Abstract
Hungary is traditionally regarded as a leishmaniasis-free country, and human or canine cases diagnosed locally have been recorded as imported. However, recent entomological surveys have verified the presence in Hungary of Phlebotomus neglectus and Phlebotomus perfiliewi perfiliewi, which have been incriminated as competent vectors of Leishmania infantum elsewhere in Europe. Following the occurrence in October 2007 of an undisputable clinical case of L. infantum canine leishmaniasis (CanL) in a 4-year-old female pug in a kennel of 20 dogs in Tolna province, an investigation was performed to assess the infection status in that canine population and to search for putative phlebotomine vectors. Another female pug became sick during the study period (May–November 2008) and L. infantum was confirmed as the causative agent. The other animals appeared clinically healthy; however, 4 additional dogs were found positive by indirect fluorescent antibody test (2 dogs), or by buffy-coat PCR (1 dog), or by both methods (1 dog). Hence the overall Leishmania infection prevalence in the kennel was 30% (6/20). All dogs were born in the same place and had been always kept outdoors. They had neither been abroad nor received a blood transfusion. No sand flies were collected with CDC Standard Miniature Light traps, Mosquito Magnet® X (MMX) dry ice-baited traps, or sticky traps placed either in or around the kennel and at nearby chicken yards during July and August of 2008 and 2009. Considering the dogs' historical background and the failure to trap any sand fly vectors in the kennel area, the origin of CanL in this site remains unexplained.
Key Words: Dog, Hungary, Indirect fluorescent antibody test, Kinetoplast DNA PCR, Leishmania infantum, Leishmaniasis, Ribosomal DNA nested PCR, Sand fly
Introduction
Canine leishmaniasis (CanL) is a vector-borne zoonotic infection caused by Leishmania (L.) infantum Nicolle 1908 (class Kinetoplastida, family Trypanosomatidae) in the Mediterranean sub-region. Although wild canids and domestic animals such as cats can be naturally infected, domestic dogs are the main reservoirs of this parasite. L. infantum causes visceral leishmaniasis (VL) or, less frequently, cutaneous leishmaniasis in humans (Gramiccia and Gradoni 2005). Moreno and Alvar (2002) estimated that about 2.5 million infected dogs exist in the endemic regions of southern Europe, most of them without any clinical signs.
L. infantum is usually transmitted between hosts by sand fly species belonging to the subgenus Phlebotomus (P.) (Larroussius) (order Diptera, subfamily Phlebotominae; Lindgren et al. 2004; Ready 2010). Both clinically ill and seropositive asymptomatic dogs are infectious to sand flies, thus posing a risk to uninfected dogs and humans (Molina et al. 1994). Vertical transmission from bitch to puppies or horizontal transmission by bites or via transfusion may also occur, but these cases are considered of limited epidemiological importance due to a lack of competent vectors (Teske et al. 2002). In northern latitudes, leishmaniasis has become more apparent in areas where sand fly vectors are either absent or present at very low densities, such as has been observed in North America (Gaskin et al. 2002; Schantz et al. 2005).
The northward spread of CanL and the vector species was observed in Western Europe during the past two decades (Ready 2010). De novo formation of CanL endemic foci, and the diffusion of sand fly species, have been clearly demonstrated in previously non-endemic provinces of northern Italy (Maroli et al. 2008). There have been other indications that a northward expansion of L. infantum is occurring in Europe. Autochthonous canine, human, equine, and feline leishmaniases have been reported from the southwestern region of Germany (Naucke and Schmitt 2004). The occurrence of CanL in non-endemic areas might be explained by dog importation from, or travel to, endemic Mediterranean regions (Teske et al. 2002). However, the spatial distribution of the leishmaniases may also be influenced by the climatic modifications associated with global climate change. These changes affect the activity and vector competence of the sand fly vector species, and parasite development in female sand flies (Ready 2010). Socio-economic alterations due to climate change might also affect the spread of L. infantum through increasing numbers of holiday travels with dogs.
Hungary has been traditionally regarded as a non-endemic country for leishmaniasis because only a few dozen imported human VL cases had been recorded (Várnai et al. 1985). In the last decade Péterfi and associates (2011) and Fried and colleagues (2003) reported VL cases diagnosed in two Hungarian persons who had spent their holidays in Dalmatia, an endemic coastal region of Croatia (Bosnić et al. 2006). Clinical CanL was diagnosed only in two dogs that returned from visits to Greece and Spain (Magdus 2004; Farkas et al. 2011). However, in the last decade, the numbers of traveling and imported dogs have increased, thereby raising concerns about the introduction of CanL to Hungary. Recently eight imported CanL cases were reported by Hungarian veterinarians during a survey (Farkas et al. 2011).
