Abstract
The purpose of this study was to develop predictive/prognostic markers for liver metastasis and recurrence following liver resection, investigating not only clinical parameters but also molecular markers that are known to be involved in the process of liver metastasis. Seventy colon cancer patients with either no distant metastasis (group A) or with resectable synchronous liver metastasis only (group B) were prospectively enrolled. All 70 patients received curative resection of the primary tumor. Group B patients underwent additional liver resection. Clinical parameters as well as serum levels of molecular markers [carcinoembryonic antigen (CEA), osteopontin, matrix metalloproteinase-7 (MMP-7), tissue inhibitor of metalloproteinase-1 (TIMP-1), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF) and E-selectin] from both tumor drainage (DV) and peripheral veins (PV) were analyzed. Results showed the clinical parameters were not significantly different between groups A and B. Nonetheless, the levels of VEGF and TIMP-1 from both DV and PV were significantly higher in group B compared to group A. In group A, 10 out of 33 (27.0%) patients developed metachronous liver metastasis. High levels of VEGF and TIMP-1 from DV were found to be significantly correlated with metachronous liver metastasis. In group B, 20 out of 33 (60.6%) patients had intrahepatic recurrence following resection of synchronous liver metastasis. The levels of VEGF from DV and the levels of TIMP-1 both from PV and DV were found to be significantly correlated with intrahepatic recurrence. Forty patients (7 from group A and 33 from group B) had liver resection and their 5-year disease-free survival rate was 15.9%. Univariate and multivariate analyses of prognostic factors revealed that the levels of VEGF and TIMP-1 from DV as well as the presence of lymph node metastasis from the primary tumor, synchronous metastasis and R1 resection were significantly associated with worse prognosis. The colon cancer patients with high levels of VEGF and TIMP-1 detected from the DV at the time of their initial surgery were found to have a high risk of metachronous liver metastasis and hepatic recurrence following the resection of synchronous liver metastasis. The high levels of VEGF and TIMP-1 were found to be significant predictive factors for poor prognosis following liver resection. These results require validation but pave the way for future transitional or clinical studies that may provide a greater understanding of colon cancer liver metastasis.
Keywords: metastasis, colon cancer, molecular marker, liver
Introduction
Liver metastasis occurs in almost 50% of patients with colon cancer during the course of the disease (1). Confined liver metastasis from colon cancer may be successfully treated by surgical resection with a 5-year survival ranging from 20 to 40% (2). However, of those patients who undergo surgical resection for liver metastasis, more than two thirds experience recurrence (3–5). Therefore, liver metastasis is a crucial issue for the treatment of colon cancer and it would be invaluable to develop predictive markers for screening high risk groups of patients for liver metastasis and prognostic markers for recurrence following liver resection. Although numerous studies have reported prognostic factors for recurrence and survival following hepatectomy and predicive factors for liver metastasis our current knowledge remains incomplete. Many studies regarding this issue have been carried out. However, the majority of previous studies investigated only clinical parameters. Recent studies in search of prognostic factors for cancer have involved molecular markers that are known to be associated with the mechanism of the disease (6,7). Thus, investigating the molecular markers that are correlated with colon cancer liver metastasis may improve our understanding of its prognosis. Currently, a limited number of studies are available that cover not only clinical parameters but also molecular markers.
The purpose of this study was to develop predictive/prognostic markers for liver metastasis and recurrence following liver resection, investigating not only clinical parameters but also molecular markers that are known to be involved in the process of liver metastasis.
Materials and methods
Patients, treatment and follow-up protocol
We prospectively enrolled 70 colon cancer patients who underwent surgery between August 2004 and June 2006. The eligibility criteria included age between 20 and 70 years, biopsy proven adenocarcinoma arising from the colon or rectum, radiologically confirmed M0 or with resectable synchronous liver metastasis (M1). Exclusion criteria were: patients with unresectable liver metastasis or extrahepatic metastasis, history of cancer within five years, poor medical condition that disallowed surgery or chemotherapy, and pregnancy. Resectability was determined by the Colorectal Tumor Board at Severance Hospital, Yonsei University Health System, Seoul, Korea, which is composed of surgical and medical oncologists as well as radiologists and pathologists. Abdominal computed tomography (CT) scans and/or positron emission tomography (PET) ruled out extrahepatic metastasis. Enrolled patients were classified into two groups: group A comprised patients without liver metastasis and group B comprised patients with synchronous liver metastasis.
The patients received neither chemotherapy nor radiation therapy prior to surgery. The patients in group A received curative surgery for the primary tumor (i.e., colon cancer) only and postoperative adjuvant chemotherapy was administered on the basis of their pathological reports. In group B, the patients received surgery for the primary tumor plus liver resection with curative intent and all were recommended to receive postoperative chemotherapy. The regimen was decided by the treating physician. Postoperative follow-up was carried out every three months. Radiological evaluation with CT and/or PET-CT was carried out every six months to detect any recurrence.
