Abstract
While trying to generate a site-directed deletion in the ORF63 latency-associated gene of varicella-zoster virus (VZV) Oka, we constructed a virus with an unexpected rearrangement. The virus has a small deletion in both copies of ORF63 and insertion of a cassette between ORFs64 and 65 containing (a) truncated ORF62, (b) ORF63 with a small deletion, and (c) full-length ORF64. The virus was not impaired for growth in cell culture, induced higher levels of VZV neutralizing antibodies in guinea pigs than parental virus, and was impaired for latency in cotton rats compared with parental virus (p=0.0022). Additional mutants containing the same truncation in ORF62, with or without the ORF63 deletion, were less impaired for latency. A VZV Oka mutant, replicating to similar titers and inducing a comparable immune response as parental virus, but impaired for latency, might serve as a safer vaccine and be less likely to reactivate to cause zoster.
Keywords: varicella-zoster virus, latency, shingles, chickenpox
Introduction
Varicella-zoster virus (VZV) causes chickenpox and the virus may reactivate later in life to cause shingles. A vaccine to prevent varicella was developed by Takahashi et al. (1974) and approved for use in the United States in 1995. A zoster vaccine was approved for use in the United States in 2006 (Oxman et al., 2005). Both of these vaccine viruses use the Oka strain of VZV, although the titer of virus in the inoculum is about 14-fold higher in the zoster vaccine than in the varicella vaccine.
The Oka varicella vaccine is usually well tolerated. The most common side effects are injection site reactions, fever, and rash. Breakthrough cases of chickenpox and herpes zoster were also frequently reported as adverse reactions (Wise et al., 2000; Sharrar et al., 2001). Rashes account for more than half of the adverse event reports. Rashes due to wild-type virus were present at a median of 8 days after vaccination, while rashes associated with vaccine virus occurred at a median of 3 weeks after vaccination (Sharrar et al., 2001). Zoster occurring after vaccination may be due to reactivation of wild-type or vaccine virus. In one study, wild-type virus was detected in zoster lesions from 12 children at a median of 3 weeks after vaccination, while vaccine virus was present in lesions from 14 children a median of 19 weeks after vaccination (Wise et al., 2000). In another study, wild-type virus was confirmed in zoster lesions from 10 patients at a median of 81 weeks after vaccination, while 22 patients had vaccine virus in zoster lesions a median of 28 weeks after vaccination (Sharrar et al., 2001). Three patients in the latter study had lesions at the site of the vaccine injection. While zoster due to vaccine virus is uncommon, it is more prevalent in immunocompromised persons who receive the varicella vaccine. Thus, a varicella vaccine that is less likely to establish latency would likely be safer in that there should a lower risk of zoster, especially in immunocompromised persons.
VZV establishes latency in cranial nerve and dorsal root ganglia. Six VZV genes, ORF4, ORF21, ORF29, ORF62, ORF63, and ORF66 are expressed during latency in humans (Cohrs et al. 2003; Kennedy et al., 2000; Meier et al. 1993). ORF63 transcripts are the most abundant viral mRNAs expressed in latently infected human ganglia (Cohrs and Gilden, 2007; Cohrs et al., 2000). Therefore, we have constructed mutants in the ORF63 gene in an attempt to construct viruses with different latency phenotypes.
Here we describe a VZV mutant that we constructed in the Oka vaccine virus which replicates to titers similar to parental virus and which is impaired for latency in a rodent model. The vaccine induces higher levels of neutralizing antibody to VZV than the parental virus in guinea pigs. Thus, the VZV Oka mutant we describe here might be safer than the current Oka vaccine in that it might be less likely to reactivate and cause zoster.
Results
Construction and growth properties of VZV ROka-NLS
In an attempt to produce VZV deleted for both copies of the carboxy-terminal nuclear localization of ORF63, melanoma cells were cotransfected with (a) the VZV insert from plasmid p63-30-4 which has a small deletion in ORF63, at the site of the carboxy-terminal nuclear localization signal, flanked by a portion of ORF62 and full-length ORF64 and (b) VZV virion DNA purified from ROka63D (which is deleted for over 95% of both copies the ORF63 gene) (Fig. 1). Homologous recombination between the plasmid and the virion DNA should result in replacement of both copies of the large deletion in ORF63 with ORF63 having a smaller deletion. After transfection, cells with CPE typical for VZV were observed and virus was passaged in melanoma cells, plaque purified 4 times so that virus only with the small deletion in ORF63 could be detected by PCR. A fifth round of plaque purification was then done and Southern blotting was performed.
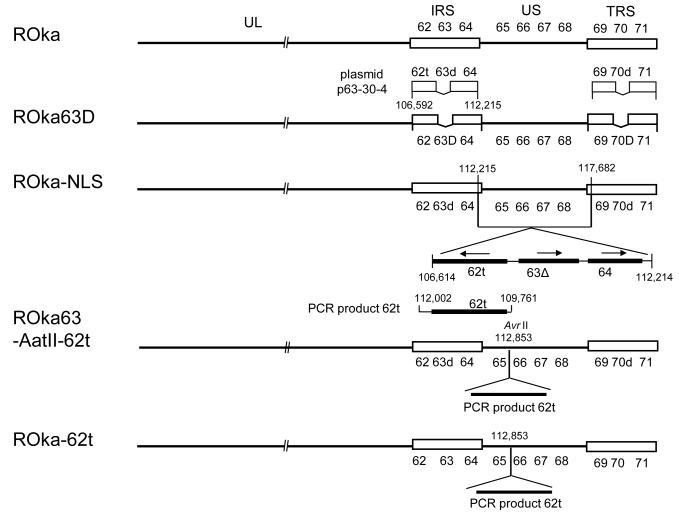
Fig. 1.