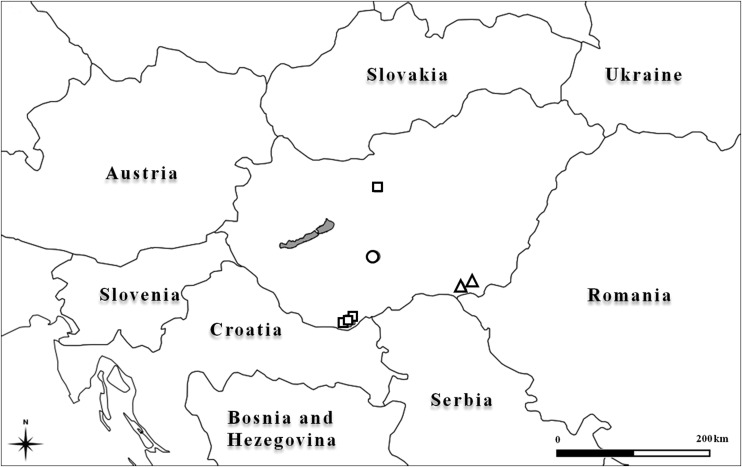
Our knowledge of the Hungarian phlebotomine sand fly fauna has been limited until recently because no thorough country-wide surveys had been conducted. From 2006 to 2009, phlebotomine sand flies were sampled during the summer months and a small number of two L. infantum vector species were recovered (Farkas et al. 2011). Phlebotomus neglectus was found in three villages near the Croatian border and in one village in northern Hungary at latitude N 47° (Fig. 1). Phlebotomus perfiliewi perfiliewi was trapped at two sites in a southeastern county in Hungary (Fig. 1), close to the sites where it was first collected in 1931–1932 (Lőrincz and Szentkirályi 1933). We report herein the first autochthonous cases of CanL confirmed in a kennel of 20 dogs in the Tolna province of Hungary.
FIG. 1.
Location of the sites of autochthonous cases of canine leishmaniasis (○), Phlebotomus neglectus (□), and Phlebotomus perfiliewi perfiliewi (▵) in Hungary.
Materials and Methods
Study site, dogs, and samples
The study was carried out in a kennel housing 20 dogs of 3 different breeds (15 pugs, 3 French bulldogs, and two chow chows), located in Tolna province, Central Hungary (Fig. 1). The dogs were kept in pens and were born in the kennel. None had been abroad nor received a blood transfusion. When a veterinarian visited in October 2007, a 4-year-old female pug (P01, the index case) showed severe clinical signs suggestive of CanL, such as alopecia, skin ulcers, uveitis, onychogryphosis, enlarged prescapular and popliteal lymph nodes, and a rapid decline in general health. A blood sample was taken for laboratory investigation. Because of deteriorating clinical conditions, dog P01 was euthanized following the owner's request and samples from popliteal lymph nodes, liver, and spleen were preserved for post-mortem analysis. Autopsy revealed enlargement of the spleen. In May 2008, clinical examinations were made and blood samples were taken from the other 19 dogs in the kennel (P2 to P20). Only one dog (P12) showed enlargement of lymph nodes, from which aspirate samples were obtained.
Blood serum and buffy coat, and biopsy/aspirate tissue samples for PCR examination were stored in the laboratory at −20°C pending the assays.
Histology and immunohistochemistry
Spleen and liver samples from dog P01 were subjected to histological and immunohistochemical examinations. Sections 4 μm thick of formalin-fixed and paraffin-embedded tissue samples were stained with hematoxylin and eosin (H&E), and examined by light microscopy.
Immunohistochemistry (IHC) was used to demonstrate the presence of Leishmania amastigotes (Tafuri et al. 2004). After deparaffinization, the sections were incubated in 3% H2O2 solution for 10 min, and then in a 2% solution of skim milk powder for 20 min. The samples were incubated overnight at 4°C with a 1:2000 dilution of anti-Leishmania lipophosphoglycan mouse monoclonal antibody (clone CA7AE; Cedarlane Laboratories, Burlington, Ontario, Canada). Antibody binding was detected by a horseradish peroxidase-labeled polymer (EnVision™+Kit; Dako, Glostrup, Denmark). A serial section incubated with phosphate buffer and a spleen sample from a dog that died from distemper virus were used as negative controls.
