Abstract
Estrogen has pleiotropic effects on the cardiovascular system. The mechanisms by which estrogen confers these pleiotropic effects on cardiovascular function is under active investigation. Until a decade ago, all estrogen signaling was thought to occur by estrogen binding to nuclear estrogen receptors (ERα and ERβ), which bind to DNA and function as ligand activated transcription factors. Estrogen binding to the receptor alters gene expression thereby altering cell function. In 2000 estrogen was also shown to bind to nuclear estrogen receptors that are tethered to the plasma membrane resulting in acute activation of signaling kinases such as PI3K. An orphan G-protein coupled receptor, GPR30, has also been shown to bind estrogen and activate acute signaling pathways. ERβ has also been reported to be localized to the mitochondria, although this has been controversial. Thus estrogen can alter cell function by binding to several estrogen receptors. There appear to be mechanisms to localize these receptors to different cellular compartments, which results in complex signaling. This paper will review the different estrogen receptors and their signaling mechanisms, will also discuss mechanisms that might regulate estrogen receptor levels and locations, and lastly will consider cardiovascular effects of estrogen signaling.
I. How Does Estrogen Alter Cell Function?
The effects of estrogen on cardiovascular function are mediated by several estrogen receptors. There are two nuclear estrogen receptors(ER), ER-α and ER -β. ERα, which is 67 kD and ERβ, which is 59 kD are highly homologous. The DNA binding domain is very conserved (~97% homologous) between ERα and ERβ, as is the ligand binding domain (60% homologous). However, ERα and ERβ differ in the amino terminal transcriptional control domain, AF-1, through which regulatory binding partners interact1. The nuclear ER can altered gene expression by direct binding to DNA, by binding DNA indirectly via other transcription factors, or by ligand independent binding (see Figure 1). In addition, the nuclear ER can be localized outside the nucleus where it can directly activate signaling kinases such as PI3K. ERβ has also been reported to be localized to the mitochondria2, 3, although this has been controversial4. Mitochondrial ERβ has been proposed to modulate mitochondrial DNA transcription3. Estrogen can also bind to an orphan G-protein coupled receptor (GPR30), which can lead to acute activation of signaling kinases5. These mechanisms will be discussed separately.
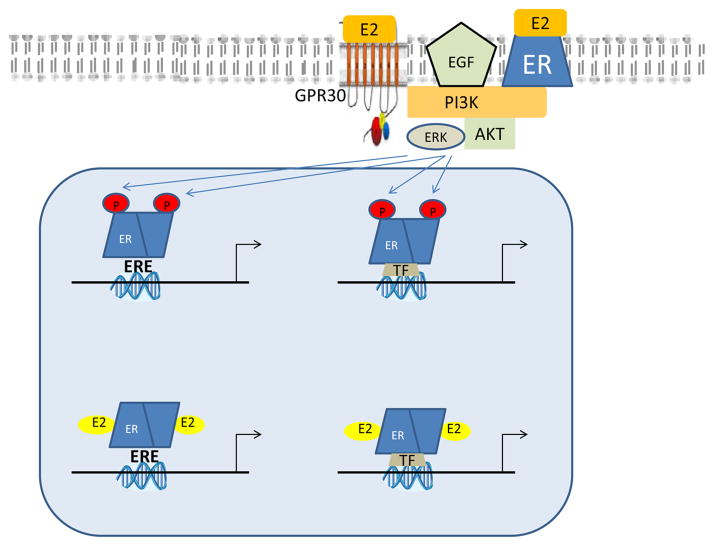
Figure 1.
Figure 1 shows the major mechanism by which ER can alter gene expression. Estrogen (E2) binds to ER resulting in dimerization and recruitment of co-regulators (not shown due to space limitations). The estrogen-ER complex binds to estrogen response elements (ERE) on the DNA resulting in altered gene transcriptions. Estrogen can also alter gene transcription by binding to transcription factors (TF) such as AP1. In addition, ER can be phosphorylated by growth factors and other plasma membrane estrogen receptors that are coupled to kinase signaling. Phosphorylated ER can activate gene transcription in a ligand-independent manner.
ER direct binding to DNA
ERα and ERβ act as ligand gated receptors to alter gene expression. Estrogen binding to the AF-2 domain of ER results in a conformational change leading to ER dimerization and binding to consensus ERE sites on the DNA. The consensus ERE sequence is 5′ GGTCAnnnTGACC 3′. Co regulators are recruited resulting in an increase or decrease in gene expression. Depending on the co-regulators (co-activators and co-repressors) present in the cell, the estrogen-ER complex can have different effects (see 6 for a more detailed review). The co-activators are also commonly shared by different nuclear receptors. The recruitment of co-regulators depends in part on the ligand bound, likely due to difference in conformation with different ligands. For example, tamoxifin is an ER agonist in endometrium because it recruits co-activators, but it is an antagonist in breast because it recruits co-repressors7.
ER indirect binding to DNA
Some genes regulated by ER do not contain an ERE. ER can also bind to DNA indirectly via transcription factors such as AP1 and Sp1 (see Figure 1). This mechanism is typically referred to as transcriptional cross talk. Estrogen can lead to both activation and inactivation of AP1 dependent transcription (see8 for details). Jakacka et al9 mutated the DNA binding site of ER to show that DNA binding was not required for estrogen modulation of the AP1 reporter. Mutations in the hinged region of ER have shown that this region is important for tethering ER to transcription factors.10
Ligand independent binding to DNA
ER can also function in a ligand independent manner to alter gene transcription (see Figure 1). ER can be phosphorylated allowing it to bind to ERE or indirectly to transcription factors and modulate gene transcription in the absence of ligand binding. It was known that growth factors such as EGF can stimulate uterine growth. Curtis et al showed that the stimulatory effects of EGF require the ER.11 Phosphorylation of ER at specific serine sites has been shown to be important for ligand independent activation of transcription12. Sinkevicius et al13 mutated ERα to decrease estrogen binding to study its ligand independent effects. Knock-in mice expressing this mutant ER exhibited a hypoplastic uterus, but growth factors were still able to increase uterine epithelial cell proliferation in OVX-KI mice13. These data show that the ligand independent pathways were active with the ER mutant with poor estrogen binding. In addition to phosphorylation of ER, growth factor dependent phosphorylation of co-activators can also be important to ligand-independent transcription14. Carascossa et al14 show that protein kinase A phosphorylates the coactivator-associated arginine methyltransferase and that phosphorylation of this co-activator is necessary for ligand-independent activation of ERα gene transcription.