Structures of VZV ROka, ROka-NLS, ROka63-AatII-62t, and ROka-62t. Compared with VZV ROka, ROka-NLS has deletions in both copies of ORF63 (ORF63 and ORF70) and insertion of a cassette containing a truncated form of ORF62, a deleted version of ORF63, and full-length ORF64 between ORF 64 and ORF65. ROka-62t has the truncated form of ORF62 present in ROka-NLS inserted between ORF65 and ORF66. ROka63-AatII-62t has the truncated form of ORF62 present in ROka-NLS inserted between ORF65 and ORF66 and the deletion in both copies of ORF63 that are present in ROka-NLS.
Digestion of virion DNA from cells infected with VZV ROka or with ROka-NLS with Not I, followed by hybridization with a probe to ORF63 showed a band of 11.8 kb in cells infected with ROka and bands of 11.8 kb and 5.6 kb in cells infected with ROka-NLS due to the insert containing an additional copy of ORF63 in ROka-NLS (Fig. 2A).
Fig. 2.
Southern blots of VZV ROka, ROka-NLS, ROka63-AatII-62t, and ROka-62t. Virion DNAs were cut with Not I (A) or BamHI (B), separated on agarose gels, transferred to nylon membranes, and hybridized with probes to ORF63 (A) or to ORF62 (near the 5′ end, corresponding to amino acids 140-360).
Sequence analysis of DNA amplified by PCR from ROka-NLS indicated that both copies of ORF63 had the small deletion in the carboxyl terminal nuclear localization sequence, and that a cassette containing VZV nucleotides 106,614 to 112,214 was inserted between ORF64 and ORF65 containing (a) a portion of the amino-terminus of ORF62 (amino acids 1-839), (b) ORF63 with a deletion in the carboxy terminal nuclear localization sequence (NLS), and (c) full-length ORF64 followed by 110 nucleotides in the region between ORF64 and ORF65 (ROka-NLS, Fig. 1). The 3′ end of the cassette (which ends after ORF64) had been inserted into the VZV genome by homologous recombination, while the 5′ end of the cassette (within ORF62) had been inserted by non-homologous recombination.
ROka-NLS grew to peak titers similar to those of ROka in melanoma cells (Fig. 3). As expected plaque sizes in cells infected with ROka-NLS were similar to those of cells infected with ROka (data not shown). Plaques in guinea pig embryo fibroblasts are irregular; therefore the area of virus-infected foci was measured using antibody to VZV glycoprotein E. Infected foci in guinea pig embryo fibroblasts infected with ROka (630,000 units of surface area) were slightly larger than those infected with ROka-NLS (460,000 units of surface area; p=0.02).
Fig. 3.
Growth of VZV ROka, ROka-NLS, ROka63-AatII-62t, and ROka-62t in cell culture. Melanoma cells were infected with each virus and at days 1 to 5 after infection the cells were treated with trypsin and the titer of cell-associated virus was determined on melanoma cells. Each data point shown is the average of two separate values.
VZV ROka-NLS expresses a truncated ORF62 transcript, but a truncated IE62 protein is not detected
Since ROka-NLS contains both full-length and truncated copies of ORF62, Northern blotting was performed to determine if both forms of ORF62 transcripts were expressed. A transcript of 4.2 kb was detected in cells infected with VZV ROka, corresponding to full-length ORF62 RNA, while transcripts of 4.2 and 2.2 kb were detected in cells infected with ROka-NLS (Fig. 4A). The ratio of the amount of 4.2 kb (full-length ORF62) RNA to 2.2kb (truncated ORF62) RNA was 1.0 in ROka-NLS (Fig. 4B), indicating that equimolar amounts of the two transcripts were expressed, even though there are two copies of the full-length ORF62 gene in ROka-NLS.
Fig. 4.
VZV ROka-NLS, ROka63-AatII-62t, and ROka-62t each express a truncated ORF62 transcript at similar levels. (A) Total RNA was isolated from melanoma cells infected with each virus, separated on an agarose gel, transferred to a nylon membrane, and hybridized with a radiolabeled probe to ORF62. The experiment was performed four times and a representative result is shown. (B) The ratio of the intensity of the ORF62 full-length transcript (4.2 kb band) to the ORF62 truncated transcript (2.2 kb band) is plotted for each of viruses. The results shown were derived from four separate experiments and the vertical lines show standard deviations.