Serology
The serum sample from index case P01 was analyzed by an indirect fluorescent antibody test (IFAT) at the IDEXX Vet Med Labor, Ludwigsburg, Germany. Later, sera from the other 19 dogs (P2–P20) were tested with a commercial IFAT kit (MegaScreen® FluoLeish; Diagnostic MegaCor GmbH, Horbranz, Austria), and in parallel, by an in-house IFAT assay using promastigotes of the WHO reference strain for L. infantum zymodeme MON-1 (MHOM/TN/80/IPT-1) as antigen. The assays followed the protocol recommended by the Office International des Epizooties (Gradoni and Gramiccia, 2004), and the cut-off titer was set at 1:80.
DNA extraction and polymerase chain reaction assays
Spleen, liver, lymph node biopsy/aspirate, and peripheral blood buffy-coat samples were examined by PCR. DNA extraction was carried out with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. DNA extracts were stored at −20°C.
Three different PCR assays were performed for Leishmania DNA amplification: ribosomal DNA single-step PCR, kinetoplast DNA PCR, and ribosomal DNA nested-PCR.
18S rDNA single step PCR
A blood sample from the P01 index case was examined first at the IDEXX Vet Med Labor, with a single-step PCR targeting the 18S rDNA locus.
Kinetoplast DNA (kDNA) PCR
A 145-bp section of L. infantum kDNA with the primer pairs RV1 and RV2 was amplified by a PCR reaction (Lachaud et al. 2002) was adapted with a modified premix. The premix was prepared with the GoTaq Core System I (Promega, Madison, WI). Concentrations in 25 μL final volume were as follows: 1× for GoTaq green buffer, 2 mM for MgCl2, and 0.2 mM for each dNTP. Primers, polymerase, and extracted DNA were administered at 25 pmole, 1 U, and 5 μL, respectively. A positive probe (prepared from the cultured IPT-1 strain of L. infantum), and two negative probes (DNA negative and buffy-coat of a healthy dog) were applied in the reaction. PCR products were sent for sequencing to Macrogen Inc. (Seoul, South Korea). Edited sequences were aligned with GenBank references (NCBI BLAST).
Small-subunit ribosomal (ssu r) DNA nested (n)-PCR
For the n-PCR assay, the first amplification was carried out in a 50-μL volume containing 10 μL DNA plus 40 μL PCR Master Mix (Promega), containing 50 pmol of the kinetoplastid-specific primers R221 and R332 of the ssu rRNA gene (van Eys et al. 1992). For the second amplification, 3 μL of the first PCR product were added to 22 μL of PCR Master Mix (Promega) containing 3 pmol of the Leishmania-specific primers R223 and R333 of the same gene (van Eys et al. 1992). Two negative (no DNA, and buffy-coat DNA from healthy dogs), and 2 positive controls (DNA from L. infantum-cultured promastigotes and buffy-coat DNA from Leishmania-infected dogs) were employed. Amplification products were analyzed on 1.5% agarose gel and visualized under UV light. Positive samples yielded a predicted n-PCR product of 358 bp.
Entomological surveys
In July and August of 2008 and 2009, three types of traps were used in and around the kennel for trapping phlebotomine sand flies. For 12 nights, two CDC Standard Miniature Light Traps (John W. Hock Co., Gainesville, FL), and one Mosquito Magnet X (MMX; American Biophysics Corp., North Kingstown, RI) dry ice-baited trap were used. CDC and MMX traps were also used in two chicken yards, both located about 400 meters away from the kennel. On the walls and doors of the dog and poultry houses, 30 sticky traps (20×20-cm squares of white paper impregnated with castor oil) were hung to monitor for the occurrence of sand flies.
Results
Histology, biochemistry, and immunohistochemistry findings in the index case (P01)
An aspiration cytology sample collected from the enlarged left popliteal lymph node was highly cellular, with numerous reactive macrophages and moderate small to medium-sized lymphocytes, as well as increased numbers of plasma cells and a few neutrophil granulocytes. Macrophages contained many organisms 1–3 μm in diameter, each having a round, deeply basophilic nucleus, and a rod shaped kinetoplast, morphologically consistent with Leishmania species amastigotes (Figs. 2 and 3).
FIG. 2.