The study was approved by the Institutional Review Board, and informed consent was obtained either from the patient or the patient’s family.
Selection of candidate molecules
To determine potential candidate molecules for this study, we searched the medical database PubMed using the keywords: colon neoplasms, liver metastasis and biological markers. We limited our search scope to studies involving adults above the age of 19 and human subjects only. We only included English language publications. Following retrieval of the list of publications we limited our focus to molecules that could be detected in the blood and that could be analyzed by enzyme-linked immune specific assay (ELISA) with commercially available antibodies. We then reviewed the body of relevant studies published and selected the seven molecules that were most widely studied and were known to be involved in each step of the liver metastatic process (Table I).
Table I.
Selected molecular markers: previous study results and the suggested relationship with colon cancer liver metastasis.
| Molecule | Previous study results and suggested mechanism (Refs.) |
|---|---|
| MMP-7 (matrilysin) | Overexpressed in liver metastasis (13,14); degrades basement membrane and activates gelatinases (15,16) |
| TIMP-1 | Higher serum level in patients with liver metastasis (10,11); growth stimulation and inhibition of apoptosis (17) |
| E-selectin | Overexpressed in patients with metastasis (18,19); anchoring to target organs (20,21) |
| CEA | Stimulates Kupffer cells to enhance cancer cell adhesion (22) |
| Osteopontin | Correlates with cancer progression, silencing suppresses metastasis (23,24) |
| VEGF | Correlates with cancer progression (8,25) |
| HGF | Correlates with cancer progression (9,17) |
CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor.
Blood sampling and ELISA
Blood samples (5 ml) were drawn from the peripheral vein (PV) and tumor drainage vein (DV) and placed in plain tubes. PV blood was obtained 1 h prior to surgical incision. DV blood was obtained prior to the ligation of any branch of the superior mesenteric vein in patients with proximal colon cancer and prior to the ligation of the inferior mesenteric vein in patients with distal colon and rectal cancer. Samples were immediately centrifuged and plasma and serum were separately stored at −70°C until analysis. ELISA for all seven molecules was performed using commercially available kits according to the manufacturer’s instructions (VEGF, HGF, E-selectin, MMP-7, TIMP-1, osteopontin: R&D Systems, Inc., MN, USA; CEA: IBL-Hamburg GmbH, Hamburg, Germany). Diluted serum was transferred to wells of plates pre-coated with primary antibody. Following the recommended incubation period, plates were washed with buffer solution, and substrate solution was added and incubated as instructed. The wells were developed with color-reagent. Stop solution was added to each well after incubation and the optical density was measured at a wavelength of 450 nm using automated optical densitometry. Each sample was run in triplicate, and the mean value was used for analysis. If the R2 of standard solutions was <0.98, data from the plate were excluded.
Statistical analysis
Analyzed clinical parameters included age, gender, the location of the tumor (right versus left colon), TNM stage, histological grade and lymphovascular invasion. Data on molecular factor levels are shown as the mean ± standard deviation (SD). The Mann-Whitney U-test, Fisher’s exact test and McNemar test were used to compare the differences in molecular factor levels between the clinical and pathological features. Cox proportional hazards analysis was used to evaluate the relationship between the pathological features, plasma molecular factor levels and overall survival. P<0.05 was considered statistically significant.
Results
Patient characteristics and factors related to synchronous liver metastasis
The mean age of group B (58.8 years) was higher than that of group A (56.5 years), but did not reach statistical significance. In both groups A and B, pT3–4 occurred more frequently than the other stages and lymph node metastasis was found equally in both groups A and B (Table II). Overall groups A and B had statistically identical clinical features with the exception of lymphovascular invasion, the frequency of which was significantly higher in group B (p=0.004). No surgery-related mortality was reported in either group.
Table II.
Patient characteristics.
| Clinical variables | Group A (n=37) | Group B (n=33) | P |
|---|---|---|---|
| Mean age (years) | 56.5±10.0 | 58.8±10.2 | 0.903 |
| Male-to-female ratio | 19:18 | 21:12 | 0.300 |
| pT | |||
| pT2 | 1 | 0 | >0.999 |
| pT3–pT4 | 36 | 33 | |
| pN | |||
| pN0 | 12 | 10 | 0.544 |
| pN1 | 9 | 5 | |
| pN2 | 16 | 18 | |
| Location | |||
| Right colon | 6 | 5 | 0.903 |
| Left colon/rectum | 31 | 28 | |
| Histology | |||
| Well/mod. diff. | 30 | 26 | 0.811 |
| Poorly/mucinous | 7 | 7 | |
| Lymphovascular invasion | 2 | 11 | 0.004 |
Right colon was defined as from the appendix and cecum to the proximal 2/3 of transverse colon. Left colon from the distal 1/3 of the transverse colon to the rectum. mod. diff., moderately differentiated.
