Incidence of HPV infection
HPV infection is prevalent and the incidence varies among age groups and by gender. The prevalence of HPV infection in a large cohort of women was 26.8%, with the prevalence increasing to 44.8% in women aged 20 to 24 [1]. In a prospective study aimed to define the natural history of HPV infection in men, in a cohort of 1159 men, at least one type of HPV DNA was detected in 50% of men prior to enrollment and the incidence of new HPV infections during the study was 38.4/1000 person months over an average of 24 months [2]. Interestingly, the incidence of acquiring a new infection in men was stable across all age groups, which differs significantly from women in whom the incidence of new HPV infections decreases with increasing age [3, 4]. This observation mirrors the declining prevalence of HPV infection in women past the peak prevalence in their mid-20s [1].
The majority of HPV infections are cleared by the immune system within 2 years, defined as an absence of HPV DNA detection on follow-up serial swabs after detection of the initial infection [2]. At 12 months, 66% of infections are cleared; this increases to 90% at 24 months. However, in men, HPV-16 has been identified as one of the slowest viral types to be cleared, and takes nearly two times longer (12 months) to be cleared than other high-risk viral types [2]. This is an interesting finding since HPV-16 is the viral type which accounts for over 90% of HPV-related oropharyngeal cancer in the United States, and this disease is more prevalent in men as compared to women, suggesting possible gender differences in the ability to mount immunologic responses against this viral type.
Persistent oral HPV infection is a risk factor for the development of HPV-related oropharyngeal cancers. The prevalence of any HPV type in the oral cavity for both men and women is approximately 6.9%. However, when separated by gender, it is significantly higher in men (10%) as compared to women (3.6%) [5]. Oral HPV infection is associated with certain sexual behaviors, with risk increasing with the number of lifetime oral sex partners [6]. In healthy individuals, the clearance rate for oral HPV infection at 6 months is approximately 40% [7].
Biology of HPV infection
Human papillomaviruses (HPV) are small, non-enveloped DNA viruses with a double-stranded genome which encodes six “early” proteins (E1, E2, E3, E4, E5, E6 and E7) and two “late” proteins (L1 and L2), which are named based on their temporal expression pattern in the viral life cycle (Figure 1).
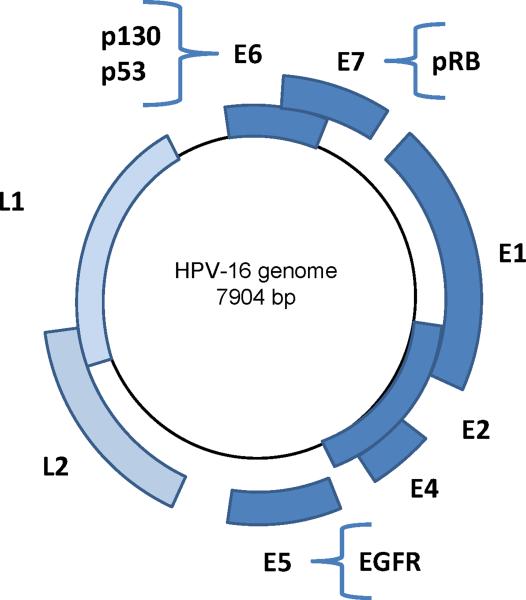
Figure 1. HPV-16 Genome.
The circular HPV-16 genome is 7904 base pairs and encodes six early (E) and two late (L) major proteins. The primary cellular targets of these proteins are indicated on the genome diagram: E6 ubiquitinates p53, E7 competes for binding with E2F to the hypophosphorylated “active” form of retinoblastoma tumor suppressor gene product (pRB), and E5 up-regulates epidermal growth factor receptor (EGFR).
