SUMMARY
Memory enables flexible use of past experience to inform new behaviors. Though leading theories hypothesize that this fundamental flexibility results from the formation of integrated memory networks relating multiple experiences, the neural mechanisms that support memory integration are not well understood. Here, we demonstrate that retrieval-mediated learning, whereby prior event details are reinstated during encoding of related experiences, supports participants’ ability to infer relationships between distinct events that share content. Furthermore, we show that activation changes in a functionally coupled hippocampal and ventral medial prefrontal cortical circuit track the formation of integrated memories and successful inferential memory performance. These findings characterize the respective roles of these regions in retrieval-mediated learning processes that support relational memory network formation and inferential memory in the human brain. More broadly, these data reveal fundamental mechanisms through which memory representations are constructed into prospectively useful formats.
INTRODUCTION
We often reflect on our past to understand current experience or predict future events. In this way, the function of memory is not merely retrospective, but rather “intrinsically prospective” (Klein et al., 2002), aimed at constructing memory representations that can be used to successfully negotiate future judgments and actions (Buckner, 2010; O’Keefe and Nadel, 1978; Tolman, 1948). From this perspective, memories do not simply consist of individual records of directly experienced events, but also include representations built by relating information acquired across multiple discrete episodes. The derived representations contained within networks of related memories would facilitate extraction of new knowledge that extends beyond direct experience to anticipate future inferential judgments about the relationships between experiences (Cohen and Eichenbaum, 1993; Eichenbaum, 1999). The flexibility to combine memories in novel ways to infer new information is essential to behavior in an ever-changing environment; yet, the neural mechanisms that underlie this constructive nature of memory are not well understood.
One potential mechanism enabling the formation of integrated networks of related memories is retrieval-mediated learning (Hall, 1996; Holland, 1981). Through retrieval-mediated learning, it has been hypothesized that individual experiences are encoded not only in the context of externally available information, but also in the context of internally generated memory representations of prior related events. By reactivating the details of prior experiences during learning, existing memories can be updated with new information to be readily applicable in novel situations.
Recent evidence indicates that hippocampus and medial prefrontal cortex (MPFC)—in particular, ventromedial prefrontal cortex (VMPFC)—both play important roles in updating existing memories through retrieval-mediated learning (Tse et al., 2007; Tse et al., 2011). Rats can rapidly learn new associations in a single trial when novel information can be integrated into a well-established memory framework (a schema), but require weeks of training when a schema (in this case, a familiar spatial layout) is not available. This facilitation of associative learning is accompanied by an up-regulation of immediate early genes in MPFC and is abolished after pharmacological inactivation of hippocampus or MPFC, providing evidence for hippocampal-MPFC involvement during retrieval-mediated learning.
In these studies, retrieval-mediated facilitation of new learning depends on the existence of a well-established associative memory network prior to new encoding. However, it remains unknown how these associative memory networks are formed initially, and whether this initial formation also relies on retrieval-mediated learning processes supported by hippocampal-MPFC interactions. Both animal (Siapas et al., 2005) and human (Ranganath et al., 2005) data indicate that hippocampus and VMPFC are functionally coupled during novel experiences. In humans, such coupling is predictive of subsequent memory (Ranganath et al., 2005), providing evidence that these regions support memory formation. Moreover, hippocampal-VMPFC connectivity is increased when encoding requires formation of a new schema relative to conditions when schemas are pre-established (van Kesteren et al., 2010). Finally, hippocampus and VMPFC activation track reactivation of the reward context of prior overlapping events during new encoding (Kuhl et al., 2010), indicating retrieval of prior related memories. Collectively, these findings provide evidence that hippocampus and VMPFC may support the initial formation of relational memory networks via retrieval-mediated learning, but several central questions remain.
First, while lesion work has documented critical roles for both hippocampus and VMPFC in inferential use of associative memories (for a review, see Zeithamova et al., 2012), the precise mechanism through which these regions contribute to flexible memory expression is unknown. In rodents, blocking hippocampal synaptic plasticity during an event that overlaps with a previous experience prevents the transfer of new knowledge to the previous context (Iordanova et al., 2011), suggesting that hippocampus supports generalization across contexts by reactivating prior experience. Converging human neuroimaging research has observed activation in hippocampus and surrounding medial temporal lobe (MTL) cortex during encoding of overlapping events that predicts subsequent inference (Greene et al., 2006; Shohamy and Wagner, 2008; Zeithamova and Preston, 2010). While these findings are commonly interpreted as indicating hippocampal-mediated retrieval of prior memories during encoding of overlapping information, they can also be explained by stronger encoding of individual associations that is reflected in increased hippocampal engagement. Thus, more direct evidence is necessary to determine whether retrieval-mediated memory integration supports inference.
Even fewer studies to date have examined how VMPFC encoding processes in particular support the inferential use of memory. Human neuroimaging research provides some initial evidence that VMPFC supports the application of knowledge acquired across multiple learning experiences during inferential test trials (Kumaran et al., 2009; Zeithamova and Preston, 2010). However, whether VMPFC also supports inferential memory performance via retrieval-mediated encoding processes is yet to be determined.
Finally, retrieval-mediated learning is hypothesized to consist of a two-stage process that involves (1) reactivation of existing memories cued by overlapping event content and (2) a binding mechanism that encodes the relationships among current events and past experience. Because existing studies on inference did not empirically isolate a critical signature of memory reactivation during new learning, it is difficult to identify the specific mechanism—reactivation or binding—through which hippocampus and VMPFC contribute to retrieval-mediated learning. Here, we implement a paradigm that enables observation of online reactivation of content-specific memories in the human brain during encoding of related events. Isolating memory reactivation and binding processes that support memory integration will enable a more detailed characterization of the neural mechanisms that underlie retrieval-mediated learning. Moreover, by quantitatively indexing reactivation during encoding, the present study provides a means of linking retrieval-mediated learning processes to future inference success.