In the DV, the serum levels of VEGF (p<0.001) and TIMP-1 (p<0.001) in group B were significantly higher than those in group A. In PV, the serum levels of E-selectin (p=0.049), VEGF (p=0.024) and TIMP-1 (p<0.001) demonstrated a significant difference (Table III and Fig. 1). Of note, the levels of VEGF and TIMP were higher in group B, whereas the levels of E-selectin were higher in group A.
Table III.
Analyses of the levels of molecular markers in tumor drainage and peripheral veins.
| Group A | Group B | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Markers | Value | Pa | Value | Pa | Pb | |
| CEA | PV | 45.15±32.19 | <0.001 | 116.70±100.31 | <0.001 | 0.116 |
| DV | 135.46±91.31 | 350.11±189.12 | 0.057 | |||
| E-selectin | PV | 41.54±19.38 | 0.009 | 20.38±12.01 | 0.280 | 0.049 |
| DV | 24.37±10.01 | 21.16±13.45 | 0.311 | |||
| Osteopontin | PV | 27.21±27.12 | 0.344 | 50.17±40.70 | 0.102 | 0.068 |
| DV | 25.44±18.48 | 31.24±19.78 | 0.528 | |||
| VEGF | PV | 258.80±179.33 | 0.471 | 459.15±319.82 | 0.529 | 0.024 |
| DV | 246.58±134.29 | 454.17±184.18 | <0.001 | |||
| HGF | PV | 891.87±712.39 | 0.367 | 1015.1±921.63 | 0.303 | 0.266 |
| DV | 748.91±532.47 | 805.69±591.17 | 0.338 | |||
| MMP-7 | PV | 3.90±1.17 | 0.059 | 4.88±2.61 | 0.075 | 0.229 |
| DV | 4.63±1.21 | 5.15±2.04 | 0.517 | |||
| TIMP-1 | PV | 74.05±31.42 | 0.194 | 233.71±97.71 | 0.010 | <0.001 |
| DV | 101.9±87.27 | 194.01±90.73 | <0.001 | |||
Values are shown as the median ± SEM;
P, comparison between DV and PV;
P, comparison between groups A and B;
CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor; MMP-7, matrix metalloproteinase-7; TIMP-1, tissue inhibitor of metalloproteinase-1; LM, liver metastasis; PV, the serum level of a molecule from the peripheral blood; DV, the serum level of a molecule from the drainage vein. Units are: ng/ml for CEA, E-selectin, osteopontin, MMP-7 and TIMP-1; pg/ml for HGF and VEGF. PV, peripheral vein; DV, drainage vein.
Figure 1.
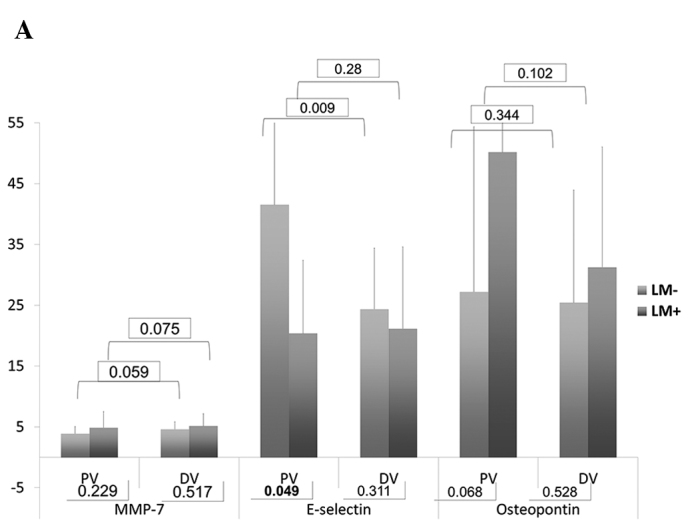
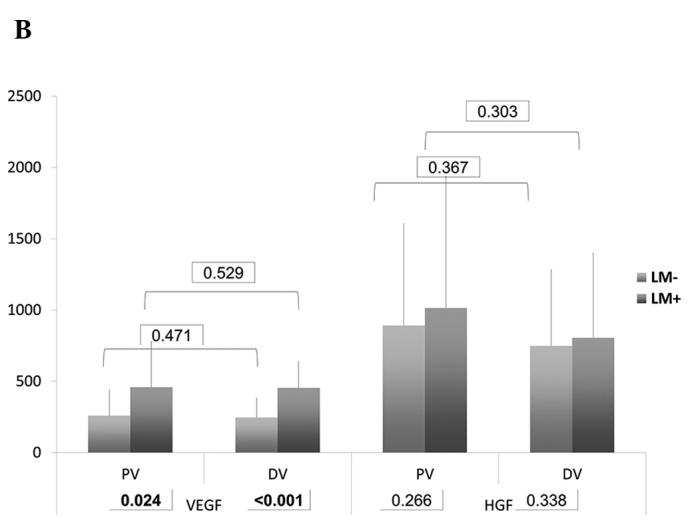
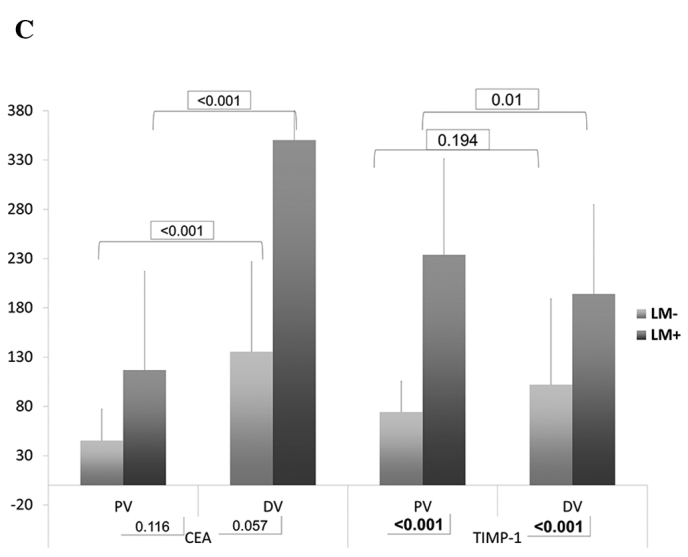
(A) Bar chart of serum levels of MMP-7, E-selectin and osteopontin. (B) Bar chart of serum levels of VEGF and HGF. (C) Bar chart of serum levels of CEA and TIMP-1. Numbers in rectangles are the P-values.
