Abstract
Aberrant cell cycle control and apoptosis deregulation are involved in biliary carcinogenesis. The tumor suppressor gene p53 and its key negative regulator murine double minute 2 (mdm2) cooperate in modulating these basic cell functions and germline p53 alteration promotes cholangiocarcinoma (CCA) formation in animal models. The potential association between common functional genetic variation in p53 (SNP72 G/C) and mdm2 (SNP309 T/G) and susceptibility to bile duct cancer, however, has not been studied. p53/SNP72 G/C (rs1042522) and mdm2/SNP309 T/G (rs2279744) were genotyped in 182 Caucasian CCA patients and 350 controls using TaqMan assays. Allelic and genotypic differences, including exploratory data analyses (according to gender, tumor localization, early onset and genotypic interactions) were compared in contingency tables using the χ2 and Fisher’s exact tests. The overall comparison of allele and genotype frequencies yielded no significant association between either SNP and CCA susceptibility. Similarly, gender- and localization-specific analyses did not reveal deviations in allelic or genotypic distributions. In carriers of the low-apoptotic p53 genotype CC, the mdm2 SNP309 T allele conferred borderline significant CCA risk [P=0.049; odds ratio (OR), 4.36; 95% CI, 0.92–20.77]. Power analysis confirmed adequate statistical power to exclude major SNP effects (each >97% for OR 1.7). Collectively, the results we obtained from the largest European CCA cohort do not support the hypothesis of a prominent role of common p53 and mdm2 variation in the genetic susceptibility to bile duct cancer. However, epistatic effects may modulate genetic CCA risk in individual subsets.
Keywords: apoptosis, biliary tract cancer, cancer susceptibility, cell cycle, cholangiocarcinoma, epistasis, gene-gene interaction, genetic risk, murine double minute 2, p53
Introduction
Cholangiocarcinoma (CCA) is a highly malignant cancer with a poor prognosis which arises from the epithelial lining of intra- or extrahepatic bile ducts (1). CCA is the second most common primary liver malignancy and epidemiological studies have reported rising incidence rates in Western countries (2,3). Since clinical presentation and diagnosis at locally advanced and/or metastatic tumor stages beyond potential surgical cure are common, the reported 5-year survival rates rarely exceed 5–10%. Notwithstanding recent progress in treatment options for advanced CCA, alternative routes to improve the prognosis may include the identification and characterization of individuals at high risk of the disease, thus allowing for tumor surveillance and early diagnosis (4–6). Clinical risk factors include, among others, primary sclerosing cholangitis, viral hepatitis and liver fluke infections in Asian countries (7,8). However, knowledge of genetic susceptibility factors, with its potential to elucidate novel carcinogenic pathways and define high-risk populations, is limited, reflecting difficulties in establishing study cohorts of informative sample sizes for this rare cancer. We previously reported the increased genetic risk of CCA in α1 antitrypsin Z allele carriers, which was in accordance with previously published pathology-based data (9–11). Although the distinct molecular pathogenesis underlying malignant cholangiocyte transformation is poorly understood, chronic cholestasis and inflammation are considered to be key elements in CCA initiation (12). Recent studies have highlighted the carcinogenic role of the activation of nuclear factor κB (NF-κB) signaling in conferring resistance to apoptosis to malignant cholangiocytes; these findings substantiate the concept of CCA as an inflammation-driven cancer (13).
The p53 tumor suppressor network is a central component of cellular stress responses and is critical for the maintenance of genomic stability in response to various stresses, including DNA damage, metabolic perturbation and oncogene activation (14). Notably, p53 has been suggested to be a negative regulator of the proinflammatory transcription factor NF-κB (15,16). Deregulation of the p53 pathway by either overexpression or somatic mutation is a common feature in a number of types of human cancer, including CCA (17–19). Furthermore, germline p53 alteration has been linked to CCA formation in animal models (20). Notably, common genetic variations, relevant to the p53 network and involved in cell cycle control and apoptosis regulation, have been identified (21). The single nucleotide polymorphism (SNP) c.309 T>G in the promoter/enhancer site of the murine double minute 2 (mdm2) gene, which is a critical negative regulator of p53 and is itself transcriptionally regulated by p53, affects the binding efficiency of the transcription factor SP1. Consequently, the SNP309 G allele has been suggested to translate into increased intracellular mdm2 levels, thereby limiting the cellular availability of functional p53 under stressful conditions (22). The p53 SNP c.72 G>C is located in a proline-rich domain and may affect the molecular structure of the p53 protein. This has been reported to have a functional impact, as the C allele is thought to induce apoptosis and suppress oncogene-induced transformation less efficiently (23).