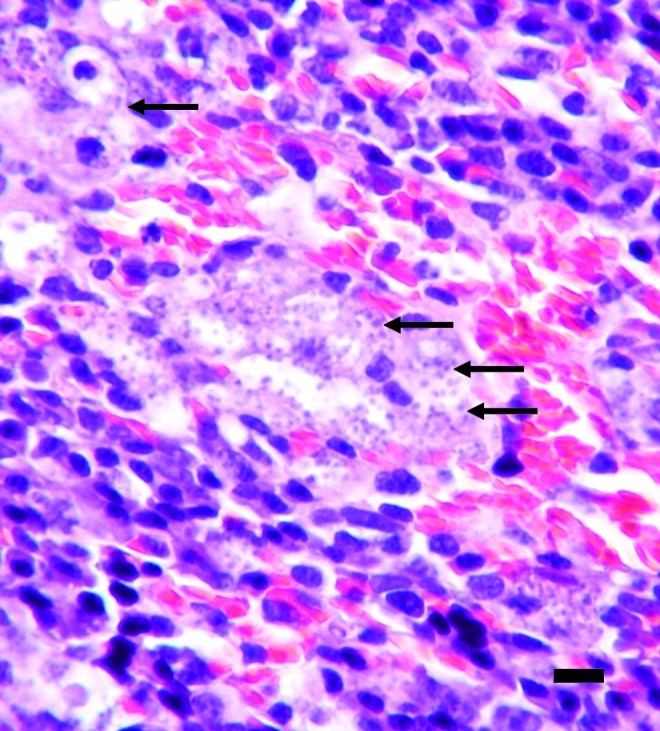
Spleen of the index case (dog P01). Macrophages of red pulp loaded with Leishmania amastigotes (arrows; hematoxylin and eosin stain; scale bar=12.5 μm). Color images available online at www.liebertpub.com/vbz
FIG. 3.
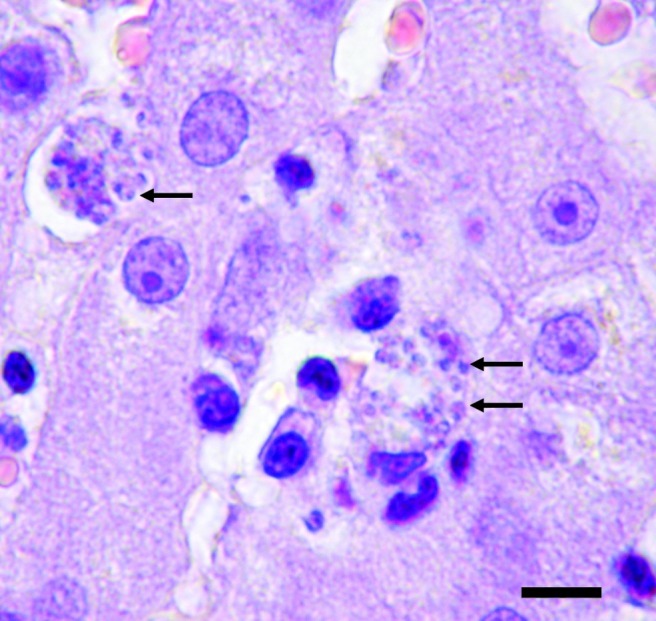
Liver of the index case (dog P01). Hepatic intralobular granuloma formation composed of macrophages loaded with Leishmania amastigotes (arrows), plasmacytes, and lymphocytes (hematoxylin and eosin stain; scale bar=12.5 μm). Color images available online at www.liebertpub.com/vbz
Blood samples were harvested and sent for analysis to a commercial laboratory (IDEXX Vet Med Labor). Routine complete blood counts revealed moderate non-regenerative anemia (red cell count 4.05 T/L [reference range: 6–9 T/L], hemoglobin 90.5 g/L [reference range: 150–190 g/L], and packed cell volume 28% [reference range: 38–55%], with marginal polychromasia), and a mildly active inflammatory leukogram (band neutrophils 0.48 G/L [reference range: <0.3 G/L).
Biochemical changes included marked hyperglobulinemia (89 g/L; reference range: 15–35 g/L), and moderate hypoalbuminemia (20 g/L; reference range: 32–47 g/L). Serum protein electrophoresis revealed that hyperglobulinemia was due to a marked polyclonal gammopathy (gamma globulins 56.9 g/L; reference range: 5–18 g/L), with a concurrent marginal increase of α2 (8.3 g/L; reference range: 5–8 g/L), and β globulins (20.8 g/L; reference range; 8–18 g/L).
The immunohistochemical reaction highlighted the parasitic organisms in tissues, increasing the sensitivity of detection compared to hematoxylin and eosin-stained sections. Amastigotes immunostained heavily in the macrophages of the spleen sample, with the parallel presence of mild background staining (Fig. 4). There was no immunostaining in the control sections.
FIG. 4.