Multivariate analyses were performed to identify clinicopathological factors and molecular markers associated with liver metastasis. The level of TIMP-1 from the DV was found to be the most significantly different factor between groups A and B (HR 4.20; p=0.001) followed by the level of VEGF from DV (HR 4.87; p=0.004). The levels of TIMP-1 from both the DV and the PV were significantly different between groups A and B (Table IV). No clinical factors were found to be significant in the multivariate analysis.
Table IV.
Multivariate analysis for factors significantly associated with the presence of liver metastasis.
| Factors | HR | 95% CI | P |
|---|---|---|---|
| Depth of invasion (pT2 vs. pT3–4) | 2.01 | 0.35–5.83 | 0.83 |
| Lymph node metastasis | 3.42 | 0.44–7.21 | 0.35 |
| Lymphovascular invasion | 2.51 | 0.87–6.33 | 0.093 |
| Histological grade (G1–2 vs. G3–4) | 1.74 | 0.18–4.00 | 0.88 |
| E-selectin (PV) (high vs. low) | 0.61 | 0.25–1.94 | 0.078 |
| VEGF (PV) (high vs. low) | 4.32 | 0.97–29.07 | 0.054 |
| VEGF (DV) (high vs. low) | 4.87 | 2.07–34.41 | 0.004 |
| TIMP-1 (PV) (high vs. low) | 3.15 | 1.66–17.86 | 0.017 |
| TIMP-1 (DV) (high vs. low) | 4.20 | 1.94–22.73 | 0.001 |
VEGF, vascular endothelial growth factor; TIMP-1, tissue inhibitor of metalloproteinase-1; high vs. low, above the median vs. the median or below; PV, the serum level of a molecule from the peripheral blood; DV, the serum level of a molecule from the drainage vein; CI, confidence interval.
Factors associated with metachronous liver metastasis
The median follow-up period for group A was 54 months (range, 33–78) and the 5-year disease-free and overall survival rates were 64.1 and 77.4%, respectively. Of the 37 patients in group A, 4 patients developed local recurrence, 10 developed systemic recurrence and 3 developed both local and systemic recurrence simultaneously. Of the 13 patients who had systemic recurrence, 10 patients (27.0%) had metachronous liver metastasis. The median time to recurrence was 27.3 months. We investigated factors correlated with metachronous liver metastasis by univariate and multivariate analyses (Table V), which revealed that only the levels of VEGF and TIMP-1 from DV were found to be independent prognostic factors for metachronous liver metastasis.
Table V.
Univariate and multivariate analyses for prognostic factors (metachronous liver metastasis) in group A.