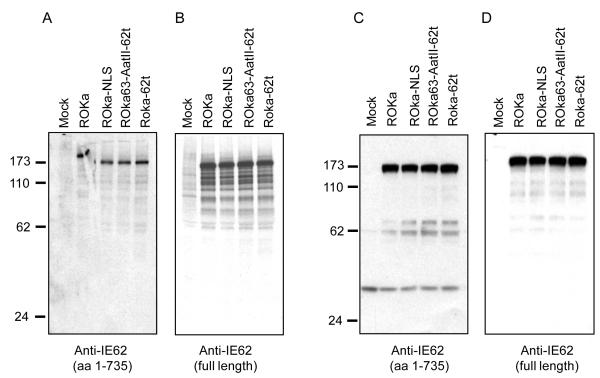
To determine if the truncated ORF62 transcript is expressed in virus-infected cells, immunoprecipitations of radiolabeled lysates from cells infected with VZV ROka or ROka-NLS were performed. Bands of about 175 kDa were detected using antibody to 1-735 of IE62 (Fig. 5A) or full-length IE62 (Fig. 5B). The smaller bands may represent degradation products of IE62. Since a truncated form of IE62 might have a conformation that is not detectable by immunoprecipitation, immunoblotting was performed using lysates from cells infected with VZV ROka and ROka-NLS. A band of approximately 175 kDa was detected in cells infected with ROka and ROka-NLS using antibody directed against amino acids 1-735 of IE62 (Fig. 5C) or full-length IE62 (Fig. 5D). Taken together these data indicate that while ROka-NLS expresses full-length IE62 protein and a truncated ORF62 transcript, the truncated IE62 protein is not expressed. Levels of VZV IE63, thymidine kinase, ORF40 major nucleocapsid protein, and gE were similar in cells infected with VZV ROka and ROka-NLS (data not shown).
Fig. 5.
Expression of IE62 is similar in cells infected with VZV ROka, ROka-NLS, ROka63-AatII-62t, and ROka-62t by immunoprecipitation or immunoblotting. Immunoprecipitation of cell lysates infected with the viruses probed with antibody to 782 amino acids of IE62 (A) or to full-length IE62 (B). Immunoblot of cells infected with the viruses probed with antibody to 782 amino acids of IE62 (C) or to full-length IE62 (D).
VZV ROka-NLS is impaired for latency in cotton rats
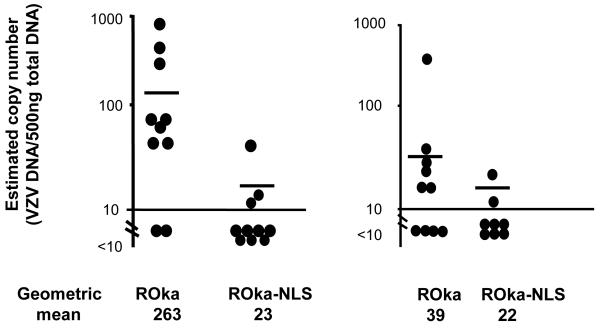
To determine if ROka-NLS is important for VZV latency, cotton rats were inoculated intramuscularly with ROka-NLS or parental ROka virus. Five to 6 weeks later, animals were sacrificed and dorsal root ganglia were examined for viral DNA. In the first experiment 80% of animals receiving VZV ROka and 30% receiving ROka-NLS had a latent VZV infection (Table 1) and the geometric mean copy numbers were 263 VZV genomes for animals infected with VZV ROka and 23 for those infected with ROka-NLS (Fig. 6A). In the second experiment 60% of animals infected with VZV ROka and 25% infected with ROka-NLS developed a latent VZV infection and the geometric mean copy numbers were 39 viral genomes for animals infected with VZV ROka and 22 for animals infected with ROka-NLS (Fig. 6B). The combined results of 5 separate experiments indicated that 35 of 50 (70%) of animals inoculated with ROka had latent VZV DNA, while 18 of 48 (38%) of animals infected with ROka-NLS had latent VZV (p=0.0022, Fisher’s exact test) (Table 1).
Table 1.
VZV ROka-NLS is impaired for latency
| No. Latently Infected Animals/Total No. Animals Infected | ||||
|---|---|---|---|---|
| Expt. | ROka | ROka-NLS | ROka63-AatII-62t | ROka-62t |
| 1 | 8/10 | 3/10 | ND | ND |
| 2 | 6/10 | 2/8 | ND | ND |
| 3 | 9/10 | 6/10 | 6/10 | 6/10 |
| 4 | 6/10 | 2/10 | 4/10 | 5/10 |
| 5 | 6/10 | 5/10 | 3/10 | 5/10 |
|
| ||||
| Total | 35/50 (70%) |
18/48 (38%) |
13/30 (43%) |
16/30 (53%) |
Fig. 6.
Estimated copy number of VZV genomes in latently infected cotton rats from experiments 1 (A) and 2 (B) in Table 1. The geometric mean number of VZV genome copy numbers per 500 ng of ganglia DNA in PCR-positive ganglia is shown at the bottom of the figure. Open circles represent samples whose copy numbers were below the limit of detection (<10 copies per 500 ng of DNA), and filled circles show the viral copy number for samples above the limit of detection.