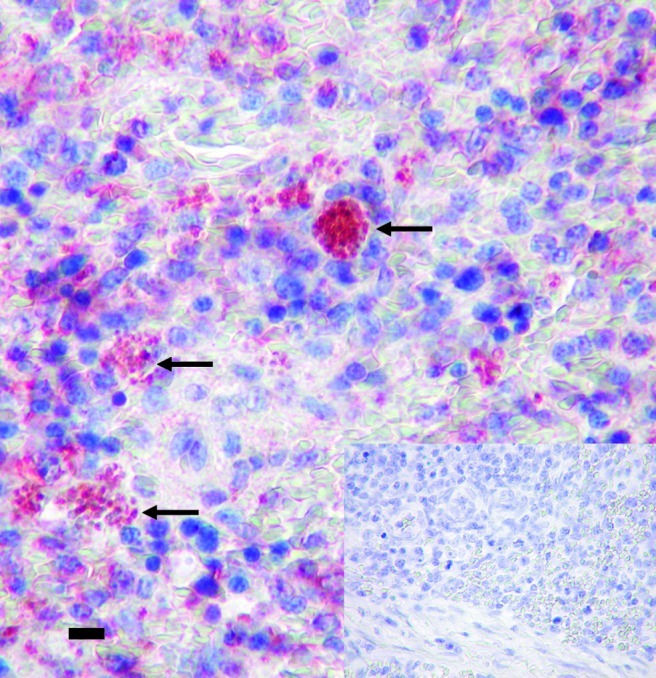
Spleen of the index case (dog P01), showing several immunolabeled Leishmania amastigotes within macrophages (inset shows spleen of a dog infected with distemper virus; no immunostaining is visible). Horseradish peroxidase-labeled polymer method (scale bar=12.5 μm). Color images available online at www.liebertpub.com/vbz
Clinical, serological, and PCR findings
At the May 2008 visit, all dogs that had previously been living with the P01 index case were found to be clinically healthy except for one dog (P12), which showed enlargement of lymph nodes. Because IFAT serology had revealed an elevated anti-leishmanial titer, dog P12 was re-evaluated clinically 6 months later, and found to exhibit typical CanL signs besides enlarged popliteal lymph nodes, such as onychogryphosis and skin and eye disorders.
The IFAT assay first performed on the index case revealed an antibody titer of 1:3200. For the other 19 dogs, the commercial IFAT kit detected one dog (P12) that was positive at a titer of 1:5120, while the remaining dogs were found to be negative, and below the 1:80 cut-off. The in-house IFAT assay confirmed the antibody titer of dog P12 (1:5120), and detected 3 other dogs that were positive at low titers (P10 and P17, titer 1:80; P20, titer 1:160), which is consistent with the higher sensitivity of the in-house fresh IFAT antigen (Table 1).
Table 1.
IFAT and PCR Findings in the Study Dogs
| Dog code | Commercial IFAT kit | In-house IFAT | Kinetoplast DNA PCR | Small-subunit ribosomal DNA nested PCR |
|---|---|---|---|---|
| P01 | NDa | NDa | Positive (liver, spleen, lymph node) | Positive (liver, spleen, lymph node) |
| P02 | neg | neg | neg (bbc) | neg (bbc) |
| P03 | neg | neg | neg (bbc) | neg (bbc) |
| P04 | neg | neg | neg (bbc) | neg (bbc) |
| P05 | neg | neg | neg (bbc) | neg (bbc) |
| P06 | neg | neg | neg (bbc) | neg (bbc) |
| P07 | neg | neg | neg (bbc) | neg (bbc) |
| P08 | neg | neg | neg (bbc) | neg (bbc) |
| P09 | neg | neg | neg (bbc) | neg (bbc) |
| P10 | neg | Positive (1:80) | neg (bbc) | Positive (bbc) |
| P11 | neg | neg | neg (bbc) | neg (bbc) |
| P12 | Positive (1:5120) | Positive (1:5120) | Positive (bbc, lymph node) | Positive (bbc, lymph node) |
| P13 | neg | neg | neg (bbc) | neg (bbc) |
| P14 | neg | neg | neg (bbc) | Positive (bbc) |
| P15 | neg | neg | neg (bbc) | neg (bbc) |
| P16 | neg | neg | neg (bbc) | neg (bbc) |
| P17 | neg | Positive (1:80) | neg (bbc) | neg (bbc) |
| P18 | neg | neg | neg (bbc) | neg (bbc) |
| P19 | Neg | neg | neg (bbc) | neg (bbc) |
| P20 | neg | Positive (1:160) | neg (bbc) | neg (bbc) |
Performed by IDEXX-Vet Med Labor; positive at titer 1:3200.
ND, not done; neg, negative; bbc, blood buffy-coat; IFAT, indirect fluorescent antibody test; PCR, polymerase chain reaction.