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
|
|
|||
| Factors | P | P | HR (95% CI) |
| pT2 vs. pT3–4 | 0.209 | 0.567 | 2.03 (0.212–24.011) |
| pN0 vs. pN1–2 | 0.028 | 0.176 | 24.41 (0.039–72.029) |
| LVI (+ vs. −) | 0.433 | 0.629 | 10.42 (0.486–48.001) |
| Disease-free interval (<1 yr vs. >1 yr) | 0.040 | 0.133 | 15.76 (0.203–62.761) |
| Adjuvant CTx (yes vs. no) | 0.108 | 0.872 | 24.03 (0.739–104.321) |
| CEA (PV) | 0.107 | 0.239 | 5.11 (0.623–31.340) |
| CEA (DV) | 0.032 | 0.118 | 7.32 (0.845–27.328) |
| VEGF (PV) | 0.059 | 0.200 | 6.09 (0.742–25.823) |
| VEGF (DV) | <0.001 | 0.002 | 6.21 (1.241–30.048) |
| TIMP-1 (PV) | 0.013 | 0.056 | 3.12 (0.893–18.295) |
| TIMP-1 (DV) | 0.008 | 0.048 | 4.01 (1.003–20.180) |
CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; TIMP-1, tissue inhibitor of metalloproteinase-1; LVI, lymphovascular invasion; PV, the serum level of a molecule from the peripheral blood; DV, the serum level of a molecule from the drainage vein; CI, confidence interval.
Factors associated with hepatic recurrence following the simultaneous resection of colon cancer and synchronous liver metastasis
In group B, 7 patients had a solitary lesion and the remaining 26 patients had multiple lesions. The median follow-up period was 45 months (range, 27–69). The 5-year disease-free and overall survival rates were 19.2 and 36.8%, respectively. A total of 27 patients had recurrence following their initial surgery, among which 20 patients (60.6%) developed intrahepatic recurrence. Eight patients had liver-only recurrence and 12 patients had liver and other organ metastasis simultaneously. The median time to recurrence was 19 months. For adjuvant chemotherapy following initial surgery, 25 patients received FOLFOX, 1 FOLFOX + bevacizumab, 6 FOLFIRI, and 1 FOLFIRI + cetuximab. We performed univariate and multivariate analyses for factors associated with intrahepatic recurrence. A significant correlation was found between the levels of VEGF from the DV and TIMP-1 from both the PV and DV (Table VI).
Table VI.
Univariate and multivariate analyses for prognostic factors (intrahepatic recurrence) in group B.
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
|
|
|||
| Factors | P | P | HR (95% CI) |
| pT2 vs. pT3–4 | 0.809 | 0.991 | 12.03 (0.011–62.074) |
| pN0 vs. pN1–2 | 0.337 | 0.728 | 9.84 (0.339–73.279) |
| LVI (+ vs. −) | 0.043 | 0.278 | 5.20 (0.641–28.311) |
| Disease-free interval (<1 yr vs. >1 yr) | 0.038 | 0.055 | 8.06 (0.403–37.461) |
| Postop. CTx. (oxaliplatin vs. irinotecan) | 0.847 | 0.895 | 33.92 (0.123–100.419) |
| Use of biological agents (yes vs. no) | 0.764 | 0.925 | 40.34 (0.250–193.004) |
| CEA (PV) | 0.711 | 0.886 | 35.61 (0.213–119.407) |
| CEA (DV) | 0.099 | 0.280 | 17.32 (0.801–47.822) |
| E-selectin (PV) | 0.069 | 0.374 | 9.07 (0.724–36.770) |
| E-selectin (DV) | 0.040 | 0.195 | 9.72 (0.857–40.226) |
| VEGF (PV) | 0.055 | 0.252 | 16.09 (0.472–52.238) |
| VEGF (DV) | <0.001 | <0.001 | 12.16 (1.431–58.348) |
| TIMP-1 (PV) | 0.009 | 0.042 | 8.12 (1.983–60.924) |
| TIMP-1 (DV) | <0.001 | 0.026 | 7.40 (1.020–28.653) |
CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; TIMP-1, tissue inhibitor of metalloproteinase-1; LVI, lymphovascular invasion; PV, the serum level of a molecule from the peripheral blood; DV, the serum level of a molecule from the drainage vein; CI, confidence interval.
Prognostic factors following liver resection
In group A, 10 patients developed metachronous liver metastasis, of which 7 patients received surgical resection or radiofrequency ablation (RFA). In group B, 33 patients developed synchronous liver metastasis, and the prognosis following resection of liver metastasis was analyzed. The median follow-up period was 18 months. The 5-year disease-free and overall survival rates for groups A and B were 15.9 and 33.2%, respectively. Of 7 patients from group A who had received surgical resection or RFA with recurrence, four had solitary lesions and the other three had multiple lesions. All 7 patients had recurrence following liver resection. Five patients had hepatic-only recurrence and the remaining 2 had other systemic recurrences in lung and paraaortic lymph nodes.