VZV ROka-NLS elicits higher levels of neutralizing antibody titers than ROka in guinea pigs
To measure VZV neutralizing antibody to VZV ROka-NLS, guinea pigs were inoculated with cell culture medium, uninfected cells, ROka-infected cells, or ROka-NLS- infected cells and after the third inoculation serum was obtained. Animals immunized with ROka-NLS had significantly higher neutralizing titers than those immunized with ROka (p=0.002, Table 2). Animals vaccinated with ROka or ROka-NLS had higher neutralizing antibody titers than animals receiving uninfected cells or medium alone (p<0.0001).
Table 2.
VZV neutralization titers in animals immunized with VZV ROka and ROka-NLSa
| Inoculum | 50% VZV-Neutralization titer after Vaccination (95% CIb) |
|---|---|
| Medium | 1.4 (1.0, 4.7) |
| Uninfected cells | 3.5 (1.8, 5.6) |
| ROka | 23 (20, 26) |
| ROka-NLS | 30 (27, 34) |
Data based on 3 animals receiving medium alone, and 5 animals each receiving uninfected cells, ROka-infected cells, or ROka-NLS infected cells
CI, confidence intervals
VZV with a truncation of ORF62 and the deletion in ORF63 is less impaired for latency than ROka-NLS
ROka-NLS has several differences from ROka that might be responsible for the reduced latency observed in rodents. Two of the changes affect proteins that are expressed during latency. ROka-NLS has a truncated form of IE62 and a deletion in the carboxy-terminal nuclear localization signal in each copy of ORF63. To determine which features of ROka-NLS are responsible for its impairment in latency, we inserted the truncated form of IE62 found in ROka-NLS into VZV ROka to generate ROka-62t (Fig. 1). We also inserted the truncated form of IE62 into ROka63-AatII30-4 which has the deletion in the carboxy terminal nuclear localization signal of ORF63 which is present in ROka-NLS to generate ROka63-AatII-62t (Fig. 1).
VZV ROka-62t and ROka63-AatII-62t had the expected genome configuration. BamHI restriction endonuclease digestion of virion DNA from ROka or ROka-NLS followed by hybridization with a probe to the 5′ end of ORF62 showed a band of 4.7 kb, while digestion of virion DNA from ROka-62t and ROka63-AatII-62t showed bands of 4.7 and 3.8 kb due to insertion of IE62t into the Avr II site located between ORF65 and ORF66 (Fig. 2B).
VZV ROka-62t and ROka63-AatII-62t grew to similar titers as parental virus and ROka-NLS (Fig. 3). ROka-62t and ROka63-AatII-62t expressed a truncated ORF62 transcript at similar levels to that seen in ROka-NLS (Fig. 4). Expression of IE62 was the same in cells infected with ROka-62t, ROka63-AatII-62t, and ROka-NLS (Fig. 5).
To determine if ROka-62t and ROka63-AatII-62t are impaired for latency similar to ROka-NLS, cotton rats were inoculated with the viruses and dorsal root ganglia were analyzed for VZV DNA 5 to 6 weeks later. 16 of 30 (53%) of animals inoculated with ROka-62t and 13/30 (43%) of animals infected with ROka63-AatII-62t had latent VZV DNA in their dorsal root ganglia, compared with 70% of animals infected with ROka or 38% of animals with ROka-NLS. The difference between ROka and ROka-62t was not significant (p=0.155, Fisher’s exact test), while the difference between ROka and ROka63-AatII-62t was significant (p=0.033, Fisher’s exact test). These data indicate that the combination of the ORF62 truncation and the ORF63 deletion contribute to the latency phenotype observed in ROka-NLS.
Discussion
We have shown that a VZV Oka mutant virus is impaired for latency in rodents, grows to peak titers similar to those seen with parental virus in vitro, and induces higher titers of neutralizing antibodies than parental virus in guinea pigs. The VZV Oka mutant grew in cell culture to similar titers as the Oka vaccine strain from which it was derived. Since the original Oka vaccine strain is attenuated in humans, we suspect that the VZV Oka mutant would also be attenuated in humans.
Vaccine virus has been isolated from zoster lesions in both children and adults vaccinated with the varicella vaccine (Liang et al., 1998; Hammerschlag et al., 1989; Plotkin et al., 1989; Quinlivan et al., 2007). The VZV Oka vaccine is less likely to reactivate to cause zoster than wild-type virus in children with leukemia (Brunell et al., 1986; Kamiya et al., 1984). Hardy et al. (1991) found that the rate of zoster among vaccinated children with leukemia was three-fold lower than the rate of zoster in children previously infected with wild-type virus. 85% of the vaccinated children with zoster had a rash associated with the vaccination or from breakthrough varicella after a household exposure before their episode of zoster. Broyer et al. (1997) reported rates of zoster in renal transplant recipients of 7% in children who had received the varicella vaccine, compared with 13% who had a history of natural varicella. While it is assumed that the vaccine virus is also less likely to reactivate to cause zoster than wild-type virus in immunocompetent children, this has not yet been demonstrated.