Acute, non-transcriptional signaling
Further complicating ER signaling, in addition to estrogen’s effects on gene transcription, estrogen has also been shown to bind to the nuclear estrogen receptor tethered to the plasma membrane, which has been shown to initiate signaling via PI3K5. ERα can undergo palmitoylation at cysteine 447, which leads to its association with caveolin. Estrogen has also been shown to bind to an orphan G-protein coupled receptor, GPR30, which can also activate rapid kinase signaling pathways such as activation of PI3K and MAPK.5 Activation of GPR30 has also been reported to reduce ischemia-reperfusion injury15 and to attenuate cardiac remodeling in salt sensitive mRen2.Lewis rats16. Chambliss used an estrogen-dendrimer conjugate, which is excluded from the nucleus to show that extracellular estrogen can stimulate endothelial cell proliferation and migration via a Gαi and eNOS dependent mechanism. 17
Mitochondrial localized ERβ
There are a number of studies suggesting a role for ER, particularly ERβ in regulation of mitochondria2, 3. ERβ has been reported to be localized in the mitochondria2, 3, 18, although the mass spectrometry identification has been questioned4. It has been suggested that mitochondrial ERβ can regulate mitochondrial genes via its association with mitochondrial DNA and/or mitochondrial transcription factors3. Estrogen has been reported to alter mitochondrial function. Yang et al19 report the ERβ is localized to the mitochondria and that knockdown of ERβ is associated with a lower mitochondrial membrane potential and increased resistance to hydrogen peroxide mediated decrease in membrane potential. A number of studies have suggested that female mitochondria generate less ROS20–24. Recently Lagranha et al reported that mitochondria from females have increased phosphorylation of mitochondrial α-ketoglutarate dehydrogenase which leads to less ROS generation by this enzyme under conditions of increased NADH24. How much the effects of estrogen on mitochondrial function are mediated by nuclear ERs versus acute signaling pathways versus mitochondrial localized ER will require further study.
The multiple layers of estrogen signaling allow for synergism between the nuclear signaling via altered gene transcription and the estrogen signaling that activates kinase signaling (see figure 2A). The ability of estrogen to alter protein expression and alter kinase signaling allows multiple levels of control. For example, in the cardiovascular system, estrogen upregulates eNOS Estrogen also acutely activates PI3K signaling leading to phosphorylation and activation of eNOS. Thus estrogen can increase NO signaling in target tissue by both increasing the level of eNOS and increasing the activity of eNOS via phosphorylation.
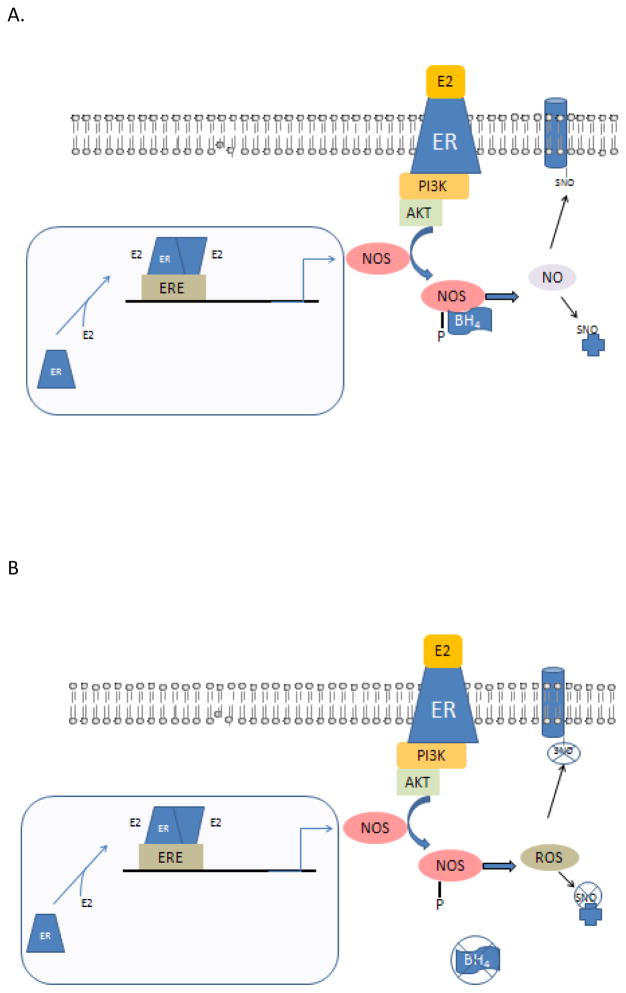
Figure 2.
Figure 2 A illustrated the interaction of the genomic and acute estrogen signaling pathways in regulating nitric oxide (NO) signaling. Estrogen (E2) can bind to ER resulting in dimerization and activation of gene transcription. Nitric oxide synthase (NOS) expression has been reported to be regulated by E2 and females have been shown to have higher levels of NOS than males. In addition, E2 active via rapid signaling pathways can activate the PI3K pathway regulating in phosphorylation and activation of NOS.
Figure 2B illustrates how this signaling might be altered with aging and disease and provides a possible explanation as to why HRT failed to protect in post-menopausal women. Tetrahydropterin (BH4) is a cofactor for NOS and in the absence of BH4, NOS now generates ROS rather than NO.
