, VK2 induces autophagy and apoptosis simultaneously, indicating that the cell expression levels of BCL-2 appear to determine the phenotype of cell death (15). In HCC Hep3B cells, VK2 induced cell cycle arrest at the G1 phase and involvement of apoptosis was suggested since results of the flow cytometric analysis revealed the sub-G1 fraction, and nuclear condensation and fragmentation appeared subsequent to VK2 treatment. VK2 activated extracellular signal-regulated kinase (ERK)1/2 in a mitogen-activated ERK-regulating kinase (MEK)-dependent manner in Hep3B (16). In SMMC-7721 HCC cells, VK2 is capable of inhibiting the growth of SMMC-7721 cells by induction of apoptosis involving p53 (3). In the present study, we found that VK2 induced p53 and increased the p21 levels, eventually leading to cell cycle arrest in the G2 phase, indicating VK2 involvement in tumor suppression.
Malignant tumors are associated with abnormalities in gene expression derived from genetic and/or epigenetic lesions, including BCL-2 overexpression (17). As an apoptotic and/or survival ‘switch’, BCL-2 is key in the balance between proapoptotic and antiapoptotic factors in the intracellular microenvironment. Overexpression of BCL-2 in tumors critically alters this balance and results in the permanent survival of tumors. It has also been reported that overexpression of BCL-2 in a leukemia cell line resulted in resistance against VK2-induced apoptosis, however, these cells still underwent differentiation via G1 arrest (18). Thus, downregulation of BCL-2 may restore this balance and further increase the ability of tumor cells to respond to the apoptotic signal induced by exogenous stimuli (19). siBCL-2 treatment reportedly increased H101 viral replication in both treated cells and tumor tissues (19), and that the downregulation of BCL-2 and cyclin D1 may enhance cisplatin sensitivity in MCF-7 human breast cancer cells (20).
In this study, we investigated the antitumor efficacy of VK2 in conjunction with siRNA to BCL-2. BCL-2-siRNA efficiently inhibited the expression of BCL-2 mRNA and protein (Fig. 1). RNAi activity was not affected by VK2 treatment. The combined action of BCL-2 knockdown and treatment with VK2 significantly inhibited tumor growth in vitro, suggesting an additional effect of the combined tumor therapy.
The mechanism underlying the additive effect of the combined therapy remains to be determined. In SMMC-7721 cells treated with the combination of siBCL-2 and VK2, we found significant changes in the cell cycle in different groups. Subsequently, we determined the expression of p53, which is mainly responsible for arrest of the cell cycle and regulation of apoptosis. In addition, we determined the level of the protein, p21, which is a transcriptional target of p53 and plays a crucial role in mediating growth arrest when cells are exposed to DNA damaging agents such as doxorubicin and γ-irradiation (21,22). It has been demonstrated that overexpression of p21 results in G1-, G2- (23), or S-phase arrest (24,25). Conversely, p21-deficient cells fail to undergo cell cycle arrest in response to p53 activation following DNA damage (26). Furthermore, p21 and p53 are essential in sustaining the G2 checkpoint following DNA damage in human cells (27). p53-mediated signaling plays an integral role in the maintenance of the G2 checkpoint delay following activation of the checkpoint. p53 is believed to exert G2 checkpoint responses through the transcriptional upregulation of the downstream target genes p21, 14-3-3 and GADD45. p21 is capable of binding to and inhibiting the cyclin B1/cdc2 complex and inhibiting cyclin-activated kinase-mediated cdc2 activation (28).
The combined treatment with siBCL-2 and VK2 down-regulated the expression of the antiapoptotic protein BCL-2, whereas the protein, p53, was overexpressed 24 h following treatment. This transcription factor plays a significant role in the regulation of the cell cycle to prevent mutations and cancer (29), as well as to control the functions of p21. It regulates the progression of the cell cycle, which was clearly increased at 24 and 36 h as a consequence of the increased p53 activity.
In conclusion, our study proved that the antitumor effect of VK2 may be improved by silencing BCL-2 expression in HCC SMMC-7721. This finding provides support for the combined use of VK2 and siBCL-2 as a promising approach in cancer gene therapy.
Acknowledgements
We thank Lu Li from the Department of Biochemistry and Molecular Biology, Anhui University of Traditional Chinese Medicine, for experimental guidance and assistance. This study was supported by the National Key Program for Basic Research of China (2010CB529902), the National Natural Science Foundation of China (10979034 and 81001008); the Science and Technology Commission of Shanghai (10JC1409100), the Shanghai Leading Academic Discipline Project (S30205); and the Shanghai Rising-Star Program (11QA1404000).