Although these two genetic variants are among the most widely studied SNPs in genetic cancer research (21), no data are currently available for CCA. In this respect, the aim of the current study was to assess the potential contribution of the common functional gene variants p53 (SNP72 G/C) and mdm2 (SNP309 T/G) to the genetic susceptibility to bile duct cancer.
Patients and methods
Patient recruitment and characteristics
In total, 182 European CCA patients (103 males and 79 females) and 350 controls (151 males and 199 females) were recruited at equal case-control proportions at three academic medical centers in Germany and Romania. Specific aspects of the study population were reported in our previous study (9). Briefly, the clinical CCA diagnosis and classification criteria were in accordance with the recommendations of the British Society of Gastroenterology (24). There was a tissue diagnosis of CCA in 84% of the subjects, while in 16% of the patients the CCA diagnosis was based on clinical assessment, taking into account imaging studies, follow-up and exclusion of alternative diagnoses, including postoperative biliary strictures. The control subjects were recruited following either negative colorectal cancer screening (n=290) or normal liver ultrasound (n=60). Written informed consent was obtained from all participants. The study was approved by the respective ethics committees and was in accordance with the revised Declaration of Helsinki.
Genomic DNA isolation and genotyping
Following venipuncture and sampling of 5 ml whole blood, genomic DNA was extracted by standard techniques using a commercial kit (QIAamp, Qiagen, Hilden, Germany). Following appropriate spectrophotometric calibration of the DNA concentrations (NanoDrop ND-1000, NanoDrop Technologies, Wilmington, DE, USA), rs1042522 (p53 SNP72) and rs2279744 (mdm2 SNP309) were genotyped using solution-phase hybridization reactions with 5′-nuclease and fluorescence detection (TaqMan assays, Applied Biosystems, Foster City, CA, USA) on an ABI 7500 Fast Real-Time polymerase chain reaction (PCR) system (Applera, Norwalk, CT, USA). Specifically, the PCR assays contained 5 μl 2X Genotyping Mastermix, 0.25 μl 40X Genotyping mix, 3.75 μl H2O and 5–50 ng/μl DNA. The amplification conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 10 sec and 60°C for 1 min.
Statistical analysis
The data were analysed using the SPSS software package (version 17.0; SPSS, Munich, Germany). The age and gender structure of the study groups were analysed using t-tests. Data are shown as the mean ± SD. Genotypic data for the two SNPs were assessed for consistency with the Hardy-Weinberg equilibrium (HWE) in controls (P>0.05) using an exact test (http://ihg.gsf.de/snps.html). The allele and genotype frequencies of the study groups were compared using χ2-tests for 2×2 and 2×3 contingency tables. Fisher’s exact tests were used as appropriate, in particular for small sample sizes. Differences in allele and genotype distributions, including exploratory data analyses stratifying for gender, tumor localization, early onset and genotypic interactions, were assessed using odds ratios (ORs) and 95% confidence intervals (CIs). P<0.05 was considered statistically significant.
To assess for adequate sample size in our study, we performed power calculations using PS: Power and Sample Size Calculation v.3.0 (http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize).
Results
Clinical parameters of the patients
Allelic discrimination for rs1042522 and rs2279744 was successful in all study subjects, yielding call rates of 100% for the two SNPs. The genotype distributions in the controls did not deviate from the Hardy-Weinberg equilibrium (P>0.05 for both SNPs). The detected allele frequencies were compatible with the Entrez SNP database entries (http://www.ncbi.nlm.nih.gov/snp). Of the cancer group, 57% of the patients were male, compared with 43% in the control group (P<0.01). The median age at CCA diagnosis/study entry was 65.8±11.4 years for cancer patients and 61.0±11.1 years for the control subjects (P<0.01). There were more patients with tumors arising from the extrahepatic biliary system than from intrahepatic localizations (141 extrahepatic vs. 41 intrahepatic localizations). The demographic and clinical data of the study population reflecting the epidemiology of CCA are shown in Table I.