II. ERα and ERβ differentially regulate gene expression
ERα and ERβ can regulate different genes in different tissues25. ERα and ERβ have been shown to regulate distinct genes in a time and tissue dependent manner26–28. These differences are attributed to differences in co-activators and co-repressors in different tissue and different levels of ERα relative to ERβ.
Within the same tissue, ERα and ERβ have been shown to differentially regulate gene expression28. Tsutsumi et al report that in vascular smooth muscle cells, iNOS expression is enhanced by ERβ and repressed by ERα29. In general there are only a few studies examining estrogen responsive genes in the cardiovascular system. O’Lone et al showed that ERα and ERβ regulate different genes in mouse aorta.26 Gene array studies to determine ER subtype regulation of gene transcription were done using mouse aorta in WT, αERKO, and βERKO from OVX mice treated with estrogen for 1 week. They reported that ERα primarily upregulates gene expression whereas ERβ results in downregulation of gene expression. They indicate that 90% of the genes showing an estrogen mediated decrease are ERβ dependent. ERβ is reported to downregulate expression of genes encoding the electron transport complexes. Jayachandran et al30 also report that ERβ regulates expression of the electron transport chain. However in contrast to O’Lone, Jayachandran et al report that electron transport chain expression was reduced in platelets from βERKO, suggesting the ERβ increases expression of the electron transport chain. This difference could be due to a differential regulation in platelets versus aorta or other differences in the model. Nikolic et al27 did gene array studies comparing gene expression in mouse hearts from OVX females that were perfused for 2 hours with either vehicle or the ERβ selective agonist DPN. In contrast to the study on aorta, 122 genes were upregulated by ERβ and only 23 genes were downregulated. Gene ontology analysis showed that DNP downregulated contractile protein genes and upregulated immune/chemokine genes and genes involved in regulating cell death. Whether the difference between O’Lone et al and Nikolic et al is due to a difference in tissue (aorta vs heart) or to the difference in model (mice null for ERβ and ERα versus treatment with an ERβ agonist) will require further study. There could also be a time dependent difference in gene expression; Nikolic acutely (for 2 hours) added an ERβ agonist to hearts from OVX mice, whereas O’Lone et al treated OVX mice with estrogen for 1 week. A number of studies have shown that estrogen regulates genes in a time dependent manner31, 32. Schnoes et al31 reported that in vascular tissue, estrogen recruits in a temporal manner specific transcription factors that propagate distinct estrogen signaling. Studies in other tissues have also shown that estrogen results in time dependent changes in gene expression32. Clearly additional studies are needed to better define the role of different ER receptors in regulating gene expression in the cardiovascular system.
Otsuki et al examined gene changes in hearts from ovariectomized females treated for 3 weeks with estrogen compared to vehicle 33. They reported an induction of seven genes and decreased expression of nine genes 33. The induced genes included lipocalin-type prostaglandin D synthase and dipeptidase I. The repressed genes included thymosin beta10 and several types of procollagen. Gabel el al. performed gene profiling to determine genes differentially expressed in hearts from mice lacking ERβ compared to WT and αERKO mice 34. Loss of ERβ was found to lead to an induction of solute carrier 4 (member 1) and decreased expression of a number of metabolism genes including SPOT14 homolog, lipoprotein lipase, ATP citrate lyase, stearoyl CoA desaturase and fatty acid synthase 34.
Other studies have used a candidate gene approach and have identified a number of genes regulated (directly or indirectly) by estrogen, including, PGC-1α 35, connexin 43 36, 37, adenine nucleotide translocator 38, heat shock proteins 39, mitochondrial complex IV 40, GLUT4 41 and MCIP1, an inhibitor of calcineurin 42. Many of these proteins have been suggested to be important in cardioprotection 43, 44. There are also data suggesting male-female differences or estrogen mediated difference in protein levels24.
III. What regulates ER levels?
Because ERα and ERβ differentially regulate gene expression, differences in the expression or activity of ERα and ERβ could have profound effects on gene expression. As discussed some genes are upregulated by ERα, but unaffected or downregulated by ERβ and vice versa. Thus by altering the relative expression or activity of ERα versus ERβ one could alter gene expression in the cell and ultimately the phenotype of the cell. GPR30 or differences in ER localization could also alter gene expression by phopshorylation of the ER receptor leading to ligand independent activity. GPR30 activation has also been reported to enhance levels of an ERα36, a variant of ERα45.
ERα and ERβ show different patterns of expression in different cell types26. Grohe et al46 have reported in cardiac myocytes that ERβ is expressed similarly in males and females but ERα is influenced by gender. ER levels have also been reported to change in the cardiovascular tissue with age and disease47. Although not studied in detail, the localization of ERα and ERβ and the activity of GPR30 can also influence tissue response to estrogen. A number of factors have been shown to regulate the expression of ERα48. Estrogen has been shown to positively regulate ER levels, and a number of other hormones such as progesterone and vitamin D have been reported to negatively regulate ER levels48. Long term estrogen treatment has been reported to increase ERα and decrease ERβ expression in the vasculature49. Differences in ER levels are reported in males and females and in pre and post-menopausal women. Gavin et al50 reported that ERα expression in vascular endothelial cells in premenopausal women is 30% lower during the early follicular phase compared to the late follicular phase. Furthermore, post-menopausal women had ERα levels that were 33% lower than in the late follicular phase. Although in the cardiovascular system we know little of the differential regulation of ER levels, this could have important implications. There are a number of different splice variants for estrogen receptors and their physiological role is poorly understood. One of the splice variants for ERβ (ERβ2) has been reported to bind poorly to ER, but to result in degradation of ERα51. Van Rooij et al also have shown that MEF2 and class II HDAC can regulate ERα expression52. Class II HDACs repress ERα via a MEF2 response element. The expression of ER levels has also been reported to change with cardiovascular disease47. Methylation of the promoter of ER has been shown to reduce ER expression53. Thus changes in the level and/or activity of different ERs are reported to occur with age and disease and such changes would have profound effects on gene expression in the cell (see Figure 3). Future studies are needed to better define changes in expression and localization of ER with age, sex and disease in the cardiovascular system.