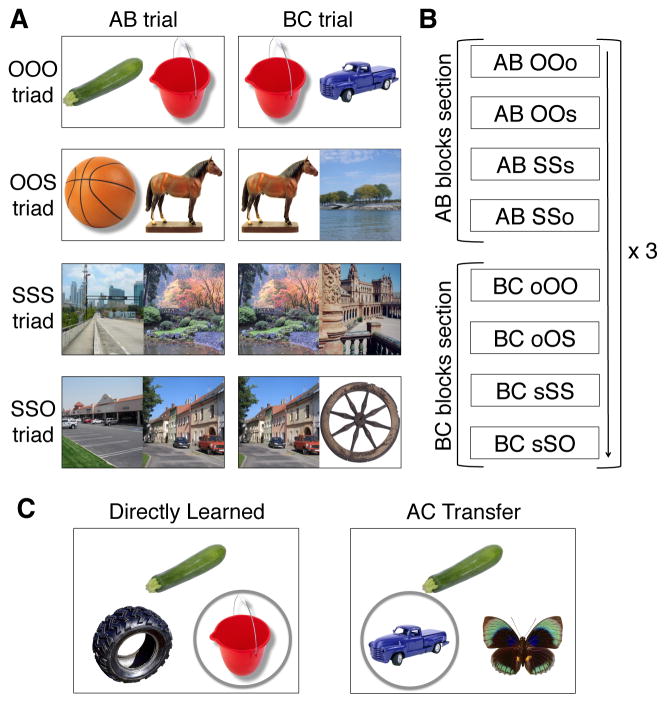
Specifically, we utilized a modified version of the associative inference task (Preston et al., 2004; Zeithamova and Preston, 2010) in combination with multivoxel pattern analysis (MVPA) (Norman et al., 2006; Polyn et al., 2005) to investigate the neural mechanisms of retrieval-mediated learning and its relationship to flexible inference about related events. The task consisted of two phases: associative encoding during block-design functional magnetic resonance imaging (fMRI) and an inferential recognition memory test after scanning. Images of objects and outdoor scenes were organized into groups of three (triads) and presented to participants as overlapping associations of image pairs (AB, BC, e.g., zucchini-pail, pail-truck, Fig. 1A). The first presented image pair from each triad consisted of stimuli of the same content class: two objects or two scenes. Images in the second pair were either of the same content class (e.g., two objects) or of mixed content (i.e., one object and one scene). Both image pairs from a given triad were presented three times each in an interleaved manner (Fig. 1B). After scanning, participants were tested using a two-alternative forced choice paradigm that included directly learned association trials (AB, BC) as well as inference trials that tested knowledge of the relationship between two discrete episodes (AC, e.g., zucchini-truck, Fig. 1C).
Figure 1.
Experimental design. (A) Color photographs of object (O) and scene (S) stimuli were organized into groups of three stimuli (triads) presented as two overlapping associations (AB, e.g., “zucchini–pail”, and BC, e.g., “pail–truck”). Triads consisted of one of four types: three objects (OOO), two objects and a scene (OOS), three scenes (SSS), and two scenes and an object (SSO). (B) Participants learned the overlapping associations from each triad during blocked-design encoding runs (see Experimental Procedures). The AB and BC associations of all triad types were repeated three times within a functional run in an interleaved manner (AB, BC, AB, BC, AB, BC). (C) After each encoding run, participants were tested on directly learned associations (AB, BC) as well as inferential relationships (AC), using a two-alternative forced-choice judgment. See also Fig. S1.
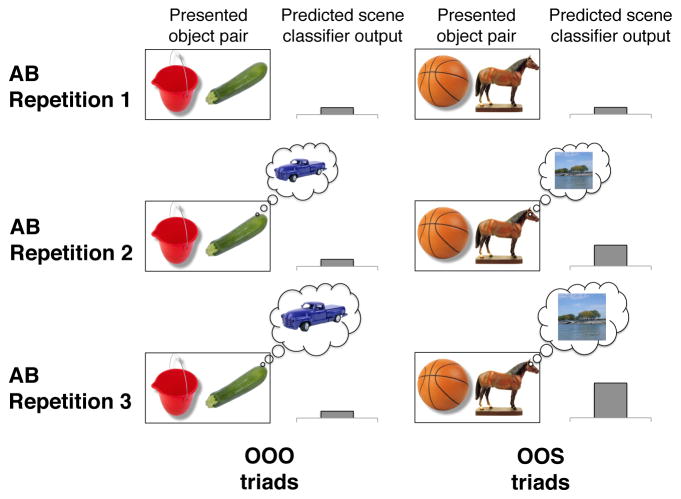
The organization of triad types enabled us to measure reactivation of related, but unseen, stimulus content in the absence of an explicit behavioral response by comparing encoding trials for which the presented information was of the same content class (e.g., two objects), but previously associated information was of a different content class (i.e., object or scene, Fig. 2). We hypothesized that reactivation of related content during overlapping events would be reflected in content-sensitive regions within the ventral temporal cortex, with the degree of reactivation predicting performance on the inferential judgments. We also examined how activation in VMPFC, hippocampus, and surrounding MTL cortices relates to the magnitude of reactivation and successful binding of overlapping experiences. By isolating signatures of memory reactivation and integration during encoding, the current study provides important insights into the specific neural mechanisms that underlie the online formation of relational memory networks via retrieval-mediated learning.
Figure 2.
Multivoxel pattern analysis (MVPA) strategy. MVPA classifiers trained to differentiate brain patterns associated with object and scene processing (see Experimental Procedures) indexed content-specific activation during each encoding condition of the associative inference task. Classifier outputs were compared across AB repetitions when presented information was from the same content class (e.g., two objects for OOO and OOS triads), but the content class of the third, unseen triad member differed (object vs. scene). AB Repetition 1. On the first AB repetition, classifier output is predicted to reflect the content of presented information and not differ for associations comprised of the same content class. AB Repetition 2 & 3. On the second and third AB repetitions, classifier output is predicted to reflect not only presented content, but also reactivated, overlapping BC associations. In this example, two objects are presented, but scene classifier output is predicted to be greater for OOS triads relative to OOO triads, reflecting the reactivation of the associated scene for OOS triads (e.g., “lake scene”), but a third object for OOO triads (e.g., “truck”). The difference in scene classifier outputs across AB repetitions of these triad types (OOO vs. OOS) serves as a critical reactivation measure. A similar analysis (not depicted) compared classifier output across AB repetitions for SSS and SSO triads.
RESULTS
Behavioral performance
Participants successfully recognized directly learned associations (mean = 91.8% correct, sd = 5.8; t(25) = 36.8, p < 0.001). Performance on the novel inference test trials was also significantly above chance (t(25) = 19.2, p < 0.001), averaging 82.3% correct (sd = 8.6). Large individual differences in inference performance were observed (range 66–98%), enabling examination of the relationship between reactivation of content-specific prior events and subsequent flexible memory performance.
Reactivation of prior experience during encoding predicts subsequent flexible use of memory
To test our hypothesis that prior related memories are reinstated during encoding and bound to current experience, we first trained an MVPA classifier to differentiate distributed patterns of neocortical activation associated with object and scene processing in an independent encoding localizer task (Fig. S1A) and validated its ability to detect reactivation of unseen stimulus content in a guided recall task (Fig. S1B,C). The trained classifier was then applied to data from the associative inference task to obtain indices of object and scene activation across AB repetitions for each encoding condition. Specifically, we compared the difference in classifier output for AB associations where the presented class of content was the same (e.g., two objects), but the content class of the third, unseen triad member (i.e., C) differed and was either an object or a scene (Fig. 2).