Results of the univariate and multivariate analyses of prognostic factors revealed that the levels of VEGF and TIMP-1 from DV as well as the presence of lymph node metastasis from the primary tumor, synchronous metastasis and R1 resection were significantly correlated with worse prognosis (Table VII and Fig. 2).
Table VII.
Univariate and multivariate analyses for prognostic factor (disease-free survival) after resection of liver metastasis.
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
|
|
|||
| Factors | P | P | HR (95% CI) |
| pT2 vs. pT3–4 | 0.632 | 0.811 | 10.22 (0.421–87.447) |
| pN0 vs. pN1–2 | <0.001 | 0.012 | 3.44 (1.003–17.019) |
| LVI (+ vs. −) | 0.043 | 0.398 | 8.90 (0.422–19.882) |
| Synchronous vs. metachronous | <0.001 | 0.003 | 5.61 (1.411–24.376) |
| Postop. CTx. (yes vs. no) | 0.729 | 0.995 | 26.43 (0.092–112.921) |
| Solitary vs. multiple metastasis | 0.037 | 0.123 | 20.540 (0.807–89.424) |
| R0 vs. R1 | <0.001 | 0.003 | 15.001 (1.596–102.531) |
| CEA (PV) | 0.612 | 0.808 | 54.72 (0.093–341.740) |
| CEA (DV) | 0.211 | 0.420 | 32.75 (0.059–199.094) |
| E-selectin (PV) | 0.091 | 0.284 | 18.78 (0.124–72.810) |
| E-selectin (DV) | 0.100 | 0.282 | 23.04 (0.097–152.376) |
| VEGF (PV) | 0.067 | 0.142 | 37.09 (0.852–212.52) |
| VEGF (DV) | <0.001 | <0.001 | 20.72 (1.781–152.377) |
| TIMP-1 (PV) | 0.044 | 0.101 | 12.12 (0.512–243.029) |
| TIMP-1 (DV) | <0.001 | <0.001 | 11.35 (1.110–57.442) |
CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; TIMP-1, tissue inhibitor of metalloproteinase-1; LVI, lymphovascular invasion; PV, the serum level of a molecule from the peripheral blood; DV, the serum level of a molecule from the drainage vein.
Figure 2.
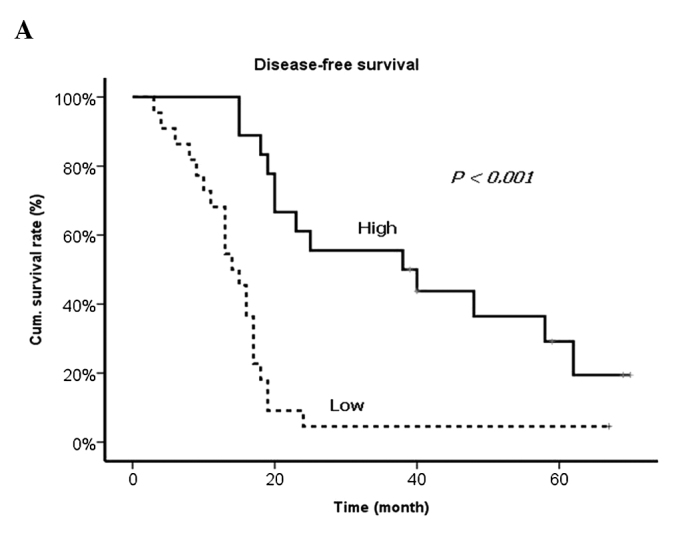
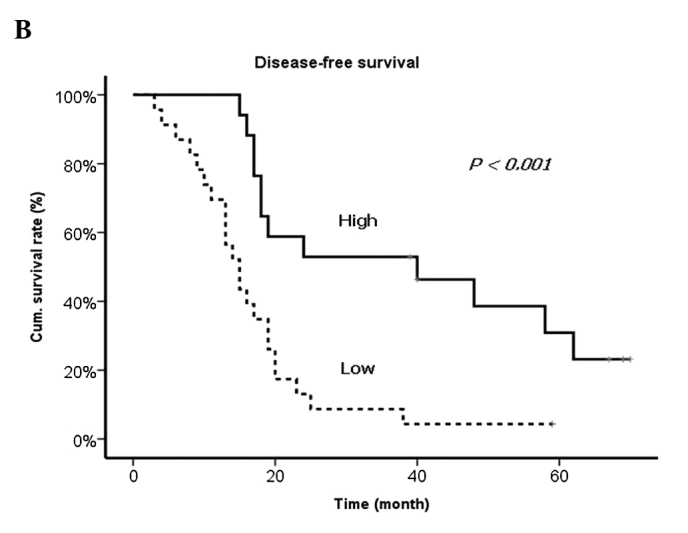
Disease-free survival curves according to the levels of (A) VEGF and (B) TIMP-1.