Table I.
Demographic and clinical parameters of the study population.
| Parameters | CCA | Controls | P-value |
|---|---|---|---|
| Subjects (n) | 182 | 350 | |
| Age, years (mean ± SD) | 65.8±11.4 | 61.0±11.1 | <0.01 |
| Gender, male/female (%) | 103/79 (57/43) | 151/199 (43/57) | <0.01 |
| Localization, intra-/extrahepatic (%) | 41/141 (23/77) | ||
| Diagnosis, tissue/clinical (%) | 152/30 (84/16) |
CCA, cholangiocarcinoma.
Effect of allelic and genotypic distributions in the study groups
A comparison of the allelic distributions yielded no significant differences in either SNP between the study groups (SNP72, 27.2 vs. 26.9%; SNP309, 37.4 vs. 36.1% in CCA patients vs. controls, respectively). Similarly, no significant difference or marked trend was noted when analyzing genotypic distributions in the total study groups (Table II). We then evaluated the potential of specific SNP effects in terms of parameters such as gender, tumor localization (extra- vs. intrahepatic) and early onset CCA, arbitrarily defined as CCA diagnosis <60 years. Similarly, in this exhaustive exploratory data analysis no association signal was obtained for either SNP (data not shown).
Table II.
Allele and genotype frequencies of p53 SNP72 and mdm2 SNP309 in CCA vs. controls.
| Frequency type | CCA n (%) | Controls n (%) | P-value | |
|---|---|---|---|---|
| Alleles | ||||
| p53 SNP72 | G | 265 (72.8) | 512 (73.1) | 0.92 |
| C | 99 (27.2) | 188 (26.9) | (0.98)a | |
| 364 (100) | 700 (100) | [0.74–1.31]b | ||
| mdm2 SNP309 | T | 228 (62.6) | 447 (63.9) | 0.70 |
| G | 36 (37.4) | 253 (36.1) | (0.95)a | |
| 364 (100) | 700 (100) | [0.73–1.23]b | ||
| Genotypes | ||||
| p53 SNP72 | GG | 94 (51.7) | 190 (54.3) | 0.49 |
| GC | 77 (42.3) | 132 (37.7) | (1.42)c | |
| CC | 1 (6.0) | 28 (8.0) | ||
| 182 (100) | 350 (100) | |||
| mdm2 SNP309 | TT | 74 (40.7) | 141 (40.3) | 0.62 |
| TG | 80 (44.0) | 165 (47.1) | (0.97)c | |
| GG | 28 (15.3) | 44 (12.6) | ||
| 182 (100) | 350 (100) | |||
Odds ratio;
95% confidence interval;
χ2 for 2×3 contingency tables.
SNP, single nucleotide polymorphism; mdm, murine double minute; CCA, cholangiocarcinoma.
Effects of epistatic SNP
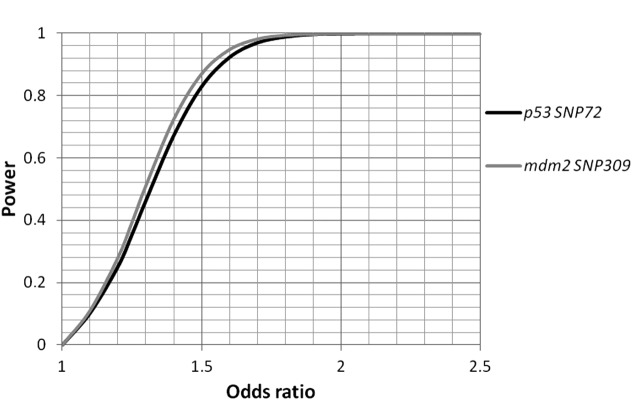
As the two variants under investigation are known to be functionally coupled, we explored the epistatic SNP effects. The results revealed a trend for the overrepresentation of SNP309 TT in carriers of the variant SNP72 genotype CC (81.8 vs. 50.0%; Fisher’s exact test P=0.24; Table III). In agreement with this trend, the allelic distributions reached borderline significance for T allele positivity in SNP72 CC homozygotes (90.9 vs. 69.6%; OR, 4.36; 95% CI, 0.92–20.77; P=0.049; Table IV). The statistical power was calculated, and sufficient power (>97% each) to pinpoint allelic association signals beyond ORs >1.7 for the two SNPs was identified (Fig. 1).