In the present study, the first AB presentation represents a novel experience comprised of two unfamiliar elements (two objects or two scenes; Fig. 1A). The pattern of brain activation during the initial AB presentation is expected to reflect the content of the present experience, regardless of the nature of the third—not yet studied—triad member. Consequently, classifier outputs for the first AB repetition would not be predicted to differ for AB associations of the same content class (e.g., OOO vs. OOS, Fig. 2A). However, subsequent presentations of AB associations are interleaved with overlapping BC associations (Fig. 1B). Based on our hypothesis, the second and third presentations of an AB association would lead to the reactivation of the third, unseen triad member (i.e., C) to promote the formation of an integrated network of related memories (i.e., A-B-C). Classifier outputs would thus be expected to reflect not only the content class of presented information, but also the content of unseen, reactivated events.
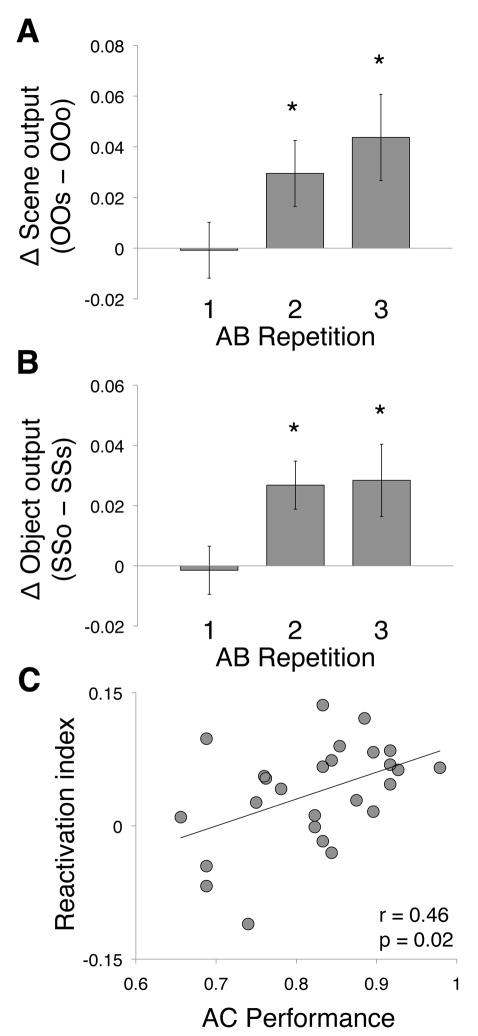
While such reactivation of related event content is expected to occur during AB repetitions for all triad types, the current experimental design enables a direct comparison of conditions where presented content is the same but the nature of the reactivated content differs (Fig. 2B,C). Classifier outputs for the second and third repetitions would be expected to differ across these conditions, providing an estimate of the degree of reactivation of related event content. Two measures of reactivation were obtained: (1) a scene reactivation estimate that compared the difference in classifier outputs for each AB repetition of OOO and OOS triads (Fig. 3A), and (2) an object reactivation estimate that compared the difference in classifier output for each AB repetition of SSS and SSO triads (Fig. 3B).
Figure 3.
Reactivation of prior event content during encoding of related associations. (A) Difference in scene classifier output across repetitions of AB associations for OOS relative to OOO triads. (B) Difference in object classifier output across repetition of AB associations for SSO relative to SSS triads. For both (a) and (b), error bars denote standard error of the mean; asterisk denotes significant difference between compared classifier outputs at p < 0.05. See also Fig. S2. (C) Across-subject correlation between reactivation index (collapsed across object and scene reactivation measures) and inference (AC) performance. Greater reactivation index was associated with superior AC accuracy.
Consistent with our predictions, classifier output for the initial AB presentation did not differ between AB associations of the same content class (scene classifier output for OOO and OOS triads t(25) = 0.07, p = 0.94, Fig. 3A; object classifier output for SSS and SSO triads t(25) = 0.17, p = 0.87, Fig. 3B). We did, however, observe differences in classifier output between triad types on the second and third AB presentations. Scene classifier output was significantly greater for AB associations from OOS triads relative to OOO triads on the second (t(25) = 2.22, p = 0.04) and third (t(25) = 2.56, p = 0.02) AB repetitions (Fig. 3A). Object classifier output was also significantly greater for AB associations from SSO relative to SSS triads on the second (t(25) = 3.51, p = 0.002) and third (t(25) = 2.44, p = 0.02) AB repetitions (Fig. 3B).
Importantly, comparing the classifier outputs across two classes of triads (i.e., OOO vs. OOS and SSS vs. SSO) controls for confounding effects of novelty that are unrelated to memory reactivation, as the number of repetitions of individual items and associations are matched across conditions (see Fig. S2A,B). Moreover, the increases in classifier output reflecting unseen, related content were not a by-product of the forced-choice nature of the 2-way MVPA classifier, as the same pattern of results was observed when we employed an alternate 3-way classification procedure (Fig. S2C–D). Finally, differences in difficulty did not drive differential classifier output when comparing within-content (OOO, SSS) and cross-content (OOS, SSO) conditions, as inferential performance was similar across the conditions (mean for within-content = 82% correct ± 2%; mean for cross-content = 83% ± 2%; t(25) = 0.58, p = 0.57).
The preceding findings demonstrate reactivation of prior related experience during overlapping event encoding, providing direct evidence for the first essential component of retrieval-mediated learning. However, to be behaviorally relevant, the reactivated memories must also be bound to the current experience. If such binding is occurring, the degree to which prior memories are reactivated during encoding should predict subsequent performance on AC judgments. We computed the change in MVPA classifier output for the unseen stimulus across repetitions (last-first AB presentation) for each condition, and then pooled the scene (ΔOOS-ΔOOO) and object reactivation estimates (ΔSSO-ΔSSS) to obtain a reactivation index for each participant. Consistent with our prediction, the reactivation index was positively correlated with AC performance across subjects (r = 0.46, p = 0.02, Fig. 3C), with greater reactivation reflecting superior inference performance.