The blood sample taken from the P01 index case in the autumn of 2007 yielded positive results for the single-step 18S rDNA PCR assay at the IDEXX Vet Med Labor (data not shown in Table 1).
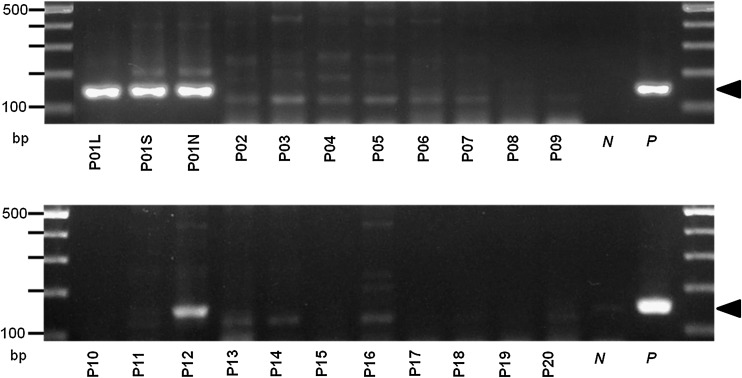
The kDNA PCR assay amplified fragments of the expected 145-bp size from the lymph node, liver, and spleen samples of index case P01, as well as from the buffy-coat and lymph node of dog P12 (Fig. 5). No other dogs in the kennel had positive results with this PCR assay. Sequence alignments supported the Leishmania origin of the PCR products. The kDNA amplicons of index case P01 showed 96% similarity with L. infantum (EU370893.1, EU370895.1, EU370899.1, and Z35272.1), and L. donovani (EU370885.1), and 94% with L. major (EU370908.1), while the strongest similarities of the amplicons of dog P12 were 95% with L. infantum (EU370893.1), and L. donovani (EU370885.1).
FIG. 5.
PCR amplicons resulting from kDNA PCR of 20 dogs (P01–P20). Positive samples of liver (P01L), spleen (P01S), and lymph nodes (P01N), of the index case (dog P01), and peripheral blood of the second diseased dog (P12) show the 145-bp-long fragments specific for L. infantum kDNA (indicated by the arrowhead; N, negative probe; P, positive probe).
The same samples were examined by ssu rDNA n-PCR. As reported in Table 1, all tissues examined from the P01 index case were positive, as were buffy-coat samples from 3 other dogs (P10, P12, and P14). The molecular positivity of dog P12 was later confirmed by ssu rDNA n-PCR carried out on follow-up lymph node samples. These results are consistent with the higher sensitivity of nested PCR.
In conclusion, 2 sick dogs (including the index case) were confirmed as symptomatic CanL cases, and 4 clinically healthy dogs were found to be Leishmania positive by IFAT (2 dogs), or by buffy-coat PCR (1 dog), or by both (1 dog). Hence the overall Leishmania infection prevalence in the kennel was 30% (6/20).
Search for sand flies
No phlebotomine sand flies were captured in any traps used in July and August of the two subsequent years, either in or around the kennel, or at the nearby chicken yards.
Discussion
Leishmaniasis in humans and dogs has been rarely reported in Hungary. Except for one old case (Makara 1942), the epidemiological evidence suggested that all human patients had acquired the infection abroad (Várnai et al. 1985; Fried et al. 2003; Péterfi et al. 2011). We were informed that some dogs taken from Hungarian shelter houses were found to be IFAT-positive in Germany (Biocontrol, Ingelheim; Vet Med Labor, Berlin, personal communication), but it is not known where these dogs became infected with L. infantum.
To the best of our knowledge, this work documents the first report of autochthonous L. infantum infection of dogs in Hungary. All dogs were born in the same kennel and were always kept outdoors. None had ever been abroad or received a blood transfusion. The possibility of transplacental transmission of leishmaniae should also be considered (Duprey et al. 2006; Boggiatto et al. 2011). The vertical transmission of L. infantum cannot be excluded, but it is unlikely that it occurred, because the parents of the infected dogs were born in the same kennel, none had ever been to endemic countries, and no symptoms of leishmaniasis occurred during their lives. On the other hand, conditions exist worldwide that call for the general revision of the importance of this means of Leishmania transmission (Boggiatto et al. 2011). Our findings depict a typical CanL focus, with less than 50% of infected dogs showing a spectrum of progressive disease (i.e., overt clinical signs, high IFAT titers, and/or microscopic demonstration of parasites), and the others characterized by sub-clinical infection (i.e., positive PCR and/or low-titer IFAT findings), with no clinical signs (Oliva et al. 2006). However, considering the historical background of the dogs and the failure to trap any sand fly vectors in and around the kennel, the origin of this focus remains unexplained. Similar confirmed or suspected autochthonous cases of leishmaniasis of unknown origin have also been reported in Austria (Kollaritsch et al. 1989) and Germany (Naucke and Schmitt 2004), where cases also occurred with no history of travel to areas where leishmaniasis is typically enzootic.