Table III.
Genotypic interaction between p53 and mdm2 variants.
| Genotypes | CCA n (%) | Controls n (%) | P-value | |
|---|---|---|---|---|
| p53 SNP72 | ||||
| GG | SNP309 | 94 (100) | 190 (100) | 0.74 |
| TT | 34 (36.2) | 69 (36.3) | (0.6)a | |
| TG | 44 (46.8) | 95 (50.0) | ||
| GG | 16 (17.0) | 26 (13.7) | ||
| GC | SNP309 | 77 (100) | 132 (100) | 0.66 |
| TT | 31 (40.3) | 58 (43.9) | (0.83)a | |
| TG | 34 (44.1) | 59 (44.7) | ||
| GG | 12 (15.6) | 15 (11.4) | ||
| CC | SNP309 | 11 (100) | 28 (100) | 0.24b |
| TT | 9 (81.8) | 14 (50.0) | ||
| TG | 2 (18.2) | 11 (39.3) | ||
| GG | 0 (0) | 3 (10.7) | ||
| mdm2 SNP309 | ||||
| TT | SNP72 | 74 (100) | 141 (100) | 0.85 |
| GG | 34 (36.2) | 69 (49.0) | (0.32)a | |
| GC | 31 (46.8) | 58 (41.1) | ||
| CC | 9 (17.0) | 14 (9.9) | ||
| TG | SNP72 | 80 (100) | 165 (100) | 0.29 |
| GG | 44 (40.3) | 95 (57.5) | (2.47)a | |
| GC | 34 (44.1) | 59 (35.8) | ||
| CC | 2 (15.6) | 11 (6.7) | ||
| GG | SNP72 | 28 (100) | 44 (100) | 0.38b |
| GG | 16 (81.8) | 26 (59.1) | ||
| GC | 12 (18.2) | 15 (34.1) | ||
| CC | 0 (0) | 3 (6.8) | ||
χ2 for 2×3 contingency tables;
Fisher’s exact test;
SNP, single nucleotide polymorphism; mdm, murine double minute; CCA, cholangiocarcinoma.
Table IV.
Allelic interaction between p53 and mdm2 variants.
| Genotypes | CCA n (%) | Controls n (%) | P-value | |
|---|---|---|---|---|
| p53 SNP72 | ||||
| GG | SNP309 | |||
| T | 112 (59.6) | 223 (58.7) | 0.86 | |
| G | 76 (40.4) | 147 (38.7) | (1.03)a | |
| 188 (100) | 380 (100) | [0.72–1.47]b | ||
| GC | SNP309 | |||
| T | 96 (62.3) | 175 (66.3) | 0.41 | |
| G | 58 (37.7) | 89 (33.7) | (0.84)a | |
| 154 (100) | 264 (100) | [0.56–1.27]b | ||
| CC | SNP309 | |||
| T | 20 (90.9) | 39 (69.6) | 0.049 | |
| G | 2 (9.1) | 17 (30.4) | (4.36)a | |
| 22 (100) | 56 (100) | [0.92–20.77]b | ||
| mdm2 SNP309 | ||||
| TT | SNP72 | |||
| G | 99 (66.9) | 196 (69.5) | 0.58 | |
| C | 49 (33.1) | 86 (30.5) | (0.89)a | |
| 148 (100) | 282 (100) | [0.58–1.36]b | ||
| TG | SNP72 | |||
| G | 122 (76.3) | 249 (75.5) | 0.84 | |
| C | 38 (23.7) | 81 (24.5) | (0.96)a | |
| 160 (100) | 330 (100) | [0.62–1.49]b | ||
| GG | SNP72 | |||
| G | 44 (78.6) | 67 (76.1) | 0.74 | |
| C | 12 (21.4) | 21 (23.9) | (0.87)a | |
| 56 (100) | 88 (100) | [0.39–1.95]b | ||
Odds ratio;
95% confidence interval.