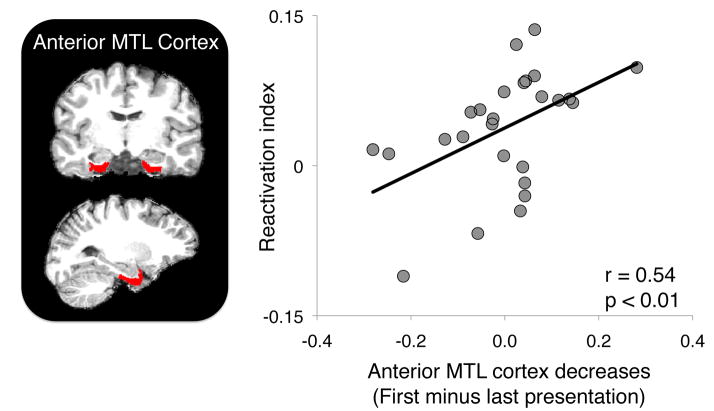
Changes in anterior MTL cortex activation are related to reactivation of prior experience
Given prior evidence linking MTL processing to memory reactivation, we further assessed how MTL regions tracked reactivation of prior memories during encoding of overlapping events. The anatomical regions of interest (ROIs) defined on individual participant brains included bilateral hippocampus, parahippocampal cortex, and anterior MTL cortex (inclusive of perirhinal and entorhinal cortices). For each region, we extracted learning-related decreases thought to reflect successful binding (Johnson et al., 2008; Kohler et al., 2005) by comparing activation during the first presentation of AB and BC associations with activation during the last presentation of AB and BC associations. We then correlated these learning-related decreases in MTL regions with reactivation in ventral temporal cortex, observing a positive relationship between the reactivation index and activation decreases in anterior MTL cortex (r = 0.54, p = 0.004, Fig. 4; p < 0.05 Bonferroni corrected). This correlation was present even when anterior MTL cortex voxels were excluded from the MVPA classification procedure used to index reactivation (Fig. S3).
Figure 4.
Across-participant correlation between activation decreases (first – last parameter estimate) in bilateral anterior MTL cortex and the reactivation index. Greater learning-related decreases in anterior MTL cortex were associated with greater reactivation of unseen, related stimulus content. See also Fig. S3.
To further assess whether the relationship between encoding activation and reactivation was unique to anterior MTL cortex, we performed the same set of analyses for our a priori VMPFC ROI and 11 additional anatomical regions in frontal, parietal, and temporal cortices that have been previously implicated in episodic memory processing (see Supplemental Experimental Procedures). The anterior MTL cortex was the only region that showed a significant relationship between changes in encoding activation and the reactivation index (all other r < 0.33, p > 0.10).
Encoding activity in hippocampus and VMPFC correlates with inference performance
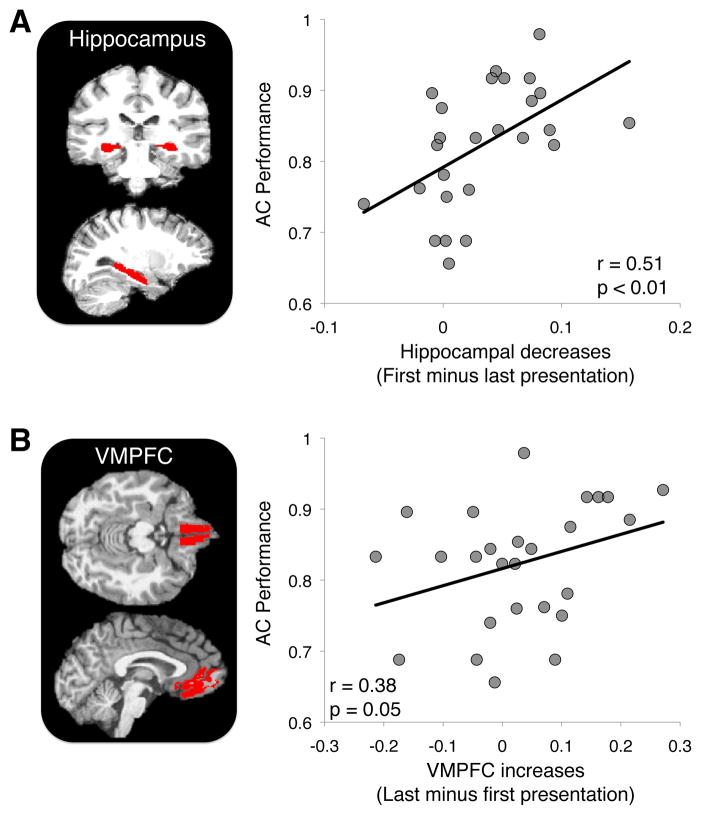
To test whether MTL regions and VMPFC are involved in binding reactivated memories with current event content, we correlated learning-related activation changes in VMPFC and each MTL subregion with inference performance. Two regions showed significant correlation with AC performance: hippocampus and VMPFC. In hippocampus, we observed a positive correlation between learning-related activation decreases and AC performance (r = 0.51, p = 0.008, Fig. 5A; p < 0.05 Bonferroni corrected). The VMPFC encoding activation showed the opposite pattern relative to hippocampus; specifically, learning-related increases in VMPFC activation were positively correlated with AC performance (r = 0.38, p = 0.05, Fig. 5B).
Figure 5.
Across-participant correlation between learning-related changes in hippocampus and VMPFC and subsequent inference performance. (A) Greater learning-related hippocampal decreases (first – last parameter estimate) across encoding repetitions were associated with greater AC performance at test. (B) Greater activation increases in VMPFC (last – first parameter estimate) across encoding repetitions were associated with greater AC performance at test.
In this task, memory for individual premise associations is an important factor for inference performance (correlation between directly learned and AC performance r = 0.76, p < 0.001). The observed relationship between hippocampal and VMPFC activation and inference performance could thus either reflect binding of individual associations or additional encoding processes specific to integration. To determine whether hippocampus and VMPFC contribute to inference above and beyond encoding of individual associations, we performed a partial correlation analysis that took into account performance on the trained premise pairs. The relationship between increases in VMPFC activation and subsequent inference performance was present even when equating for differences in memory for directly learned associations (partial r = 0.53, p = 0.007; p < 0.05 Bonferroni corrected). The relationship between hippocampal activation decreases and inference performance was only significant in right hippocampus when accounting for performance on directly learned associations (bilateral hippocampus partial r = 0.22, p = 0.29; right hippocampus partial r = 0.39, p = 0.05). No other brain region demonstrated a significant relationship between changes in activation (increases or decreases) across AB repetitions when controlling for performance on directly learned associations, though a statistical trend was observed in inferior frontal gyrus pars orbitalis (r = 0.38, p = 0.06). These findings indicate that the relationship between right hippocampal and VMPFC encoding activation and subsequent inference goes above and beyond learning of directly experienced associations, suggesting that these regions mediate binding of current experiences to reactivated memories.