Discussion
Findings of the present study have shown that the levels of VEGF and TIMP-1, detected in the blood from the vein that directly drains from the primary tumor, were correlated with synchronous and metachronous liver metastasis and hepatic recurrence following R0 resection of liver metastasis more significantly than any other clinical parameters. VEGF is a well-known angiogenic molecule that is overexpressed in the majority of solid organ cancers. It is known to act specifically on endothelial cells to promote new vessel formation and is uniformly reported to be associated with cancer progression (8,9). TIMP-1 is the primary inhibitor of MMP-9 and an imbalance in the MMP-9/TIMP-1 ratio has been proposed to be a potential reason for progression of adenoma to carcinoma (10). Several studies have shown that TIMP-1 level was increased in colon cancer patients and correlated with poor prognosis (10,11).
To the best of our knowledge, this may be the first study to address the clinical importance of molecular levels from the tumor drainage vein (DV), rather than the peripheral vein (PV) in liver metastasis. The rationale behind this finding is that molecules that are expressed and secreted by the primary tumor circulate through the capillaries of the liver, lungs and the rest of the body prior to reaching the peripheral veins and during that circulation a large part of the molecules may be metabolized or decayed. Our hypothesis was that the level of these molecules detected in the DV might carry more accurate information regarding the tumor status and prognosis than in the PV. Tien et al (12) analyzed the relationship between the level of angiogenic factors in both the DV and the PV and prognosis in colon cancer patients. These authors found that the level of VEGF in the DV was an independent prognostic factor. Results of the present study are in concordance with those of Tien et al (12).
A previous study by Yoon et al (9) successfully demonstrated the clinical implication of angiogenic molecular markers in colon cancer patients with liver metastasis. These authors measured the circulating levels of angiogenic molecules such as VEGF, bFGF, EGF and HGF preoperatively and on the third day, first and third month postoperatively. They also found that the preoperative VEGF and HGF levels were significant prognostic factors for recurrence-free survival. High levels of either of these molecular markers were prognostic of poor survival. The fact that a high VEGF level was correlated with poor prognosis is in concordance with our results, whereas the VEGF level found in the study by Yoon et al (9) was measured only in the peripheral vein. In our study, univariate analysis revealed that the VEGF level from both the tumor drainage vein and peripheral vein were significantly correlated with the prognosis. However, multivariate analysis revealed that the VEGF level from only the tumor drainage vein was an independently significant prognostic factor. This might result from the fact that the peripheral VEGF level was significantly influenced by the tumor drainage vein VEGF level. However, the correlation between peripheral and tumor drainage vein VEGF levels remains to be determined, as well as whether we can predict VEGF level in the tumor drainage vein from that in the peripheral vein, which is a crucial issue in terms of clinical practice.
This study has limitations. Although carried out prospectively, only a small number of patients were enrolled. The ELISA technique for the detection of circulating molecular marker is the gold standard. However, the paradigm has now shifted in such a way that biological processes are not expected to be explicated through the investigation of individual molecules. Rather it is the complex interplay between molecules as addressed by high-throughput technologies that is expected to yield complex results. Although we found that the VEGF and TIMP-1 levels in the tumor drainage vein have a significant relationship with synchronous and metachronous liver metastasis and hepatic recurrence following liver resection more than any other clinical parameters, blood from the tumor drainage vein is difficult to obtain, especially in non-surgical patients.
Nonetheless, despite its limitations, the strong point of this study might be the well-defined homogenous patient subsets and long-term follow-up results. This study may also be one of few to cover not only clinical parameters but also molecular markers in developing predictive/prognostic factors for colon cancer liver metastasis.
In conclusion, on the basis of the results from the current study, colon cancer patients who had a high level of VEGF and TIMP-1 detected from the tumor drainage vein at their initial surgery were found to have a high risk of metachronous liver metastasis and hepatic recurrence following the resection of synchronous liver metastasis. The high levels of VEGF and TIMP-1 were also found to be significant prognostic factors that predict poor prognosis following liver resection. These results require validation but may pave the way for future transitional or clinical studies that may further elucidate colon cancer liver metastasis.
Acknowledgements
This study was supported by a National Research Foundation of Korea grant funded by the Korean Government (KRF-E00148).