It is possible that subclinical reactivation of the vaccine virus might occur and be a valuable feature of the vaccine in that it would result in boosting of the immune response to the virus over time. Krause and Klinman (2000) found that titers of antibody to VZV rose over time in vaccinees with low VZV titers at higher rates than would be expected from exposure to wild-type varicella, suggesting that the vaccine virus was undergoing subclinical reactivation with boosting of the immune response. However, this hypothesis is controversial and other investigators feel that the rise in antibody titers years after vaccination is more likely due to exposure to wild-type virus than reactivation of vaccine virus (Seward et al., 2000; LaRussa et al., 2000). In addition, the recent approval of a second dose of varicella vaccine for children at age 4-6 years after the first dose at 12-15 months (Centers for Disease Control, 2007) suggests that a single dose does not provide full protection which might occur if the vaccine virus was able to undergo subclinical reactivation and boosting.
The frequency of zoster occurring after varicella vaccination of healthy adults is unknown. In a passive, voluntary postmarketing study of varicella vaccinees after licensure in the United States, 205 cases of zoster occurred more than 6 weeks after vaccination. Of the 32 cases in which the origin of the virus was determined by PCR, 22/32 (69%) of cases were due to vaccine virus and 10/32 (31%) were due to wild-type virus (Sharrar et al., 2001). Thus while the Oka vaccine can reactivate to cause zoster, the true incidence of this phenomenon, especially in adults, is not known.
We found that ROka63-AatII-62t was partially impaired for latency, but to a lesser extent than ROka-NLS. ROka63-AatII-62t has both the truncated version of ORF62 and the deletion in both copies of ORF63 that are found in ROka-NLS. Insertion of the truncated version of ORF62 into ROka had a minimal effect on latency (ROka-62t, Table 1), and a prior study showed that virus with only the carboxy terminal nuclear localization signal deletion in both copies of ORF63 (ROka63-AatII30-4) was not significantly impaired for latency (Cohen et al., 2005a). Thus, both the ORF62 truncation and ORF63 deletion contribute to the latency phenotype observed in ROka-NLS. While the ORF62 protein has been shown to interact with the ORF63 protein (Lynch et al., 2002), the interaction domain in the ORF63 protein is at amino acids 55 to 67 (Baiker et al., 2004) of the ORF62 protein, not in the area deleted in ROka-NLS.
The contribution of the ORF62 truncation to the latency phenotype in ROka-NLS is intriguing in view of experiments comparing viral transcription in cells infected with parental and vaccine strain Oka viruses. Transcription of ORF62 is reduced in cells infected with Oka vaccine virus, compared with parental Oka virus (Cohrs et al, 2006; Grinfeld et al. 2009). While we did not detect a difference in ORF62 transcription in cells infected with ROka-NLS in vitro, it is possible that differences might occur during lytic or latent infection in vivo. Transcripts were detected from the truncated copy of ORF62; however, truncated protein could not be detected. While truncated forms of ORF62 can be expressed by transient transfection using a different viral promoter (Baudoux et al., 1995), it is possible that (a) the truncated protein is not made or is less stable in the context of virus infection, or (b) that the expression level of truncated mutants is lower when expressed from its native promoter.
Analysis of several other VZV mutants showed that most are dispensable for latency in rodents. VZV unable to express ORFs 1, 2, 10, 13, 14, 17, 32, 47, 57, 61, 66, or 67 was dispensable for latency in rodents (Grinfeld et al., 2004; Sato et al., 2002a,b; Sato et al., 2003a, b). While deletion of ORFs 4, 29, or 63 resulted in viruses which were impaired for latency in cotton rats (Cohen et al., 2005a, b; Cohen et al., 2007), each of these mutants was either replication defective or markedly impaired for growth in cell culture. Only one other VZV mutant, which expresses ORF29 protein from a CMV promoter is also impaired for latency in rodents and replicates to titers similar to parental virus (Cohen et al., 2007). Thus ROka-NLS is only the second VZV mutant that is impaired for latency in an animal model, but not for replication in cell culture.
Materials and Methods
Cells, viruses, plasmids, and transfections
Human melanoma (MeWo) cells were a gift from Charles Grose (U. Iowa) and were used for transfections and propagation of virus. VZV recombinant Oka virus (ROka) and recombinant Oka virus deleted for both copies of ORF63 (ROka63D) have been described previously (Cohen et al., 2004).
Cosmids VZV NotIA, NotIB, MstIIA, and MstIIB are derived from the Oka strain of VZV and encompass the entire genome. Cosmid MstIIA-63AatII30-4 has a deletion in the carboxy-terminal nuclear localization of both copies of ORF63 (Cohen et al., 2005a).
Plasmid p63-30-4 contains a portion of ORF62, a deletion (VZV nucleotides 111,335 to 111,379) in ORF63 which removes the carboxy-terminal nuclear localization signal, and ORF64 and was described previously (Cohen et al., 2005a) (Fig. 1)
A plasmid containing a truncated version of ORF62 was constructed by PCR amplication of ROka-NLS using primers 5′-GCATACCTAGGTTACGTGAACACCACAAC (containing an Avr II site [underlined] and VZV nucleotides 109,761-109,742) and 5′ GCATACCTAGGCCGCGCCGAATTATATGACC (containing an Avr II site [underlined] and VZV nucleotides 112,002-112,021), cutting the PCR product with Avr II and cloning the DNA into the Avr II site of LITMUS 28i (New England Biolabs, Beverly, MA) to create plasmid 62t.