SNP, single nucleotide polymorphism; mdm, murine double minute; CCA, cholangiocarcinoma.
Figure 1.
Statistical power as a function of the effect sizes (odds ratios) and the respective minor allele frequencies of p53 SNP72 (black) and mdm2 SNP309 (grey). α=0.05. SNP, single nucleotide polymorphism; mdm, murine double minute.
Discussion
Given the relative rarity of bile duct cancer and the ensuing lack of informative study populations, there is a paucity of information concerning genetic risk factors for CCA development. As yet, CCA is one of the few cancer types for which no genome-wide association study (GWAS) is available (25). Using a multi-institutional approach we have established a cohort comprising 182 cancer patients, which is currently the largest European-based cohort. However, this sample size may still be regarded as limited with respect to genetic association studies (26). In consideration of our overall negative association signals for either SNP, power estimations were important to ascertain adequate sample size. In our study, the study power exceeded 97% for the two variants with an OR set at 1.7, thus reasonably excluding major SNP effects in CCA susceptibility. The study power to firmly refute associations within distinct exploratory subgroups was limited, meaning that specific risk modulations for subsets of patients may not have been detectable in our data set. For example, there are data indicating gender-specific effects of SNP309 in the mdm2 promoter, which has been suggested to be regulated by estrogens (27–29). Moreover, the two SNPs have been reported to modulate the age of onset in certain types of cancer, e.g., in head and neck and hereditary non-polyposis colorectal cancer (HNPCC) (30,31). Bile duct cancer is characterized by late onset, with a median age at diagnosis of >70 years, which is compatible with the age structure of our study population (32).
However, we observed a potential genotype-specific interaction between p53 SNP72 and mdm2 SNP309. Specifically, in the presence of the apoptosis-deficient p53 variant genotype SNP72 CC, the mdm2 SNP309 major allele T increased the genetic risk of CCA (P=0.049; OR, 4.36; 95% CI, 0.92–20.77). Thus, it appears that in this specific genotypic context the relative attenuation of cellular mdm2 availability (related to the T allele of SNP309) with increased degradation of variant p53 in SNP72 CC homozygous individuals may result in the SNP309 T variant becoming a risk allele. In this respect, it should be noted that, beyond its low-apoptotic capacity, the C allele of p53 SNP72 has been reported to be a stronger inducer of transcription and cell-cycle arrest. These are potential pro-oncogenic effects that may become relevant in a genetic setting of less effective p53 suppression mediated by the mdm2 SNP309 T variant (33,34). However, the association signal was weak and based on a limited number of individuals. With regard to such complex gene-gene interactions, further confirmatory studies in larger cohorts are necessary to ascertain such epistatic SNP effects.
In conclusion, our data do not support the hypothesis of a prominent role of p53 SNP72 and mdm2 SNP309 in the genetic architecture of bile duct cancer. A subtle risk modulation by specific genotype-specific interactions of these functionally coupled genes may modify CCA susceptibility and this requires confirmatory studies in different cohorts.
Acknowledgements
This study was supported by HOMFOR (to V.Z.) and preliminary results were presented, in part, at the Annual Meeting of the American Association for the Study of Liver Diseases (AASLD) in Boston, MA, USA, in October 2009.