Replication cycle
The replication cycle of HPV has been well-studied in the epithelium of the cervix, and has been found to be tightly linked to the differentiation of the epithelium that it infects. After infection of the undifferentiated epithelial cells within the basal cell layer, the E1 and E2 proteins are expressed and regulate viral replication and expression of the other early viral genes. As the infected cell migrates toward the superficial layers of the squamous epithelium, the E6 and E7 oncogenes are expressed and modify the cell cycle to retain the differentiating host keratinocyte in a state which is favorable for amplification of the viral genome. The E6 oncoprotein ubiquitinates p53, thereby flagging it for proteosomal degradation via the ubiquitin-proteasome pathway. The E7 protein competes for binding with E2F to the hypophosphorylated, “active” form of the retinoblastoma tumor suppressor gene product, pRB, thus releasing the transcription factor E2F to bind and activate its targets to facilitate cell cycle progression. The binding affinity of E6 and E7 to p53 and pRB, respectively, differentiates the “low” and “high” risk types of HPV, which is based on the risk of the infected cell to progress to malignant transformation.
Expression of late proteins
Upon cellular differentiation to the granular epithelial layer, the late proteins, L1 and L2, which consist of the major and minor capsid proteins respectively, are expressed and encapsidate the newly synthesized viral genomes. L1 spontaneously forms pentamers which assemble with the L2 protein to form the viral capsule [8]. These capsid proteins are linked by disulfide bonds to provide structural stability and protection against environmental insults when the virus is shed from the superficial epithelium [9]. The L2 protein is highly conserved amongst viral types, and is exposed during binding to a cell surface receptor during initial infection [10], which completes the viral lifecycle.
Effect on epithelium
In the head and neck region, HPV infects the basal cell layer of the reticulated squamous epithelium of the deep crypts within the lingual and palatine tonsils. The reticulated epithelium is a modified form of stratified squamous epithelium, which contains lymphocytes, plasma cells, macrophages, and interdigitating cells which migrate between the reticulated epithelium and underlying lymphoid stroma. Therefore, the basement membrane of the squamous epithelium lining the deep crypts is disrupted to allow for the passage of lymphocytes and antigen-present cells from the external environment of the oropharynx to the tonsillar lymphoid tissue. Based on the function of the tonsil, its microanatomy leaves the basal cell layer vulnerable to HPV infection.
Cellular Progression to Dysplasia and Cancer
Many HPV infections are either cleared by the immune system or result in latent infections of the basal cell layer, with low viral copy numbers maintained indefinitely, or until injury or immunosuppression induces active infection. Integration of viral DNA into the host genome is a strong predictor of risk of progression from viral infection to neoplastic disease [11]. Late genes (L1 and L2) and some early genes (E1 and E2) are commonly deleted with viral integration and with the disruption of E2 expression, there is unregulated expression of the E6 and E7 oncoproteins [12, 13]. Concurrently, E5 up-regulates the expression of epidermal growth factor receptor (EGFR) within the cell [14]. This leads to the overexpression of proto-oncogenes and repression of p21 (cyclin-dependent kinase inhibitor 1A) expression, a regulatory protein that controls cell apoptosis and differentiation [14]. The interruption of cellular mechanisms that regulate apoptosis and the cell cycle results in dysregulated cell cycle proliferation, delayed cellular differentiation, increased frequency of spontaneous and mutagen-induced mutations, and increased chromosomal instability [15]. Thus, the over-expression of the viral oncoproteins, E6 and E7, drives and maintains the neoplastic process.
Immune System and HPV
Several lines of evidence highlight the importance of a functioning immune system in controlling HPV infection and its associated neoplasms.
Foremost is the observation that the majority of immune-competent individuals infected with HPV are able to clear the infection without any clinical manifestation, and it is only in 10% of infected individuals who can go on to develop HPV-related lesions [2].
Histologic examination of spontaneously regressing HPV-related lesions demonstrates infiltration of CD4+ and CD8+ T cells, whereas these immune cells are lacking in the lesions of patients with persistent disease [16]. Immunocompromised individuals such as organ transplant recipients on immunosuppressive medications [17] and patients infected with human immunodeficiency virus (HIV) [7] have been documented to have significantly increased rates of HPV infections and of HPV-related diseases [18, 19]. Once these individuals stop their immunosuppressive medications or recover their immune cell counts, they are able to clear the infection and associated lesions.
Preclinical studies have reported that animals immunized with vaccines which elicit HPV-specific CD8+ T cells demonstrate regression of established HPV-related cancers [20, 21].