Cosmid MstIIA-62t was constructed by cutting cosmid Mst IIA with Avr II and inserting the Avr II fragment with the truncated portion of ORF62 from ROka-NLS obtained from plasmid 62t. The resulting cosmid MstIIA-62t has two full-length copies of ORF62 and one truncated copy of ORF62.
Cosmid MstIIA-63AatII30-4-62t was constructed by cutting cosmid MstIIA-63AatII30-4 with Avr II and inserting the Avr II fragment with the truncated portion of ORF62 from plasmid 62t as described above. The resulting cosmid has two full-length copies of ORF62, one truncated copy of ORF62, and a deletion in the carboxy-terminal nuclear localization signal of both copies of ORF63.
Recombinant viruses with the truncated copy of ORF62 were made by transfecting melanoma cells with the MstIIA cosmids with the ORF62 truncation along with cosmids MstIIB, NotIA, and NotIB and plasmid pCMV62 by the calcium phosphate transfection procedure (Cohen et al., 2005a).
Southern blotting, Northern blotting, immunoblotting, and immunoprecipitations
Southern blotting was performed using virion DNA isolated from VZV mutants, digesting the DNA with restriction enzymes, fractionating the DNA on agarose gels, and transferring the DNA to nylon membranes. The membranes were probed with [32P]dCTP radiolabeled probes corresponding to ORF62 nucleotides 421-1,081 or full-length ORF63.
For Northern blotting, RNA was isolated from VZV-infected cells using TRIzol reagent (Invitrogen, Carlsberg, CA), separated on a formaldehyde gel, transferred to nitrocellulose, and hybridized to a [32P]dCTP radiolabeled probe corresponding to VZV nucleotides 421-1,081 of ORF62. Radioactivity in DNA bands on the gel were quantified using a PhosphorImager and ImageQuant software (Molecular Dynamics, Piscataway, NJ).
Immunoblots were performed using lysates of VZV-infected melanoma cells prepared in RIPA buffer (0.01 M Tris pH 7.4, 0.15 M NaCl, 1% NP-40, 1% DOC, 0.1% SDS). After fractionation on SDS-polyacrylamide gels and transfer to nylon membranes, the blots were incubated with rabbit polyclonal antibodies to IE62. One antibody, #18 (a gift from William T. Ruyechan) was targeted to amino acids 1-735 of IE62 (Spengler et al., 2000), while the other antibody (a gift from Paul R. Kinchington), was made by immunizing rabbits with a mixture of purified proteins (amino acids 1 to 161, 162 to 506, 506 to 1310, and 417 to 824 representing full-length VZV IE62 [Kinchington et al., 2000]).
For immunoprecipitations, cells were radiolabeled with [35S]methionine and lysed in RIPA buffer. Antibody to IE62 was added to the lysates followed by protein G, the immune complexes were separated on SDS-polyacrylamide gels, and autoradiography was performed.
Virus growth studies
Flasks of melanoma cells were infected with VZV mutants at 37oC and 1 to 5 days later the cells were treated with trypsin, and serial dilutions of cells were used to infect melanoma cells in 6-well plates. One week later the plates were stained with crystal violet and the number of plaques was counted. Immunofluorescent foci were measured by fixing virus-infected cells with methanol-acetone (1:1 [vol/vol]), incubating with mouse monoclonal antibody to VZV glycoprotein E (Chemicon, Temecula, CA) followed by Alexa 488-conjugated anti-mouse antibody (Molecular Probes, Eugene, OR), and measuring the size of immunofluorescent foci using Image J software (http://rsb.info.nih.gov/ij/).
Analysis of immune responses
Four- to 6-week old female Hartley guinea pigs were vaccinated subcutaneously with cell culture medium or human diploid fibroblasts (MRC-5 cells) that were uninfected or infected with 1 × 105 PFU of VZV ROka or ROka-NLS. Animals received three doses of vaccine at monthly intervals. Serum was obtained 5 weeks after the third vaccination. To determine the neutralizing titer, serum was diluted 1:4, 1:8, 1:16, 1:32, 1:64, and 1:128 in SPGC (10% fetal bovine serum, 0.1% sodium glutamate, 5% sucrose in PBS) and each dilution (0.3 ml) was mixed with 100 PFU of VZV Oka vaccine strain (0.3 ml). The mixture was incubated for 1 hr on ice and 0.5 ml of the mixture was added to each well of a 6-well plate of melanoma cells. After incubation for 1 hr at 37°C, 3.5 ml of additional medium was added. After 10 days the cells were fixed and stained with crystal violet. VZV neutralizing antibody titers were defined as the concentration of guinea pig serum required to reduce the number of plaques by 50% compared to PBS.
Analysis of latent infection
Four- to 6-week-old female cotton rats were inoculated intramuscularly along the spine with VZV-infected melanoma cells containing 1.75 × 105 PFU of VZV. Five to 6 weeks later animals were sacrificed and DNA was isolated from pooled dorsal root ganglia. PCR was performed using 500 ng of dorsal root ganglia DNA or serial dilutions of VZV cosmid NotIA in 500 ng of DNA from uninfected cotton rats and primers corresponding to ORF 21 (Cohen et al., 2004). PCR products were analyzed on a Southern blot using a radiolabeled probe to ORF21. The lower limit of detection of DNA was 10 copies of viral DNA when the viral DNA was mixed with 500 ng of salmon sperm DNA. Copy numbers were estimated by densitometry using a phosphorimager.