Hippocampal-VMPFC interactions increase across learning of overlapping experiences
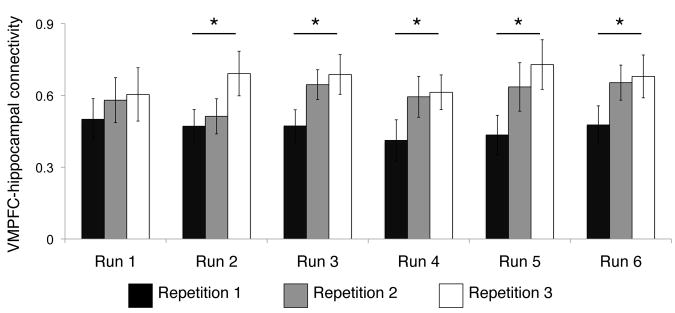
In line with recent rodent research (Iordanova et al., 2011; Iordanova et al., 2007; Tse et al., 2007; Tse et al., 2011), the present findings indicate that hippocampus and VMPFC are both engaged in support of retrieval-mediated learning. To further test for learning-related changes in hippocampal–VMPFC coupling, we performed a functional connectivity analysis using bilateral hippocampus as the seed region to determine whether the pattern of connectivity between hippocampus and VMPFC changed across repeated presentations of overlapping associations. Within each individual functional run, we constructed separate regressors corresponding to the first, second, and third repetitions of individual associations for each participant. A repeated measures ANOVA revealed that hippocampal–VMPFC connectivity increased across repetitions of overlapping associations irrespective of the functional run (repetition linear trend F(1,21) = 9.78, p = 0.005). Importantly, hippocampal–VMPFC connectivity did not change over the course of the experiment (run linear trend F < 1); rather, increases in hippocampal-VMPFC connectivity were specific to repetitions of overlapping events within each run (repetition x run interaction F(1,21) = 1.74, p = 0.20; Fig. 6), suggesting increased functional connectivity between hippocampus and VMPFC during the online formation of integrated memory representations. Three additional regions—frontal pole, precuneus, and superior parietal cortex—showed increased connectivity with hippocampus across repetitions of overlapping associations (Fig. S4); however, unlike VMPFC, encoding activation in these regions was not related to inference performance (all r < 0.14, p > 0.5).
Figure 6.
Functional connectivity between hippocampus and VMPFC across encoding repetitions displayed separately for each run. A significant increase in hippocampal-VMPFC connectivity was observed across encoding repetitions, but connectivity between these regions did not change as a factor of functional run. Asterisk denotes significant increase in connectivity within an individual run. See also Fig. S4.
DISCUSSION
A challenge in memory research has been to understand how we build rich, cohesive memory representations that relate different experiences. Here, we provide a direct link between retrieval-mediated encoding processes and flexible memory expression in the human brain. Using a multivoxel pattern analysis, we demonstrate that prior experience is reactivated during encoding of related events, and that such online reactivation of memories is predictive of individuals’ ability to infer novel relationships between two discrete, overlapping episodes. We further show that reactivation within content-sensitive higher-order visual areas is related to anterior MTL cortex activation, suggesting that responses in this region may influence the extent and specificity of retrieved memories.
Extensive controversy exists as to whether encoding activation relating to subsequent inference reflects memory integration during encoding or strengthening of individual directly learned associations, leading to improved “on the fly” inference at retrieval (for a review, see Zeithamova et al., 2012). Here, decreasing hippocampal and increasing VMPFC engagement across repetitions of overlapping events were associated with superior inference even when controlling for memory of premise associations, providing a strong evidence for online integration of related memories as they are encoded. Furthermore, we observed increased connectivity between hippocampus and VMPFC across interleaved presentations of overlapping events. These findings illustrate how a functionally coupled hippocampal–VMPFC circuit supports binding of reactivated memories with current experience, forming integrated memories that relate overlapping experiences. These relational memory networks enable the predictive application of memory by grouping related elements from multiple experiences in support of future inferential judgments.
Reactivation during encoding as a means of relational memory network formation
The present study organically builds upon and significantly extends prior studies examining the neural mechanisms supporting retrieval-mediated learning. Prior rodent research has shown that the existence of a well-learned spatial schema speeds acquisition of new object-place associations (Tse et al., 2007; Tse et al., 2011). Another recent report demonstrated that blocking hippocampal plasticity during contextual fear conditioning prevents the transfer of a newly acquired fear response to a previously experienced, overlapping spatial context (Iordanova et al., 2011). The presumption in each of these studies is that existing memories are reactivated during new learning and updated with new information, resulting in facilitated encoding and generalization. However, without an empirical measure of memory reactivation, such a presumption is only speculative. The methods employed in the current study enabled us to directly observe memory reactivation during encoding of related events, providing a key index of a process critical to retrieval-mediated learning.
Furthermore, in contrast to prior studies, our findings emphasize the beneficial function of retrieval-mediated learning. Reactivation of existing memories both prior to (Diekelmann et al., 2011; Hupbach et al., 2007; Schwabe and Wolf, 2009) and during (Kuhl et al., 2011) new encoding has typically been linked to increased susceptibility to interference. For example, reactivation of memories prior to encoding of overlapping events has been associated with increased forgetting of reactivated memories (Diekelmann et al., 2011). However, one recent report demonstrated that reactivation of reward contexts associated with prior experiences during encoding of related events tracked the retention of originally learned information (Kuhl et al., 2010), providing speculative evidence that memory reactivation plays a role in reducing forgetting. The present data fundamentally extend this work by demonstrating an alternate adaptive function of reactivation that supports memory integration and successful inference.
Moreover, the current study provides evidence for the role of anterior MTL cortex in the reactivation of prior event details during related experiences. Existing rodent (Ji and Wilson, 2007; Karlsson and Frank, 2009) and human (Kuhl et al., 2010) research has primarily linked memory reactivation with hippocampal responses. In the present study, activation changes in anterior MTL cortex, but not hippocampus, correlated with the degree of overlapping memory reactivation across participants. We propose that hippocampus drives memory reactivation within ventral temporal regions through interactions with anterior MTL cortex. Anatomical evidence reveals that information from content-sensitive ventral temporal regions reaches the hippocampus primarily through inputs from entorhinal cortex, which in turn receives visual information from perirhinal and parahippocampal cortices (Suzuki and Amaral, 1994; Witter and Amaral, 1991). The output of hippocampal processing reaches ventral temporal regions through reciprocal pathways. This anatomical connectivity suggests that reactivation of prior experience within hippocampus would first impact anterior MTL cortex responses, which in turn would influence processing in ventral temporal cortex. Thus, reactivation within ventral temporal cortex may be more closely coupled with anterior MTL cortex responses than with hippocampal activation. In the present study, changes in encoding activation within hippocampus were correlated with activation changes in anterior MTL cortex across participants (r = 0.46, p = 0.02), consistent with the idea of an indirect hippocampal influence on reactivation through anterior MTL cortex. As a second step in retrieval-mediated learning, the hippocampus would then bind reactivated memory content with the current event. Therefore, while anterior MTL cortex would track the degree of reactivation, it would be hippocampal responses that determine subsequent inference success. Future high-resolution fMRI studies of MTL function that utilize multivariate measures (Diana et al., 2008; Liang et al., 2012) may provide a means by which to test this model of retrieval-mediated learning by isolating hippocampal responses that are differentially related to reactivation and binding.