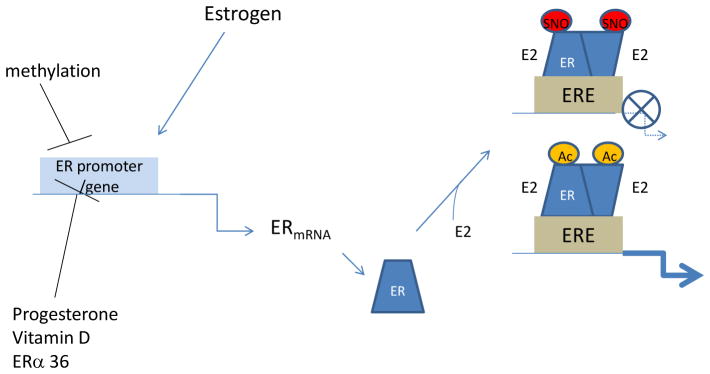
Figure 3.
Figure 3 illustrated the different mechanisms that can regulate the level and activity of ER. ER expression is regulated by methylation of the ER promoter region. In many cell types estrogen has been shown to increase ERa transcription and progesterone, vitamin D and ERα36 have been reported to decrease expression of ER. ER transcriptional activity can be regulated by post-translational modifications such as acetylation (Ac), which activates transcriptional activity or by S-nitrosylation (SNO) which tends to inhibit translational activity.
IV. Post-translational modifications of ER
As discussed the activity of the ER can be modulated by post-translational modifications. Phosphorylation of ER can result in ligand-independent modulation of gene transcription. The ER can be phosphorylated on a number of sites54. Phosphorylation of serine 118, 104 and 106 of ERα by ERK1/2 are important for ligand independent activation12. The precise role of each phosphorylation site of ER is not known, but they appear to have important functional consequences. For example, phosphorylation of ERα on serine 118 has been shown to be important for tamoxifin inhibition of gene expression in breast cancer55.
The ER can also be S-nitrosylated, which has been reported to inhibit ER binding to selective EREs56. It has been suggested that S-nitroyslation of ER could shift the signaling from the nucleus to non-genomic signaling mechanisms. Because ER can also lead to an increase in nitric oxide synthase (NOS) and an increase in activity of NOS via acute kinase signaling mechanisms, the S-nitrosylation of ER could serve as a negative feedback regulator.
ERs can also be modified by acetylation. Acetylation has been reported to increase the transcriptional activity of ERα57, 58. BRCA1, the breast cancer susceptibility gene has been shown to result in decreased acetylation of ERα, which would reduce ERα transcriptional activity. The mechanism by which BRCA1 decreases acetylation of ERα is still under investigation,59 but it has been suggested that BRCA1 can increase mono-ubiquitination on the same lysines that are targets for acetylation. Thus BRCA1 mediated ubiquitination of ERα would reduce its acetylation and transcriptional activation. The promoter of ER can also be methylated which is reported to decrease ER levels and inactivate ER transcriptional activity (see Figure 3).53
V. Effects of Estrogen on the Cardiovascular System
Estrogen has many important effects on the cardiovascular system. Premenopausal females have reduced cardiovascular disease, and the incidence of disease increases after menopause. Estrogen has been shown to improve the lipid profile, thereby reducing the development of atherosclerosis. Estrogen increases NO signaling in the vasculature and to improve vessel responsiveness. Estrogen has also been reported to reduce the onset of type 2 diabetes, to improved insulin responsiveness, to alter glucose metabolism, and to alter mitochondrial biogenesis.
1. Estrogen and Hypertrophy
There are a number of studies showing that estrogen can slow the development of hypertrophy. OVX female C57B6 mice receiving estrogen via minipumps exhibited reduced hypertrophy following transaortic constriction (TAC) compared to mice receiving vehicle60. Consistent with this finding, females have been reported to have less hypertrophy than males at 2 weeks after TAC in C57B6 mice61. Skavdahl et al further showed that the reduced hypertrophy observed in intact females compared to males was lost in female mice lacking ERβ, suggesting a role for ERβ in the reduction in hypertrophy61. Similarly, Babiker et al reported that OVX females from WT mice and mice lacking ERα showed less hypertrophy when treated with estrogen than with vehicle; however mice lacking ERβ treated with estrogen did not show a reduction in hypertrophy62. Taken together these data suggest a role for ERβ in reducing hypertrophy in females.
The details of the mechanism by which estrogen reduced hypertrophy is still under investigation. Donaldson et al63 reported that OVX females treated with estrogen had less hypertrophy than OVX females treated with vehicle; they further showed that estrogen treatment resulted in a decrease in calcineurin A, which has been shown to enhance hypertrophy. They also showed that the inhibitory effects of estrogen were lost in mice lacking calcineurin A, suggesting that the beneficial effects of estrogen in limiting hypertrophy is due to estrogen mediated degradation of calcineurin A.