References
- 1.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 2.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010;2:407–416. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 5.Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol. 2010;28:3531–3540. doi: 10.1200/JCO.2009.27.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortner MA. Photodynamic therapy for cholangiocarcinoma: overview and new developments. Curr Opin Gastroenterol. 2009;25:472–476. doi: 10.1097/MOG.0b013e32832e6e1f. [DOI] [PubMed] [Google Scholar]
- 7.Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi: 10.1016/j.dld.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–150. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Mihalache F, Hoblinger A, Grunhage F, et al. Heterozygosity for the alpha1-antitrypsin Z allele may confer genetic risk of cholangiocarcinoma. Aliment Pharmacol Ther. 2011;33:389–394. doi: 10.1111/j.1365-2036.2010.04534.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Ortiz-Pallardo ME, Ko Y, Fischer HP. Is heterozygous alpha-1-antitrypsin deficiency type PIZ a risk factor for primary liver carcinoma? Cancer. 2000;88:2668–2676. doi: 10.1002/1097-0142(20000615)88:12<2668::aid-cncr4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Hoblinger A, Lammert F. Genetics of biliary tract diseases: new insights into gallstone disease and biliary tract cancers. Curr Opin Gastroenterol. 2008;24:363–371. doi: 10.1097/MOG.0b013e3282f79b32. [DOI] [PubMed] [Google Scholar]
- 12.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 15.Komarova EA, Krivokrysenko V, Wang K, et al. p53 is a suppressor of inflammatory response in mice. FASEB J. 2005;19:1030–1032. doi: 10.1096/fj.04-3213fje. [DOI] [PubMed] [Google Scholar]
- 16.Gudkov AV, Komarova EA. Dangerous habits of a security guard: the two faces of p53 as a drug target. Hum Mol Genet. 2007;16(Spec No 1):R67–R72. doi: 10.1093/hmg/ddm052. [DOI] [PubMed] [Google Scholar]
- 17.Khan SA, Taylor-Robinson SD, Carmichael PL, Habib N, Lemoine NR, Thomas HC. Analysis of p53 mutations for a mutational signature in human intrahepatic cholangiocarcinoma. Int J Oncol. 2006;28:1269–1277. [PubMed] [Google Scholar]
- 18.Khan SA, Thomas HC, Toledano MB, Cox IJ, Taylor-Robinson SD. p53 Mutations in human cholangiocarcinoma: a review. Liver Int. 2005;25:704–716. doi: 10.1111/j.1478-3231.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 19.Tannapfel A, Weinans L, Geissler F, et al. Mutations of p53 tumor suppressor gene, apoptosis, and proliferation in intrahepatic cholangiocellular carcinoma of the liver. Dig Dis Sci. 2000;45:317–324. doi: 10.1023/a:1005412626515. [DOI] [PubMed] [Google Scholar]
- 20.Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, DePinho RA. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622–6627. doi: 10.1158/0008-5472.CAN-05-4609. [DOI] [PubMed] [Google Scholar]
- 21.Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9:95–107. doi: 10.1038/nrc2584. [DOI] [PubMed] [Google Scholar]
- 22.Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Dumont P, Leu JI, Della Pietra AC, III, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartman M, Loy EY, Ku CS, Chia KS. Molecular epidemiology and its current clinical use in cancer management. Lancet Oncol. 2010;11:383–390. doi: 10.1016/S1470-2045(10)70005-X. [DOI] [PubMed] [Google Scholar]
- 26.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 27.Bond GL, Hirshfield KM, Kirchhoff T, et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006;66:5104–5110. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- 28.Bond GL, Levine AJ. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene. 2007;26:1317–1323. doi: 10.1038/sj.onc.1210199. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer V, Widmann T, Muller M, et al. Genotypic interaction and gender specificity of common genetic variants in the p53/mdm2 network in Crohn’s disease. Digestion. 2010;81:246–251. doi: 10.1159/000241413. [DOI] [PubMed] [Google Scholar]
- 30.Jones JS, Chi X, Gu X, Lynch PM, Amos CI, Frazier ML. p53 polymorphism and age of onset of hereditary nonpolyposis colorectal cancer in a Caucasian population. Clin Cancer Res. 2004;10:5845–5849. doi: 10.1158/1078-0432.CCR-03-0590. [DOI] [PubMed] [Google Scholar]
- 31.Shen H, Zheng Y, Sturgis EM, Spitz MR, Wei Q. P53 codon 72 polymorphism and risk of squamous cell carcinoma of the head and neck: a case-control study. Cancer Lett. 2002;183:123–130. doi: 10.1016/s0304-3835(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 32.Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999;19:1092–1100. doi: 10.1128/mcb.19.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pim D, Banks L. p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer. 2004;108:196–199. doi: 10.1002/ijc.11548. [DOI] [PubMed] [Google Scholar]