Hippocampal contributions to relational memory network formation and inference
Decreasing hippocampal engagement across repeated encoding of individual associations has been attributed to the rapid binding of associative information contained within single events (Johnson et al., 2008; Kohler et al., 2005). Here, decreased hippocampal engagement across repetitions of overlapping events was related to individuals’ ability to infer relationships between separate events, even when accounting for memory of the individual associations. These findings demonstrate that the specific role of hippocampus in memory integration extends beyond its contribution to within-event associative binding.
Hippocampal, but not prefrontal, encoding activation during an event overlapping with a prior experience has been associated with subsequent inference success in a single trial associative inference paradigm (Zeithamova and Preston, 2010), suggesting a unique role of the hippocampus in rapid integration of events that are experienced only once. In the present study, greater initial engagement of the hippocampus in successful participants may similarly reflect rapid integration as overlapping events are initially experienced. Decreasing activation across repetitions then occur as integrated memories become more established, reflecting the decreased need for binding (Johnson et al., 2008; Kohler et al., 2005). Alternatively, hippocampal decreases across repetitions may reflect progressively more efficient coding of integrated memories (Goshen et al., 2011; Karlsson and Frank, 2008). Consistent with this latter possibility, hippocampal replay in animals is associated with relatively sparse neural firing that may reflect tuning of memory representations through enhanced efficiency (e.g., Karlsson and Frank, 2008); such sparse firing at the cellular level may translate into repetition-related reductions in hippocampal activation observed in the present fMRI study. Recent findings linking hippocampal deactivation to increased memory search (Reas et al., 2011) might further suggest that hippocampal activation decreases in the present study reflect memory search for related event content as events are repeated. This interpretation is consistent with the observed increase in functional coupling between hippocampus and default network regions that have also been implicated in memory search and successful retrieval (Huijbers et al., 2011).
Notably, initial studies on the role of the hippocampus in inference focused on its contribution to performance at the time of retrieval (for a review, see Zeithamova et al., 2012). The current study contributes to a growing body of literature linking inference to hippocampal encoding processes (Greene et al., 2006; Shohamy and Wagner, 2008; Zeithamova and Preston, 2010) but goes beyond prior work to demonstrate a specific mechanism: retrieval-mediated memory integration. Our findings provide further insights into recent electrophysiological findings in rodents demonstrating experience-dependent generalized firing patterns that respond to similar locations in overlapping environments (Singer et al., 2010). Such generalized firing patterns suggest that hippocampal neurons develop representations that link different experiences by coding the similarities between events, although the precise mechanism by which such codes emerge is not known. The present findings suggest that retrieval-mediated encoding processes may underlie the formation of similar hippocampal representational codes for related events to include information beyond what is directly experienced (Gupta et al., 2010).
VMPFC encoding processes that support inference
The VMPFC receives direct input from the hippocampus and has an extensive network of connections with a diverse set of sensory, limbic, and subcortical structures (Cavada et al., 2000). This pattern of anatomical connectivity suggests that the VMPFC may be essential for the integration of information from the distributed cortical and subcortical networks that support episodic memories. However, few studies to date have directly examined the contributions of VMPFC to memory integration. Recent lesion studies have shown that MPFC damage impairs performance on tasks that require the inferential use of memories (DeVito et al., 2010b; Iordanova et al., 2007; Koscik and Tranel, 2012), but whether MPFC contributes to performance through the retrieval-mediated learning process set forth here could not be determined. Moreover, these lesion studies do not address whether the contribution of MPFC to inferential performance arises from interactions with hippocampus, a region also critical for inference (Bunsey and Eichenbaum, 1996; DeVito et al., 2010a; Dusek and Eichenbaum, 1997).
In the present study, VMPFC demonstrated increased functional coupling with hippocampus as related events were interleaved during learning. Moreover, increasing VMPFC engagement across repetitions was related to the ability to successfully infer relationships between overlapping events, even when accounting for memory of directly learned events. Prior reports have implicated hippocampal–VMPFC interactions in the use of memory schemas that resulted in speeded acquisition of new associative information (Tse et al., 2007; Tse et al., 2011) and flexible transfer of knowledge to new experimental settings (Kumaran et al., 2009). Utilizing MVPA measures of memory reactivation, the present findings extend this research by providing evidence that hippocampal–VMPFC interactions also underlie the initial formation of relational memory networks through a retrieval-mediated encoding process that enables subsequent inference.
In light of existing literature (Tse et al., 2007; Tse et al., 2011), we further propose that hippocampus and VMPFC may play complementary roles during relational memory network formation. The observation that VMPFC activation increases across repetitions of overlapping associations (in contrast to hippocampal activation decreases) were related to successful inference is consistent with the notion that hippocampus rapidly binds event elements into integrated representations that are then transferred to the VMPFC for permanent storage and future use (Frankland and Bontempi, 2005; Takashima et al., 2009; Takashima et al., 2006; Takehara-Nishiuchi and McNaughton, 2008). The proposition that VMPFC is recruited after initial memory integration by hippocampus is also supported by the fact that hippocampal, but not VMPFC, encoding activation predicted inference success in single-trial associative learning (Zeithamova and Preston, 2010). Alternatively, VMPFC increases in the present study may reflect organization or resolution of overlapping memory representations (Hasselmo and Eichenbaum, 2005; Ross et al., 2011) that leads to their integration in the current paradigm.
Hippocampal connectivity with default network regions
Increased hippocampal–VMPFC functional coupling across repetitions of overlapping events was accompanied by corresponding increases in hippocampal functional connectivity with precuneus, superior parietal cortex, and frontal pole. These regions—along with the hippocampus and VMPFC—are considered part of the default network (Raichle et al., 2001) that is also engaged during simulation of future events (Addis et al., 2009; Andrews-Hanna et al., 2010) and successful episodic remembering (Buckner et al., 2005; Greicius et al., 2004), in particular during recollection of specific event details (Vincent et al., 2006). Based on this evidence, it has been proposed that the default network supports the formation of mental models of significant events, particularly when judgments about those events depend on inferred content (Buckner et al., 2008). The default network would support the reactivation of prior events that could then be recombined and recoded into prospectively useful models of experience (Buckner, 2010). The present findings provide support for this hypothesis, demonstrating increased coupling between hippocampus and other components of the default network during retrieval-mediated formation of relational memory networks.