2. Estrogen Signaling and Cardioprotection
Acute in vivo administration of estrogen just prior to ischemia has been reported to reduce infarct size24, 64–68. Hale et al65, 66 showed that a 10ug IV bolus of β-estradiol reduced infarct size in both male and female rabbits. Similarly Booth et al64 showed that 20ug estrogen administration reduced infarcts size in rabbits, and Das and Sarkar67 found that 10ug/kg IV estrogen administered prior to coronary ligation to also reduced infarct size in rabbits. Sbarouni et al showed estrogen treatment of OVX rabbits decreased infarct size compared to vehicle treatment in rabbits fed normal and a cholesterol enriched diet68. Lagranha et al also showed that treatment of male rats with estrogen for 2 weeks reduced infarct size24. Wang et al showed that estradiol improved contractile function in rat hearts following ischemia and reperfusion69. In addition to studies, primarily in rabbit, showing that addition of estrogen can reduce infarct size, a number of studies have reported reduced infarct size in females compared to males following ischemia and reperfusion27, 34, 70–73. The mechanism by which estrogen reduces ischemia-reperfusion injury is still under investigation. There are data suggesting a role for signaling via ERβ and ERα, although the results are mixed. Estrogen has also been reported to upregulate nitric oxide synthase which appears to be important in protection observed in females25, 71, 72, 74. A role for S-nitrosylation of several proteins has been suggested to play a role in the protection observed in females71, 72. There are also data suggesting an important role for estrogen activation of kinase signaling pathways such as PI3K. A role for GPR30 in activation of these signaling mechanisms has also been suggested15, 75, 76. It is likely that estrogen mediated protection involved upregulation of important target genes as well as acute activation of kinase signaling pathways (see Figure 2A). It is also likely that these pathways synergize to enhance cardioprotection. The observation that acute addition of estrogen, just prior to ischemia can reduce infarct size would suggest that acute signaling pathways play an important role in protection. It is possible that different estrogen receptors are important for the chronic versus the acute protection observed with estrogen. This would account for some of the discrepancies in the literature.
Although pre-menopausal women have reduced incidence of cardiovascular disease, there are data suggesting those women who have angioplasty have worse outcomes than men. The reasons for this difference, which are discussed in detail elsewhere, include technical issues relating to smaller vessel size in females, and increased co-morbidities in premenopausal females who develop cardiovascular disease (for discussion see77). The number of pre-menopausal women who develop cardiovascular disease is small and it has been proposed that they may have more underlying risk factors. It has also been suggested that although estrogen might reduce the risk of cardiovascular disease, primarily by improving lipid profile or by vascular effects, estrogen may increase the injury resulting from ischemia, either by increased cell death pathways or by adverse remodeling. As mentioned previously, acute administration of estrogen in animal models has been shown to reduce cell death, so this explanation seems less likely. However, it is likely that the effects of estrogen signaling have both beneficial and detrimental effects on ischemia-reperfusion injury and it is important to better understand how estrogen alters cell death in this context.
3. Estrogen effects on Cardiac Physiology
It is well established that estrogen results in improved vascular function and lipid profile78. Estrogen can also regulate the inflammatory response. Sex hormones have been shown to alter inflammatory cytokines79. Kararigas et al have reported a significant increase in activation of inflammatory signaling in mice lacking ERβ80. These data are consistent with the study of Nikolic et al who reported that an ERβ agonist decreased levels of inflammatory cytokines27.
Furthermore, estrogen has a number of effects on metabolism. Estrogen has been shown to reduced diabetes. In fact although hormone replacement therapy (HRT) was not beneficial in terms of reducing cardiovascular risk, HRT did improve insulin sensitivity and reduced type-2 diabetes.81–83 These data are consistent with data showing that loss of ERα in humans has been associated with the development of type 2 diabetes84.
Estrogen has recently been shown to promote stem cell survival.85 Estrogen receptors have been shown to play an important role in cardiac repair by bone marrow-derived endothelial progenitor cells following infarction86, 87. Estrogen receptor has been reported to increase survival of cardiomyocytes following myocardial infarction88.
VI. Why does HRT fail to protected?
Premenopausal women have been shown to have reduced cardiovascular disease and the incidence of cardiovascular disease rises after menopause. As discussed, estrogen has a number of beneficial effects on the cardiovascular system such as improved lipid profile, improved vascular health and improved insulin responsiveness. The beneficial effects of estrogen in the cardiovascular system and reduced cardiovascular disease in premenopausal females led to the hypothesis that estrogen is cardioprotective and formed the basis for the use of hormone replacement therapy (HRT) to reduce cardiovascular disease. It was therefore surprising when several large prospective studies, the WHI and the HERS study found that HRT was not beneficial89, 90. Interestingly, in the WHI and HERS trials, HRT resulted in an improvement in lipid profile90 and a reduction in type 2 diabetes,81, 82 but in spite of these effects HRT did not improve cardiovascular outcomes.
When WHI and HERS trial data were initially released, several proposals were put forth to explain possible reasons why estrogen did not protect in post menopausal women, in contrast to reduced cardiovascular disease in premenopausal women. One popular hypothesis, known as the “timing hypothesis” centered on the observation that the mean age at which HRT was initiated in WHI was 63.91–93 It was suggested that these women were likely post menopausal (i.e. with low levels of estrogen) for a number of years before estrogen was restarted. It was proposed that continuous treatment with estrogen might have a different outcome than reinstatement of estrogen after it falls during menopause. The WHI data have been re-analyzed to address this issue and the re-analyzed data did not support the concept that initiating HRT soon after menopause has a beneficial effect on cardiovascular disease and stroke94, 95.
It has been suggested that perhaps there are age related changes that occur that reduce the protection afforded by estrogen96. One mechanism by which estrogen improves both vascular health and reduces cardiomyocytes death is by activation of eNOS (see Figure 2). Tetrahydrobiopterin (BH4) is a co-factor for eNOS and in the absence of BH4, eNOS becomes uncoupled, a mode in which it generates superoxide and very little nitric oxide97. Presumably, activation of NOS in the absence of BH4 would be detrimental rather than cardioprotective (see Figure 2B). Loss or reduction of BH4 has been suggested to be a contributing factor as to why many cardioprotective drugs lose their protection with aging or diseases, which reduce BH4 levels98. Tetrahydrobiopterin has been shown to be reduced with aging leading to uncoupling of NOS resulting in decreased nitric oxide production and increased ROS production99–101. The lack of nitric oxide production by NOS could interfere with cardioprotective signaling, and the increase in ROS could exacerbate the injury.
It is also possible that there are age related changes in ER levels or activity or in the proportion of ERα versus ERβ versus GRP30. As mentioned, ER can undergo a number of post-translational modifications that can alter its activity. ER can undergo acetylation which tends to increase its transcriptional activity. Methylation of the ER promoter reduces levels of ER. Furthermore, ER levels are themselves transcriptionally regulated by estrogen, vitamin D and other hormones. Thus level, activity or composition of ER levels could alter with age.