Although no phlebotomine vectors were trapped, their presence, and hence their role in transmission, cannot be discounted. First, the infections detected in the study dogs in October 2007 and May 2008 must have been acquired at least 1 year earlier (i.e., in summer 2007, or even before). Sand flies were no longer in the vicinity when the symptoms were diagnosed. Second, low densities of competent L. infantum vectors have recently been found in Hungary (Farkas et al. 2011). Specimens of P. neglectus were caught less than 100 kilometers south of the kennel. This species may occasionally occur around the kennel site, where the annual mean temperature is >10°C, and the monthly mean temperature in July and August is >20°C (Mersich et al. 2003). These are beneficial temperature ranges for sand fly adults (Lindgren et al. 2004). Furthermore, the lack of sand flies in our trapping survey could be explained by the frequent mosquito control measures applied in the region, which may have significantly reduced local sand fly populations for long periods (Killick-Kendrick, 1999). An alternative explanation for the transmission might be the accidental importation of infected sand flies from endemic areas of Croatia or Serbia, which are only a few hundred kilometers from the study site (Bosnić et al. 2006). Although direct transport of infected sand flies by tropospheric winds has not been demonstrated, Hendrickx and colleagues (2008) demonstrated that the wind dispersal capacity of Culicoides species, which are similar in size and flight capacity to sand flies (Killick-Kendrick, 1999), can reach up to 300–400 kilometers over land. Further investigations are needed to elucidate these capabilities of phlebotomine sand flies.
The source of infection could also be infected asymptomatic dogs traveling in the area with foreign tourists or hunters during the summer season. Wild canids, such as red foxes (Vulpes vulpes), jackals (Canis aureus), and wolves (Canis lupus; Criado-Fornelio et al. 2000; Mohebali et al. 2005), may also have played a role in the introduction of L. infantum in our study area.
The high CanL prevalence found in the kennel (6/20 dogs, 30%) is also indicative of direct dog-to-dog transmission. This cycle was recently proposed for kennels with an apparent absence of active vector transmission (Gaskin et al. 2002; Duprey et al., 2006). Infected dogs can serve as a source of infection for uninfected animals through bites and abrasions.
Our findings, together with other reports (Kollaritsch et al. 1989; Naucke and Schmitt 2004), indicate that autochthonous leishmaniasis can spread northward from endemic regions of southern Europe. This zoonotic disease represents a potential threat to public and canine health in Hungary, because the presence of two competent vector species, P. neglectus and P. perfiliewi perfiliewi, in some areas might support the spread of L. infantum. Although only low densities of the vector species were caught, they could be important from an epidemiological point of view. The lack of knowledge of CanL of local veterinarians, coupled with an increasing number of infected asymptomatic dogs traveling from endemic areas, could play important roles in the future epidemiology of leishmaniasis in Hungary.
Acknowledgments
We wish to thank the owners of the dog kennel and the properties where the mosquito traps were used. We also express our grateful thanks to Dr. Jerome A. Hogsette for kindly providing MMX traps and advice on their field use. This study was partially funded by EU grant FP7-261504 EDENext and is catalogued by the EDENext Steering Committee as EDENext 023. The contents of this article are the sole responsibility of the authors and do not reflect the views of the Eureopean Commission.
Author Disclosure Statement
No competing financial interests exist.