Conclusions
Several leading theories hypothesize that the fundamental flexibility of episodic memory results from our ability to form networks of related memories that link discrete events (Buckner, 2010; Cohen and Eichenbaum, 1993; O’Keefe and Nadel, 1978; Tolman, 1948). Despite the theoretical importance of this question, much empirical memory research has focused solely on encoding processes that mediate memories for individual events. Here, we demonstrate that memories for distinct experiences are integrated through a retrieval-mediated encoding mechanism, with prior related memories being reactivated and bound to the current experience during encoding. Our data also highlight the importance of hippocampal interactions with VMPFC during the formation of such integrated memory networks, thus broadening our understanding of how these structures work in concert to support the flexibility of episodic memory. Together, these findings afford a deeper understanding of how remembering the past influences what we experience and learn in the present. More broadly, the results emphasize the adaptive nature of memory, whereby memory representations are constructed to anticipate, and successfully negotiate, future judgments.
EXPERIMENTAL PROCEDURES
Participants
Thirty-four healthy volunteers (age 18–29, 17 females) participated after giving consent in accordance with a protocol approved by the University of Texas at Austin Institutional Review Board. All participants were right-handed, native English speakers and received $25/hour for their involvement. Data from four participants were excluded for excessive motion; one participant was excluded because of excessive noise in the fMRI timeseries due to scanner artifact; three participants were excluded for poor performance (failure to reach 75% accuracy on directly learned associations). Data from the remaining 26 participants were used in all reported analyses.
Materials and procedures
The encoding and recognition task was a modified version of the associative inference paradigm (Preston et al., 2004; Zeithamova and Preston, 2010). Stimuli were color photographs of common objects (O) and outdoor scenes (S) organized into groups of three stimuli (triads). Triads consisted of one of four types (Fig. 1A): three objects (OOO), two objects and a scene (OOS), three scenes (SSS), two scenes and an object (SSO). A total of 24 triads of each type were used in the experiment. Stimuli from each triad were presented as two overlapping associations (AB, BC).
Participants intentionally encoded overlapping associations from each triad during six block-design functional runs. Each functional run consisted of 24 associative encoding blocks along with baseline blocks. Encoding blocks were 12 s long, comprised of four associative encoding trials. On each trial, a pair of stimuli was presented for 2.5 s followed by 0.5 s of fixation. The initial four blocks within each functional run consisted of AB associations, one block for each triad type (OOO, OOS, SSS, SSO; Fig. 1B) in a counterbalanced order within and across participants. The following four blocks consisted of the corresponding BC associations. The alternating presentation of AB and BC associations was then repeated two additional times within a run to allow for three interleaved presentations of the overlapping associations (AB, BC, AB, BC, AB, BC). The left-right position of A and B stimuli was randomized across repetitions. The organization of stimuli into triads and the trial order were randomized across participants by creating six randomization groups. Odd/even digit baseline (Stark and Squire, 2001) blocks occurred at the beginning and end of each run and between each encoding block. Baseline blocks lasted 12 s and consisted of four trials. On each trial, a single digit between 1 and 8 was presented for 2.5 s followed by 0.5 s of fixation; participants indicated whether the digit was odd or even.
Each encoding run was followed by a non-scanned recognition test. Participants were tested on the directly learned (16 AB, 16 BC) and inference (16 AC) associations for each triad type (Fig. 1C). On each self-paced test trial, a cue was presented on the top of the screen (e.g., an A stimulus) and two choice probes were presented at the bottom of the screen (e.g., two B stimuli from different triads). Participants indicated which of the two choice stimuli was associated with the cue. Participants were instructed that on inference trials, the association between the cue (A) and the correct choice (C) was indirect, mediated through a third stimulus (B) that shared an association with both the cue and the correct choice during encoding. To control for familiarity, the incorrect choice was a familiar item, but one that was not [directly or indirectly] associated with the cue.
The order of test trials was pseudorandom, with the constraint that individual inference trials were tested before the corresponding AB and BC associations to ensure that an AC association was not formed during the test. Because of the repeated study-test nature of the design, participants were instructed prior to scanning that they would be tested on the directly learned associations as well as the indirect relationships. Participants practiced the encoding and test phases prior to scanning using stimuli different from those used during fMRI data collection.
In a separate scanning session (separated by 1–7 days), an object/scene encoding localizer and guided recall task was collected for multivoxel pattern classifier training and validation (see Supplementary Experimental Procedures).
fMRI data acquisition
Whole-brain imaging data were acquired on a 3.0T GE Signa MRI system (GE Medical Systems). During each session, structural images were acquired using a T2-weighted flow-compensated spin-echo pulse sequence (TR = 3 s; TE = 68 ms; 256 × 256 matrix, 1 × 1 mm inplane resolution) with 31 3 mm thick oblique axial slices (0.6 mm gap), approximately 20 degrees off the AC-PC line. Functional images were acquired using a GRAPPA parallel EPI sequence using the same slice prescription as the structural images (TR = 2 s; TE = 30 ms; flip angle = 90°; 64 × 64 matrix; 3.75 × 3.75 mm inplane resolution, interleaved slice acquisition). For each functional scan, the first six EPI volumes were discarded to allow for T1 stabilization. An additional high-resolution T1-weighted SPGR scan (sagittal plane, 1.3 mm slice thickness, 1 mm2 inplane resolution) was acquired during the first scanning session. Head movement was minimized using foam padding.
Preprocessing of fMRI data
Data were preprocessed and analyzed using SPM5 (Wellcome Department of Cognitive Neurology) and custom Matlab routines. Functional images were corrected to account for differences in slice acquisition times by interpolating the voxel time series using sinc interpolation and resampling the time series using the first slice as a reference point. For each session, functional images were realigned to the first volume in the time series to correct for motion and co-registered to the T2-weighted structural image from the corresponding scan session. To coregister images across the two scanning sessions, the T2-weighted structural images from each session were coregistered to the T1 SPGR image, and the coregistration parameters were applied to the corresponding functional images from the same session. Functional images were then resliced to the space of the mean functional image from the second session, high-pass filtered (128s), and converted to percent signal. All analyses were performed in the native space of each participant; no spatial smoothing was applied.
Multivoxel pattern analysis of fMRI data
Pattern classification analyses were implemented using the Princeton MVPA toolbox (http://code.google.com/p/princeton-mvpa-toolbox/) and custom MATLAB code. An anatomically defined mask comprised of the visually selective areas of the ventral temporal lobe was used for MVPA classification. A cortical parcellation of the high-resolution T1 SPGR image was obtained for each participant using FreeSurfer (Martinos Center for Biomedical Imaging, MGH, Charlestown, MA) and the resulting left and right inferotemporal cortex, fusiform gyrus, and parahippocampal gyrus were combined to serve as the mask for MVPA classification. The classifier was first trained to differentiate object and scene processing on data from the encoding localizer task; we then validated the classifier’s ability to measure reactivation of unseen, recalled content by applying it to data from the guided recall task (see Fig. S1 and Supplementary Experimental Procedures).