References
- 1.Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71:4252–4266. doi: 10.1002/1097-0142(19930615)71:12+<4252::aid-cncr2820711815>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Choi HJ, Hyun MS, Jung GJ, Kim SS, Hong SH. Tumor angiogenesis as a prognostic predictor in colorectal carcinoma with special reference to mode of metastasis and recurrence. Oncology. 1998;55:575–581. doi: 10.1159/000011915. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 4.Bozzetti F, Doci R, Bignami P, Morabito A, Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg. 1987;205:264–270. doi: 10.1097/00000658-198703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno H, Mochizuki H, Hashiguchi Y, Hatsuse K, Fujimoto H, Hase K. Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br J Surg. 2004;91:327–333. doi: 10.1002/bjs.4429. [DOI] [PubMed] [Google Scholar]
- 6.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Barozzi C, Ravaioli M, D’Errico A, et al. Relevance of biologic markers in colorectal carcinoma: a comparative study of a broad panel. Cancer. 2002;94:647–657. doi: 10.1002/cncr.10278. [DOI] [PubMed] [Google Scholar]
- 8.Baker EA, Bergin FG, Leaper DJ. Plasminogen activator system, vascular endothelial growth factor, and colorectal cancer progression. Mol Pathol. 2000;53:307–312. doi: 10.1136/mp.53.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon SS, Kim SH, Gonen M, et al. Profile of plasma angiogenic factors before and after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 2006;13:353–362. doi: 10.1245/ASO.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 10.Waas ET, Wobbes T, Ruers T, Lomme RM, Hendriks T. Circulating gelatinases and tissue inhibitor of metalloproteinase-1 in colorectal cancer metastatic liver disease. Eur J Surg Oncol. 2006;32:756–763. doi: 10.1016/j.ejso.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Oberg A, Hoyhtya M, Tavelin B, Stenling R, Lindmark G. Limited value of preoperative serum analyses of matrix metalloproteinases (MMP-2, MMP-9) and tissue inhibitors of matrix metalloproteinases (TIMP-1, TIMP-2) in colorectal cancer. Anticancer Res. 2000;20:1085–1091. [PubMed] [Google Scholar]
- 12.Tien YW, Chang KJ, Chiu YF, Huang KW, Lee PH. Comparison of angiogenic factor levels in tumor drainage and peripheral venous blood from colorectal cancer patients. Ann Surg Oncol. 2006;13:1357–1363. doi: 10.1245/s10434-006-9042-8. [DOI] [PubMed] [Google Scholar]
- 13.Adachi Y, Yamamoto H, Itoh F, Hinoda Y, Okada Y, Imai K. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut. 1999;45:252–258. doi: 10.1136/gut.45.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masaki T, Matsuoka H, Sugiyama M, et al. Matrilysin (MMP-7) as a significant determinant of malignant potential of early invasive colorectal carcinomas. Br J Cancer. 2001;84:1317–1321. doi: 10.1054/bjoc.2001.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 16.Zeng ZS, Shu WP, Cohen AM, Guillem JG. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: evidence for involvement of MMP-7 activation in human cancer metastases. Clin Cancer Res. 2002;8:144–148. [PubMed] [Google Scholar]
- 17.Bueno M, Salgado S, Beas-Zarate C, Armendariz-Borunda J. Urokinase-type plasminogen activator gene therapy in liver cirrhosis is mediated by collagens gene expression down-regulation and up-regulation of MMPs, HGF and VEGF. J Gene Med. 2006;8:1291–1299. doi: 10.1002/jgm.961. [DOI] [PubMed] [Google Scholar]
- 18.Alexiou D, Karayiannakis AJ, Syrigos KN, et al. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer. 2001;37:2392–2397. doi: 10.1016/s0959-8049(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 19.Uner A, Akcali Z, Unsal D. Serum levels of soluble E-selectin in colorectal cancer. Neoplasma. 2004;51:269–274. [PubMed] [Google Scholar]
- 20.Wittig BM, Kaulen H, Thees R, et al. Elevated serum E-selectin in patients with liver metastases of colorectal cancer. Eur J Cancer. 1996;32A:1215–1218. doi: 10.1016/0959-8049(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 21.Roselli M, Guadagni F, Martini F, et al. Association between serum carcinoembryonic antigen and endothelial cell adhesion molecules in colorectal cancer. Oncology. 2003;65:132–138. doi: 10.1159/000072338. [DOI] [PubMed] [Google Scholar]
- 22.Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703–712. doi: 10.1023/a:1006576627429. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal D, Chen T, Irby R, et al. Osteopontin identified as colon cancer tumor progression marker. CR Biol. 2003;326:1041–1043. doi: 10.1016/j.crvi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Wai PY, Mi Z, Guo H, et al. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis. 2005;26:741–751. doi: 10.1093/carcin/bgi027. [DOI] [PubMed] [Google Scholar]
- 25.Behzadian MA, Windsor LJ, Ghaly N, Liou G, Tsai NT, Caldwell RB. VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. FASEB J. 2003;17:752–754. doi: 10.1096/fj.02-0484fje. [DOI] [PubMed] [Google Scholar]