References
- 1.Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci. 1992;16:307–343. [PubMed] [Google Scholar]
- 2.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515–521. doi: 10.1359/jbmr.2000.15.3.515. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Qi Z, Qian J, et al. Induction of apoptosis in hepatocellular carcinoma Smmc-7721 cells by vitamin K(2) is associated with p53 and independent of the intrinsic apoptotic pathway. Mol Cell Biochem. 2010;342:125–131. doi: 10.1007/s11010-010-0476-8. [DOI] [PubMed] [Google Scholar]
- 4.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 5.Smeenk L, Lohrum M. Behind the scenes: Unravelling the molecular mechanisms of p53 target gene selectivity (Review) Int J Oncol. 2010;37:1061–1070. doi: 10.3892/ijo_00000757. [DOI] [PubMed] [Google Scholar]
- 6.Wu JN, Huang J, Yang J, Tashiro S, Onodera S, Ikejima T. Caspase inhibition augmented oridonin-induced cell death in murine fibrosarcoma l929 by enhancing reactive oxygen species generation. J Pharmacol Sci. 2008;108:32–39. doi: 10.1254/jphs.fp0072079. [DOI] [PubMed] [Google Scholar]
- 7.Kim R, Tanabe K, Emi M, Uchida Y, Toge T. Potential roles of antisense therapy in the molecular targeting of genes involved in cancer (Review) Int J Oncol. 2004;24:5–17. [PubMed] [Google Scholar]
- 8.Zusman I, Gurevich P, Gurevich E, Ben-Hur H. The immune system, apoptosis and apoptosis-related proteins in human ovarian tumors (A review) Int J Oncol. 2001;18:965–972. doi: 10.3892/ijo.18.5.965. [DOI] [PubMed] [Google Scholar]
- 9.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 10.Futami T, Miyagishi M, Seki M, Taira K. Induction of apoptosis in HeLa cells with siRNA expression vector targeted against bcl-2. Nucleic Acids Res. 2002;(Suppl):251–252. doi: 10.1093/nass/2.1.251. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Huang S, Zhang H, Wang H, Guo H, Qian G, Fan X, Lu J, Hoffman AR, Hu JF, Ge S. Targeted knockdown of Bcl2 in tumor cells using a synthetic TRAIL 3′-UTR microRNA. Int J Cancer. 2010;126:2229–2239. doi: 10.1002/ijc.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SL, Wu Y, Yu H, et al. Inhibition of Bcl-2 expression by a novel tumor-specific RNA interference system increases chemosensitivity to 5-fluorouracil in Hela cells. Acta Pharmacol Sin. 2006;27:242–248. doi: 10.1111/j.1745-7254.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Erster S, Zaika A, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 14.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama T, Miyazawa K, Naito M, et al. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy. 2008;4:629–640. doi: 10.4161/auto.5941. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Okano J, Nagahara T, Murawaki Y. Apoptosis of liver cancer cells by vitamin K2 and enhancement by MEK inhibition. Int J Oncol. 2006;29:1501–1508. [PubMed] [Google Scholar]
- 17.Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 18.Miyazawa K, Yaguchi M, Funato K, et al. Apoptosis/differentiation-inducing effects of vitamin K2 on HL-60 cells: dichotomous nature of vitamin K2 in leukemia cells. Leukemia. 2001;15:1111–1117. doi: 10.1038/sj.leu.2402155. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Wang H, Zhang J, et al. Enhanced therapeutic efficacy by simultaneously targeting two genetic defects in tumors. Mol Ther. 2009;17:57–64. doi: 10.1038/mt.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yde CW, Issinger OG. Enhancing cisplatin sensitivity in MCF-7 human breast cancer cells by down-regulation of Bcl-2 and cyclin D1. Int J Oncol. 2006;29:1397–1404. [PubMed] [Google Scholar]
- 21.El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 22.El-Deiry WS, Harper JW, O’Connor PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 23.Niculescu AB, III, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogryzko VV, Wong P, Howard BH. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4877–4882. doi: 10.1128/mcb.17.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radhakrishnan SK, Feliciano CS, Najmabadi F, et al. Constitutive expression of E2F-1 leads to p21-dependent cell cycle arrest in S phase of the cell cycle. Oncogene. 2004;23:4173–4176. doi: 10.1038/sj.onc.1207571. [DOI] [PubMed] [Google Scholar]
- 26.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 27.Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 28.Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 29.Harms KL, Chen X. The C terminus of p53 family proteins is a cell fate determinant. Mol Cell Biol. 2005;25:2014–2030. doi: 10.1128/MCB.25.5.2014-2030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]