VII. Unresolved Questions and Future Directions
Estrogen can signal by at least 3 different receptors. These receptors can differentially regulate transcription; thus depending on the relative levels of receptors, estrogen can increase, decrease or have no effect on transcription. These receptors can localized to different parts of the cell and this can alter their signaling and thus the response to estrogen. Estrogen receptor signaling also depends on co-regulators and thus by altering co-regulator levels and/or post translational modification of ER and/or the co-regulators, estrogen can have different responses. Thus differential post translational modifications of ERα relative to ERβ could alter the response of the cell to estrogen. Based on the complexity of estrogen signaling it is not surprising that estrogen could have different effects as a function of age and disease, which could be important in the lack of protection with HRT in post -menopausal females.
Nitric oxide has been shown to be an important component of estrogen signaling and cardioprotection, and NOS has been shown to become uncoupled with age and disease, typically due to loss or oxidation of the co-factor BH4. Uncoupled nitric oxide synthase generates ROS rather than nitric oxide and this could also contribute to the lack of protection of HRT with age and disease.
What are the key unresolved questions regarding ER signaling in the cardiovascular system? We need to know the levels of post translational modification of ERα, ERβ and GPR30 in cardiovascular targets as a function of age, sex, and disease. We need to know the genes that are regulated by ERα, ERβ and GPR30 and we need to determine if and how this changes with time of estrogen treatment. We need to understand the localization of the different estrogen receptors and how this affects estrogen signaling. We also need to better understand the mechanisms by which estrogen regulates cardiovascular function. How does estrogen regulate endothelial function, lipid profiles, mitochondrial function, response to ischemia, insulin sensitivity, and hypertrophic responses? We need to understand how estrogen regulates these processes in young healthy women before we can understand why estrogen was not beneficial in aging post-menopausal women.
Abbreviations
- AF-1
activator function-1
- AF-2
activator function-2, ligand binding region of ER
- AP1
A transcription factor complex of c-fos and c-jun
- BH4
tetrahydrobiopterin
- BRCA1
breast cancer gene 1
- DPN
2,3-bis(4-hydroxyphenyl)-propionitrile
- ER
estrogen receptor
- ERE
estrogen response element
- ERK
extracellular regulated kinase
- GPR30
G-protein coupled receptor 30, also known as G-protein estrogen receptor
- HDAC
histone deacetylase
- HERS
heart and estrogen/progestin replacement study
- HRT
hormone replacement therapy
- MAPK
mitogen activated protein kinase
- MEF2
myocyte enhancer factor 2
- OVX
ovariectomized
- NOS
nitric oxide synthase
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- Sp1
Specificity factor 1, a transcription factor
- TAC
transaortic constriction
- WHI
women’s health initiative
- WT
wild type
Reference List
- 1.McInerney EM, Katzenellenbogen BS. Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem. 1996;271(39):24172–24178. doi: 10.1074/jbc.271.39.24172. [DOI] [PubMed] [Google Scholar]
- 2.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17(5):2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria--physiological and pathological implications. Mol Cell Endocrinol. 2008;290(1–2):51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwend T, Gustafsson JA. False positives in MALDI-TOF detection of ERbeta in mitochondria. Biochem Biophys Res Commun. 2006;343(3):707–711. doi: 10.1016/j.bbrc.2006.02.164. [DOI] [PubMed] [Google Scholar]
- 5.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 7.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 8.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 9.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276(17):13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 10.Burns KA, Li Y, Arao Y, Petrovich RM, Korach KS. Selective mutations in estrogen receptor alpha’s D-domain alters nuclear translocation and non-ERE gene regulatory mechanisms. J Biol Chem. 2011 doi: 10.1074/jbc.M110.187773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci U S A. 1996;93(22):12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas RS, Sarwar N, Phoenix F, Coombes RC, Ali S. Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity. J Mol Endocrinol. 2008;40(4):173–184. doi: 10.1677/JME-07-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149(6):2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carascossa S, Dudek P, Cenni B, Briand PA, Picard D. CARM1 mediates the ligand-independent and tamoxifen-resistant activation of the estrogen receptor alpha by cAMP. Genes Dev. 24(7):708–719. doi: 10.1101/gad.568410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297(5):H1806–1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2010;5(11):e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120(7):2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800(10):1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW. Estrogen receptor beta as a mitochondrial vulnerability factor. J Biol Chem. 2009;284(14):9540–9548. doi: 10.1074/jbc.M808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74(3):456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68(4):959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 22.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borras C, Gambini J, Vina J. Mitochondrial oxidant generation is involved in determining why females live longer than males. Front Biosci. 2007;12:1008–1013. doi: 10.2741/2120. [DOI] [PubMed] [Google Scholar]