The main goal of the MVPA approach was to assess whether events that overlap with existing memories lead to the reactivation of unseen, related content. To do so, MVPA classifiers trained on the encoding localizer were applied to the encoding data from associative inference paradigm to provide a measure of content-specific reactivation during overlapping events. For each participant, a regressor matrix labeled the timeseries by encoding condition (e.g., first repetition of AB associations for OOO triads, first repetition of AB associations for OOS triads, etc.; 36 timepoints per condition). To account for the hemodynamic lag, condition labels were shifted back by three scans (6 seconds) with respect to the functional timeseries. The mean classifier output for each content class (object, scene) was then extracted for each experimental condition. As the critical measure of reactivation, we assessed the change in classifier output across repetitions of AB associations (last–first AB presentation) where the presented class of content was the same (e.g., two objects for OOO and OOS triads), but the content class of the third, unseen triad member differed (i.e., object vs. scene; Fig. 2). This analysis yielded two measures of reactivation across AB repetitions: an estimate of scene reactivation (ΔOOS–ΔOOO) and an estimate of object reactivation (ΔSSO–ΔSSS). The two reactivation estimates were then pooled into an overall reactivation index score to assess the behavioral significance of the content-specific reactivation. Cross-participant correlation, using Spearman correlation coefficient, assessed the relationship between the reactivation index and inference performance (AC).
Medial temporal lobe and VMPFC region-of-interest analysis
An additional ROI analysis assessed MTL and VMPFC contributions to reactivation and encoding processes in the associative inference paradigm. For each participant and ROI, learning-related activation changes across repetition were extracted and correlated with (1) the reactivation index and (2) AC inference performance across subjects. To assess the specificity of the findings, we performed similar analyses on 11 additional anatomical regions. See Supplementary Experimental Procedures for full details of the ROI analyses.
Functional connectivity analyses
To assess changes of functional connectivity between hippocampus and VMPFC during encoding of overlapping associations, we performed functional connectivity analyses using hippocampus as a seed. The timecourse of hippocampal activation within each run was split into thirds, and functional connectivity was extracted for each third of a run (corresponding to the first, second and third repetition of individual associations). Repeated measures ANOVA was used to assess the effect of repetition on functional connectivity (see Supplementary Experimental Procedures for full details).
Supplementary Material
HIGHLIGHTS.
Shared content across experiences triggers reactivation of overlapping memories
Anterior medial temporal lobe cortex tracks fidelity of retrieval during encoding
Hippocampus and ventromedial prefrontal cortex interact to integrate related events
Retrieval-mediated memory integration enables inference across experiences
Acknowledgments
This work was supported by a National Science Foundation CAREER Award (A.R.P.), Army Research Office Grant 55830-LS-YIP (A.R.P.), the National Alliance for Research on Schizophrenia and Depression (A.R.P.), and NIH-NIMH National Research Service Award F32MH094085 (D.Z.). We thank Sasha Wolosin and Jackson Liang for help with data collection, Christine Manthuruthil and Arjun Mukerji for help with data analysis, and Margaret Schlichting for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual review of psychology. 2010;61:27–48. C21–28. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. Cambridge, MA: MIT; 1993. [Google Scholar]
- DeVito LM, Kanter BR, Eichenbaum H. The hippocampus contributes to memory expression during transitive inference in mice. Hippocampus. 2010a;20:208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Lykken C, Kanter BR, Eichenbaum H. Prefrontal cortex: Role in acquisition of overlapping associations and transitive inference. Learning & memory (Cold Spring Harbor, NY) 2010b;17:161–167. doi: 10.1101/lm.1685710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18:536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nature neuroscience. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural brain research. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature reviews. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. An fMRI Analysis of the Human Hippocampus: Inference, Context, and Task Awareness. Journal of Cognitive Neuroscience. 2006;18:1156–1173. doi: 10.1162/jocn.2006.18.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. Learning about associatively activated stimulus representations: Implications for acquired equivalence and perceptual learning. Anim Learn Behav. 1996;24:233–255. [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Acquisition of Representation-Mediated Conditioned Food Aversions. Learn Motiv. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 2011;6:e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learning & memory (Cold Spring Harbor, NY) 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. J Neurosci. 2011;31:7156–7162. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Killcross AS, Honey RC. Role of the medial prefrontal cortex in acquired distinctiveness and equivalence of cues. Behavioral neuroscience. 2007;121:1431–1436. doi: 10.1037/0735-7044.121.6.1431. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature neuroscience. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Asthana S, Gluck MA, Myers C. Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:129–145. doi: 10.1080/13825580601139444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci. 2008;28:14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nature neuroscience. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Cosmides L, Tooby J, Chance S. Decisions and the evolution of memory: multiple systems, multiple functions. Psychological review. 2002;109:306–329. doi: 10.1037/0033-295x.109.2.306. [DOI] [PubMed] [Google Scholar]
- Kohler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. The Human Ventromedial Prefrontal Cortex Is Critical for Transitive Inference. Journal of Cognitive Neuroscience. 2012;24:1191–1204. doi: 10.1162/jocn_a_00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. Fidelity of neural reactivation reveals competition between memories. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5903–5908. doi: 10.1073/pnas.1016939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature neuroscience. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content Representation in the Human Medial Temporal Lobe. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in cognitive sciences. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; 1978. [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science (New York, NY) 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JD. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Reas ET, Gimbel SI, Hales JB, Brewer JB. Search-Related Suppression of Hippocampus and Default Network Activity during Associative Memory Retrieval. Front Hum Neurosci. 2011;5:112. doi: 10.3389/fnhum.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Sherrill KR, Stern CE. The hippocampus is functionally connected to the striatum and orbitofrontal cortex during context dependent decision making. Brain research. 2011;1423:53–66. doi: 10.1016/j.brainres.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. New episodic learning interferes with the reconsolidation of autobiographical memories. PLoS One. 2009;4:e7519. doi: 10.1371/journal.pone.0007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci. 2010;30:11586–11604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernandez G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science (New York, NY) 2008;322:960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychological review. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science (New York, NY) 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. Schema-dependent gene activation and memory encoding in neocortex. Science (New York, NY) 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Fernandez G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Flexible memories: differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. J Neurosci. 2010;30:14676–14684. doi: 10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: Building memories to navigate future decisions. Frontiers in Human Neuroscience. 2012:6. doi: 10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.