Statistics
To determine the 50% neutralization titers from the dilutions tested, we assumed that the number of plaques follows a Poisson distribution with a mean value depending on the dilution proportion. An exponential mean function is used, where the mean titer of neutralizing antibody is an exponential function of an intercept and the log dilution proportion. We assume that the Poisson mean is given by e(a + b log(X)) where X is the dilution number. We estimate the unknown terms a and b, using the method of maximum likelihood, which provides a smooth monotone estimate of the true mean number of plaques for any dilution X. We then use this smoothed estimate to determine the 50% neutralization dilution by comparison of serum from infected to uninfected guinea pigs. The average number of plaques in serum from uninfected guinea pigs was 90, based on two replicates. Thus the estimated titer that neutralizes 50% of virus plaques, say X50 solves e{a+b log(X50)}/90 = 0.50. Multiplying both sides by 90 yields e{a+b log(X50)} = 45. Taking the log of both sides yields a+b (log (X50)=log(45). After subtracting a and dividing by b, the equation becomes log(50)={log(45)−a}/b. Then taking the anti-log of both sides we get 50= e[{log(45)−a}/b]. A 95% confidence interval for the 50% neutralization dilution is constructed by the likelihood ratio method. Comparisons between estimated 50% neutralizations under different conditions were conducted by the likelihood ratio test statistic.
Acknowledgements
This study was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases and the NIH Clinical Center. We thank Drs. Paul Kinchington (University of Pittsburgh, Pittsburgh, PA) and William T. Ruyechan (University at Buffalo SUNY, Buffalo, NY) for antibodies to IE62, and Dr. Qingxue Li for assistance with the immunofluorescent focus assay.
References
- Baiker A, Bagowski C, Ito H, Sommer M, Zerboni L, Fabel K, Hay J, Ruyechan W, Arvin AM. The immediate-early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J. Virol. 2004;78:1181–1194. doi: 10.1128/JVI.78.3.1181-1194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoux L, Defechereux P, Schoonbroodt S, Merville MP, Rentier B, Piette J. Mutational analysis of varicella-zoster virus major immediate-early protein IE62. Nucleic Acids Res. 1995;23:1341–9. doi: 10.1093/nar/23.8.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyer M, Tete MT, Guest G, Gagnadoux MF, Rouzioux C. Varicella and zoster in children after kidney transplantation: long term results of vaccination. Pediatrics. 1997;99:35–39. doi: 10.1542/peds.99.1.35. [DOI] [PubMed] [Google Scholar]
- Brunell PA, Taylor-Wiedeman J, Geiser CF, Frierson L, Lydick E. Risk of herpes zoster in children with leukemia: varicella vaccine compared with history of chickenpox. Pediatrics. 1986;77:53–56. [PubMed] [Google Scholar]
- Centers for Disease Control Recommended immunization schedules for persons aged 0-18 years - United States. Morb Mortal Wkly Rep. 2007;55(51):Q1–Q4. [Google Scholar]
- Cohen JI, Cox E, Pesnicak L, Srinivas S, Krogmann T. The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency. J. Virology. 2004;78:11833–11840. doi: 10.1128/JVI.78.21.11833-11840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Bontems S, Sadzot C, Pesnicak L. Regions of the varicella-zoster virus ORF63 latency-associated protein important for efficient replication in vitro are also critical for efficient establishment of latency. J. Virol. 2005a;79:5069–5077. doi: 10.1128/JVI.79.8.5069-5077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Pesnicak L, Ali MA. Absence or overexpression of the varicella-zoster virus (VZV) ORF29 latency-associated protein impairs late gene expression and reduces latency in a rodent model. J. Virol. 2007;81:1586–1591. doi: 10.1128/JVI.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Ross JP, Pesnicak L, Prikhodko E. Varicella-zoster virus ORF4 latency-associated protein is important for establishment of latency. J. Virol. 2005b;79:6969–6975. doi: 10.1128/JVI.79.11.6969-6975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed VZV genes in human ganglia. J. Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH, Gomi Y, Yamanishi K, Cohen JI. Comparison of virus transcription during lytic infection of the Oka parental and vaccine strains of Varicella-Zoster virus. J Virol. 2006;80:2076–82. doi: 10.1128/JVI.80.5.2076-2082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 2003;77:6660–5. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van Der Keyl H, Tal-Singer R. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J. Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinfeld E, Ross A, Forster T, Ghazal P, Kennedy PG. Genome-wide reduction in transcriptomal profiles of varicella-zoster virus vaccine strains compared with parental Oka strain using long oligonucleotide microarrays. Virus Genes. 2009;38:19–29. doi: 10.1007/s11262-008-0304-3. [DOI] [PubMed] [Google Scholar]