- 24.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106(11):1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, Grohe C. 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res. 1999;43(3):666–674. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 26.O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21(6):1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 27.Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42(4):769–780. doi: 10.1016/j.yjmcc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17(2):203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsumi S, Zhang X, Takata K, Takahashi K, Karas RH, Kurachi H, Mendelsohn ME. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J Endocrinol. 2008;199(2):267–273. doi: 10.1677/JOE-07-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayachandran M, Preston CC, Hunter LW, Jahangir A, Owen WG, Korach KS, Miller VM. Loss of estrogen receptor beta decreases mitochondrial energetic potential and increases thrombogenicity of platelets in aged female mice. Age (Dordr) 32(1):109–121. doi: 10.1007/s11357-009-9119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnoes KK, Jaffe IZ, Iyer L, Dabreo A, Aronovitz M, Newfell B, Hansen U, Rosano G, Mendelsohn ME. Rapid recruitment of temporally distinct vascular gene sets by estrogen. Mol Endocrinol. 2008;22(11):2544–2556. doi: 10.1210/me.2008-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17(10):2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 33.Otsuki M, Gao H, Dahlman-Wright K, Ohlsson C, Eguchi N, Urade Y, Gustafsson JA. Specific regulation of lipocalin-type prostaglandin D synthase in mouse heart by estrogen receptor beta. Mol Endocrinol. 2003;17(9):1844–1855. doi: 10.1210/me.2003-0016. [DOI] [PubMed] [Google Scholar]
- 34.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38(2):289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh YC, Choudhry MA, Yu HP, Shimizu T, Yang S, Suzuki T, Chen J, Bland KI, Chaudry IH. Inhibition of cardiac PGC-1alpha expression abolishes ERbeta agonist-mediated cardioprotection following trauma-hemorrhage. Faseb J. 2006;20(8):1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- 36.Chung TH, Wang SM, Wu JC. 17beta-estradiol reduces the effect of metabolic inhibition on gap junction intercellular communication in rat cardiomyocytes via the estrogen receptor. J Mol Cell Cardiol. 2004;37(5):1013–1022. doi: 10.1016/j.yjmcc.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Yu W, Dahl G, Werner R. The connexin43 gene is responsive to oestrogen. Proc Biol Sci. 1994;255(1343):125–132. doi: 10.1098/rspb.1994.0018. [DOI] [PubMed] [Google Scholar]
- 38.Too CK, Giles A, Wilkinson M. Estrogen stimulates expression of adenine nucleotide translocator ANT1 messenger RNA in female rat hearts. Mol Cell Endocrinol. 1999;150(1–2):161–167. doi: 10.1016/s0303-7207(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 39.Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003;285(2):H687–692. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh YC, Yu HP, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Upregulation of mitochondrial respiratory complex IV by estrogen receptor-beta is critical for inhibiting mitochondrial apoptotic signaling and restoring cardiac functions following trauma-hemorrhage. J Mol Cell Cardiol. 2006;41(3):511–521. doi: 10.1016/j.yjmcc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006;103(5):1605–1608. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280(28):26339–26348. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boengler K, Schulz R, Heusch G. Connexin 43 signalling and cardioprotection. Heart. 2006;92(12):1724–1727. doi: 10.1136/hrt.2005.066878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shamaei-Tousi A, Halcox JP, Henderson B. Stressing the obvious? Cell stress and cell stress proteins in cardiovascular disease. Cardiovasc Res. 2006 doi: 10.1016/j.cardiores.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24(4):709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grohe C, Kahlert S, Lobbert K, Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol. 1998;156(2):R1–7. doi: 10.1677/joe.0.156r001. [DOI] [PubMed] [Google Scholar]
- 47.Nordmeyer J, Eder S, Mahmoodzadeh S, Martus P, Fielitz J, Bass J, Bethke N, Zurbrugg HR, Pregla R, Hetzer R, Regitz-Zagrosek V. Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation. 2004;110(20):3270–3275. doi: 10.1161/01.CIR.0000147610.41984.E8. [DOI] [PubMed] [Google Scholar]
- 48.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24(11):4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res. 2002;91(9):814–820. doi: 10.1161/01.res.0000038304.62046.4c. [DOI] [PubMed] [Google Scholar]
- 50.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94(9):3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao C, Matthews J, Tujague M, Wan J, Strom A, Toresson G, Lam EW, Cheng G, Gustafsson JA, Dahlman-Wright K. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67(8):3955–3962. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 52.van Rooij E, Fielitz J, Sutherland LB, Thijssen VL, Crijns HJ, Dimaio MJ, Shelton J, De Windt LJ, Hill JA, Olson EN. Myocyte enhancer factor 2 and class II histone deacetylases control a gender-specific pathway of cardioprotection mediated by the estrogen receptor. Circ Res. 106(1):155–165. doi: 10.1161/CIRCRESAHA.109.207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43(4):985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 54.Atsriku C, Britton DJ, Held JM, Schilling B, Scott GK, Gibson BW, Benz CC, Baldwin MA. Systematic mapping of posttranslational modifications in human estrogen receptor-alpha with emphasis on novel phosphorylation sites. Mol Cell Proteomics. 2009;8(3):467–480. doi: 10.1074/mcp.M800282-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kok M, Holm-Wigerup C, Hauptmann M, Michalides R, Stal O, Linn S, Landberg G. Estrogen receptor-alpha phosphorylation at serine-118 and tamoxifen response in breast cancer. J Natl Cancer Inst. 2009;101(24):1725–1729. doi: 10.1093/jnci/djp412. [DOI] [PubMed] [Google Scholar]
- 56.Garban HJ, Marquez-Garban DC, Pietras RJ, Ignarro LJ. Rapid nitric oxide-mediated S-nitrosylation of estrogen receptor: regulation of estrogen-dependent gene transcription. Proc Natl Acad Sci U S A. 2005;102(7):2632–2636. doi: 10.1073/pnas.0409854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20(7):1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson BJ, Tremblay AM, Deblois G, Sylvain-Drolet G, Giguere V. An acetylation switch modulates the transcriptional activity of estrogen-related receptor alpha. Mol Endocrinol. 2010;24(7):1349–1358. doi: 10.1210/me.2009-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y, Fan S, Hu C, Meng Q, Fuqua SA, Pestell RG, Tomita YA, Rosen EM. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Mol Endocrinol. 2010;24(1):76–90. doi: 10.1210/me.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104(12):1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- 61.Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288(2):H469–476. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- 62.Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohe C, Doevendans PA. Estrogen receptor beta protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol. 2006;26(7):1524–1530. doi: 10.1161/01.ATV.0000223344.11128.23. [DOI] [PubMed] [Google Scholar]