References
- Boggiatto PM. Gibson-Corley KN. Metz K, et al. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl Trop Dis. 2011;4:e1019. doi: 10.1371/journal.pntd.0001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnić S. Gradoni L. Khoury C, et al. A review of leishmaniasis in Dalmatia (Croatia) and results from recent surveys on phlebotomine sand flies in three southern counties. Acta Trop. 2006;99:42–49. doi: 10.1016/j.actatropica.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A. Gutierrez-Garcia L. Rodriguez-Caabeiro F, et al. A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara, Spain. Vet Parasitol. 2000;92:245–251. doi: 10.1016/s0304-4017(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Duprey ZH. Steurer FJ. Rooney JA, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas R. Tánczos B. Bongiorno G, et al. First surveys to investigate the presence of canine leishmaniasis and its phlebotomine vectors in Hungary. Vector Borne Zoonotic Dis. 2011;11:823–834. doi: 10.1089/vbz.2010.0186. [DOI] [PubMed] [Google Scholar]
- Fried K. Todorova R. Pintér E. Humán visceralis leishmaniosis megbetegedés Magyarországon. Epinfo. 2003;10:1. [Google Scholar]
- Gaskin AA. Schantz P. Jackson J, et al. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34–44. doi: 10.1892/0891-6640(2002)016<0034:vliany>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Gradoni L. Gramiccia M. Manual of Standards for Diagnostic Tests and Vaccines. Paris, France: Office International des Epizooties; 2004. Leishmaniosis. [Google Scholar]
- Gramiccia M. Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Invited review. Int J Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Hendrickx G. Gilbert M. Staubach C, et al. A wind density model to quantify the airborne spread of Culicoides species during north-western Europe bluetongue epidemic, 2006. Prev Vet Med. 2008;87:162–181. doi: 10.1016/j.prevetmed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- Kollaritsch H. Emminger W. Zaunschirm A, et al. Suspected autochthonous kala-azar in Austria. Lancet. 1989;1:901–902. doi: 10.1016/s0140-6736(89)92895-x. [DOI] [PubMed] [Google Scholar]
- Lachaud L. Marchergui-Hammami S. Chabbert E, et al. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol. 2002;40:210–215. doi: 10.1128/JCM.40.1.210-215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren E. Naucke T. Menne B. Climate variability and visceral leishmaniasis in Europe. Report of the Scientific Working Group meeting on Leishmaniasis; Geneva, Switzerland. Feb 2–4;2004 ; pp. 88–93. [Google Scholar]
- Lőrincz F. Szentkirályi Z. Phlebotomus macedonicus (Adler és Theodor, 1931) előfordulása Magyarországon. Állattani Közlemények. 1933;3–4:160–169. [Google Scholar]
- Magdus M. A kutya leishmaniosisa (leishmaniosis canum) Állatorvosi praxis. 2004;5:5–9. [Google Scholar]
- Makara Gy. Érdekes human-parazitológiai esetek. Leishmania donovani fertőzés hazánkban. Orvosi Hetilap. 1942;83:562. [Google Scholar]
- Maroli M. Rossi L. Baldelli R, et al. The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop Med Int Health. 2008;13:256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- Mersich I. Práger T. Ambrózy P, et al. Climate Atlas of Hungary. Budapest: Hungarian Meteorological Service; 2003. A nyári és a téli félév középhőmérséklete; pp. 13–14. [Google Scholar]
- Mohebali M. Hajjaran H. Hamzavi Y, et al. Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet Parasitol. 2005;129:243–251. doi: 10.1016/j.vetpar.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Molina R. Amela C. Nieto J, et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994;88:491–493. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Moreno J. Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- Naucke TJ. Schmitt C. Is leishmaniasis becoming endemic in Germany? Int J Med Microbiol. 2004;293(Suppl 37):179–181. doi: 10.1016/s1433-1128(04)80036-6. [DOI] [PubMed] [Google Scholar]
- Oliva G. Scalone A. Foglia Manzillo V, et al. Incidence and time course of Leishmania infantum infections examined by parasitological, serologic, and nested-PCR techniques in a cohort of naïve dogs exposed to three consecutive transmission seasons. J Clin Microbiol. 2006;44:1318–1322. doi: 10.1128/JCM.44.4.1318-1322.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péterfi Z. Nemes Z. Vigvári S, et al. Visceral leishmaniasis in an immunocompetent Hungarian adult patient. Health. 2011;3:1–5. [Google Scholar]
- Ready PD. Leishmaniasis emergence in Europe. Euro Surveill. 2010;15:19505. [PubMed] [Google Scholar]
- Schantz PM. Steurer FJ. Duprey ZH, et al. Autochthonous visceral leishmaniasis in dogs in North America. J Am Vet Med Assoc. 2005;226:1316–1322. doi: 10.2460/javma.2005.226.1316. [DOI] [PubMed] [Google Scholar]
- Tafuri WL. Santos RL. Arantes RM, et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immun Methods. 2004;292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Teske E. van Knapen F. Beijer EG, et al. Risk of infection with Leishmania spp. in the canine population in the Netherlands. Acta Vet Scand. 2002;43:195–201. doi: 10.1186/1751-0147-43-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eys GJ. Schoone GJ. Kroon NC, et al. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 1992;51:133–142. doi: 10.1016/0166-6851(92)90208-2. [DOI] [PubMed] [Google Scholar]
- Várnai F. Fülöp É. Bánhegyi D. Leishmania cutanea magyar állampolgárok által importált esetei. Orvosi Hetilap. 1985;126:2535. [PubMed] [Google Scholar]