- Grinfeld E, Sadzot-Delvaux C, Kennedy PG. Varicella-zoster virus proteins encoded by open reading frames 14 and 67 are both dispensable for the establishment of latency in a rat model. Virology. 2004;323:85–90. doi: 10.1016/j.virol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Hammerschlag MR, Gershon A, Steinberg S, Clarke L, Gelb L. Herpes zoster in an adult recipient of live attenuated varicella vaccine. J. Infect. Dis. 1989;160:535–537. doi: 10.1093/infdis/160.3.535. [DOI] [PubMed] [Google Scholar]
- Hardy I, Gershon AA, Steinberg SP, LaRussa P, the Varicella Vaccine Collaborative Study Group The incidence of zoster after immunization with live attenuated varicella vaccine. N. Engl. J. Med. 1991;325:1545–1550. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Kato T, Isaji M, et al. Immunization of acute leukemic children with a live varicella vaccine (Oka strain) Biken J. 1984;27:99–102. [PubMed] [Google Scholar]
- Kennedy PG, Grinfeld E, Bell JE. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 2000;74:11893–11898. doi: 10.1128/jvi.74.24.11893-11898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington PR, Fite K, Turse SE. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 2000;74:2265–2277. doi: 10.1128/jvi.74.5.2265-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PR, Klinman DM. Varicella vaccination: evidence for frequent reactivation of the vaccine strain in healthy children. Nature Med. 2000:451–454. doi: 10.1038/74715. [DOI] [PubMed] [Google Scholar]
- LaRussa P, Steinberg SP, Shapiro E, Vazquez M, Gershon AA. Varicella vaccine revisted [letter] Nature Med. 2000;12:1299. doi: 10.1038/82070. [DOI] [PubMed] [Google Scholar]
- Liang MG, Heidelberg KA, Jacobson RM, McEvoy MT. Herpes zoster after varicella vaccination. J. Am. Acad. Dermatol. 1998;38:761–763. doi: 10.1016/s0190-9622(98)70206-3. [DOI] [PubMed] [Google Scholar]
- Lynch JM, Kenyon TK, Grose C, Hay J, Ruychen WT. Physical and functional interaction between the varicella-zoster virus IE63 and IE62 proteins. Virology. 2002;302:71–82. doi: 10.1006/viro.2002.1555. [DOI] [PubMed] [Google Scholar]
- Meier JL, Holman RP, Croen KD, Smialek JE, Straus SE. Varicella-zoster virus transcription in human trigeminal ganglia. Virology. 1993;193:193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Plotkin SA, Starr SE, Connor K, et al. Zoster in normal children after varicella vaccine. J. Infect. Dis. 1989;159:1000–1001. doi: 10.1093/infdis/159.5.1000. [DOI] [PubMed] [Google Scholar]
- Quinlivan ML, Gershon AA, Al Bassam MM, et al. Natural selection for rash-forming genotypes of the varicella-zoster vaccine virus detected within immunized human hosts. Proc. Natl. Acad. Sci. U S A. 2007;104:208–12. doi: 10.1073/pnas.0605688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Callanan LD, Pesnicak L, Krogmann T, Cohen JI. Varicella-zoster virus (VZV) ORF17 protein induces RNA cleavage and is critical for replication of VZV at 37°C, but not 33°C. J Virol. 2002b;76:11012–11023. doi: 10.1128/JVI.76.21.11012-11023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Pesnicak L, Cohen JI. Varicella-zoster virus open reading frame 2 encodes a membrane phosphoprotein that is dispensable for viral replication and for establishment of latency. J. Virol. 2002a;76:3575–3578. doi: 10.1128/JVI.76.7.3575-3578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Pesnicak L, Cohen JI. Varicella-zoster virus ORF47 protein kinase which is required for replication in human T cells, and ORF66 protein kinase which is expressed during latency, are dispensable for establishment of latency. J. Virol. 2003a;77:11180–11185. doi: 10.1128/JVI.77.20.11180-11185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Pesnicak L, Cohen JI. Use of a rodent model to show that varicella-zoster virus ORF61 is dispensable for establishment of latency. J. Med. Virol. 2003b;70(Suppl 1):S79–S81. doi: 10.1002/jmv.10326. [DOI] [PubMed] [Google Scholar]
- Seward J, Jumann A, Schmid S. Varicella vaccine revisted [letter] Nature Med. 2000;12:1298–1299. doi: 10.1038/82068. [DOI] [PubMed] [Google Scholar]
- Sharrar RG, LaRussa P, Galea SA, Steinberg SP, Sweet AR, Keatley M, et al. The postmarketing safety profile of varicella vaccine. Vaccine. 2001;19:916–923. doi: 10.1016/s0264-410x(00)00297-8. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Ruyechan WT, Hay J. Physical interaction between two varicella zoster virus gene regulatory proteins, IE4 and IE62. Virology. 2000;272:375–381. doi: 10.1006/viro.2000.0389. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Otsuka T, Okunu Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Wise R,P, Salive ME, Braun MM, Mootrey GT, Seward JF, Rider LG, et al. Postlicensure safety surveillance for varicella vaccine. JAMA. 2000;284:1271–1279. doi: 10.1001/jama.284.10.1271. [DOI] [PubMed] [Google Scholar]