- 63.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res. 2009;104(2):265–275. doi: 10.1161/CIRCRESAHA.108.190397. 211p following 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Booth EA, Marchesi M, Kilbourne EJ, Lucchesi BR. 17Beta-estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther. 2003;307(1):395–401. doi: 10.1124/jpet.103.054205. [DOI] [PubMed] [Google Scholar]
- 65.Hale SL, Birnbaum Y, Kloner RA. beta-Estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. Am Heart J. 1996;132(2 Pt 1):258–262. doi: 10.1016/s0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- 66.Hale SL, Birnbaum Y, Kloner RA. Estradiol, Administered Acutely, Protects Ischemic Myocardium in Both Female and Male Rabbits. J Cardiovasc Pharmacol Ther. 1997;2(1):47–52. doi: 10.1177/107424849700200106. [DOI] [PubMed] [Google Scholar]
- 67.Das B, Sarkar C. Similarities between ischemic preconditioning and 17beta-estradiol mediated cardiomyocyte KATP channel activation leading to cardioprotective and antiarrhythmic effects during ischemia/reperfusion in the intact rabbit heart. J Cardiovasc Pharmacol. 2006;47(2):277–286. doi: 10.1097/01.fjc.0000202563.54043.d6. [DOI] [PubMed] [Google Scholar]
- 68.Sbarouni E, Iliodromitis EK, Bofilis E, Kyriakides ZS, Kremastinos DT. Estrogen alone or combined with medroxyprogesterone but not raloxifene reduce myocardial infarct size. Eur J Pharmacol. 2003;467(1–3):163–168. doi: 10.1016/s0014-2999(03)01627-3. [DOI] [PubMed] [Google Scholar]
- 69.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol. 2006;40(2):205–212. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Cross HR, Murphy E, Steenbergen C. Ca(2+) loading and adrenergic stimulation reveal male/female differences in susceptibility to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;283(2):H481–489. doi: 10.1152/ajpheart.00790.2001. [DOI] [PubMed] [Google Scholar]
- 71.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98(3):403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 72.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120(3):245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther. 2005;315(3):1125–1135. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 74.Chakrabarti S, Lekontseva O, Peters A, Davidge ST. 17beta-Estradiol induces protein S-nitrosylation in the endothelium. Cardiovasc Res. 2010;85(4):796–805. doi: 10.1093/cvr/cvp368. [DOI] [PubMed] [Google Scholar]
- 75.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298(1):H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery. 148(2):436–443. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75(3):478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 78.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89(12A):12E–17E. doi: 10.1016/s0002-9149(02)02405-0. discussion 17E–18E. [DOI] [PubMed] [Google Scholar]
- 79.Corcoran MP, Meydani M, Lichtenstein AH, Schaefer EJ, Dillard A, Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 206(2):217–224. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kararigas G, Fliegner D, Gustafsson JA, Regitz-Zagrosek V. The role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol Genomics. doi: 10.1152/physiolgenomics.00199.2010. [DOI] [PubMed] [Google Scholar]
- 81.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 82.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 83.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 122(1–3):74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 85.Nelson WD, Zenovich AG, Ott HC, Stolen C, Caron GJ, Panoskaltsis-Mortari A, Barnes SA, 3rd, Xin X, Taylor DA. Sex-dependent attenuation of plaque growth after treatment with bone marrow mononuclear cells. Circ Res. 2007;101(12):1319–1327. doi: 10.1161/CIRCRESAHA.107.155564. [DOI] [PubMed] [Google Scholar]
- 86.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114(21):2261–2270. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- 87.Dawn B, Bolli R. Increasing evidence that estrogen is an important modulator of bone marrow-mediated cardiac repair after acute infarction. Circulation. 2006;114(21):2203–2205. doi: 10.1161/CIRCULATIONAHA.106.658260. [DOI] [PubMed] [Google Scholar]
- 88.Brinckmann M, Kaschina E, Altarche-Xifro W, Curato C, Timm M, Grzesiak A, Dong J, Kappert K, Kintscher U, Unger T, Li J. Estrogen receptor alpha supports cardiomyocytes indirectly through post-infarct cardiac c-kit+ cells. J Mol Cell Cardiol. 2009;47(1):66–75. doi: 10.1016/j.yjmcc.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 89.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 90.Shlipak MG, Chaput LA, Vittinghoff E, Lin F, Bittner V, Knopp RH, Hulley SB. Lipid changes on hormone therapy and coronary heart disease events in the Heart and Estrogen/progestin Replacement Study (HERS) Am Heart J. 2003;146(5):870–875. doi: 10.1016/S0002-8703(03)00412-5. [DOI] [PubMed] [Google Scholar]
- 91.Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. Am J Epidemiol. 2007;166(5):506–510. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- 92.Merz CN, Johnson BD, Berga SL, Braunstein GD, Azziz R, Yang Y, Reis SE, Bittner V, Hodgson TK, Pepine CJ, Sharaf BL, Sopko G, Kelsey SF. Total estrogen time and obstructive coronary disease in women: insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Womens Health (Larchmt) 2009;18(9):1315–1322. doi: 10.1089/jwh.2008.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356(25):2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 94.Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banks E, Canfell K. Invited Commentary: Hormone therapy risks and benefits--The Women’s Health Initiative findings and the postmenopausal estrogen timing hypothesis. Am J Epidemiol. 2009;170(1):24–28. doi: 10.1093/aje/kwp113. [DOI] [PubMed] [Google Scholar]
- 96.White RE, Gerrity R, Barman SA, Han G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75(11):788–793. doi: 10.1016/j.steroids.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273(40):25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 98.Tang XL, Takano H, Xuan YT, Sato H, Kodani E, Dawn B, Zhu Y, Shirk G, Wu WJ, Bolli R. Hypercholesterolemia abrogates late preconditioning via a tetrahydrobiopterin-dependent mechanism in conscious rabbits. Circulation. 2005;112(14):2149–2156. doi: 10.1161/CIRCULATIONAHA.105.566190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297(5):H1829–1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pierce GL, Larocca TJ. Reduced vascular tetrahydrobiopterin (BH4) and endothelial function with ageing: is it time for a chronic BH4 supplementation trial in middle-aged and older adults? J Physiol. 2008;586(Pt 11):2673–2674. doi: 10.1113/jphysiol.2008.154229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]