Humoral immune response
Clinically, systemic immune responses against HPV infection are often detected as humoral responses generated against the configured L1 pentamer, but this response is weak, inconsistent, and may not even protect against future re-infection [22]. For high-risk types, the seroconversion rate is only 30 to 50% following documented infection [23] and, in patients with HPV-associated cancer, 30 to 50% have detectable antibody levels against the L1 protein from the causative viral type [24]. If present, the antibody titers can persist for many years even after the infection is cleared, so seropositivity is a useful marker for past infection rather than current infection.
Humoral immune responses have also been detected to the “early” viral proteins. Patients with cervical cancer can have detectable antibodies to E7 [25] and HPV-related head and neck cancer patients have detectable antibodies to E6 (unpublished data, Dr. Sara Pai). Serum analysis for antibodies against the full viral proteome therefore has some promise as a screening method for HPV-associated oropharyngeal cancer [26]. However, patients with pure hypogammaglobulinemia appear to be at no higher risk of developing HPV-related diseases than patients with normal immune function, suggesting that humoral responses do not play a major role in clearing established HPV infections.
Cell-mediated immune response
Rather, it is the cell-mediated immune responses, or HPV-specific CD4+ and CD8+ T cells, which are most critical in clearing established lesions (Figure 2). The virus-specific CD4+ and CD8+ T cells coordinate to clear chronic viral infections [27], and patients with evidence of previously cleared HPV-16 infections have strong detectable T-cell responses to viral proteins [28]. Deficits in T-cell response have been documented in patients with cervical cancer and in patients with cervical intraepithelial neoplasia [29, 30]. The relative contributions to this lack of T-cell response from inherent host genetic factors [31] and/or viral mechanisms to escape immune recognition are not known.
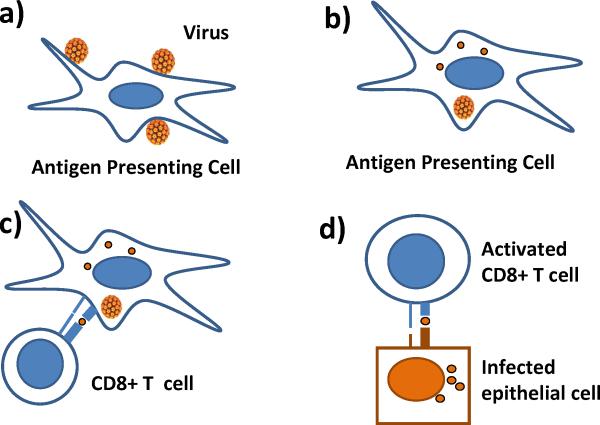
Figure 2. Cell-mediated Immune Responses.
Cell-mediated clearance of virally-infected cells begins with A) antigen presenting cells encountering viral particles or proteins. B) The virus is engulfed by the antigen presenting cell and undergoes intracellular degradation. C) The viral peptides are then presented to immature CD8+ T cells via MHC class I molecules. D) These activated cytotoxic CD8+ T cells then recognize and eliminate virally-infected epithelial cells.[78]
Viral Mechanisms to Evade the Immune System
HPV has evolved multiple mechanisms to evade host immunological responses [32, 33], thereby leading to successful establishment of HPV-related lesions.
Coordination of viral replication to cellular differentiation
The first adaptive mechanism for escaping immune surveillance is the coordination of viral replication to cellular differentiation.
In the uterine cervix, within the organization of stratified squamous epithelium, the degree of immune surveillance decreases considerably in the superficial, keratinized layers. HPV takes advantage of this organization by tightly regulating its own replication with differentiation of the keratinocyte. The virus evades cytotoxic T lymphocyte (CTL) responses by expressing a minimal level of viral gene products in the keratinocytes of the basal cell layer and up-regulates expression of viral gene products with differentiation and upward migration of keratinocytes, away from areas of active immune surveillance. In addition, HPV does not cause lysis of keratinocytes— rather, virions are released through the mechanical breakage of surface epithelium and thereby minimize any associated inflammatory response. In this way, HPV replication is a local phenomenon with minimal systemic immune activation.
In the tonsil, as mentioned previously, HPV infects the reticulated epithelium lining the deep tonsillar crypts. Recent data suggests that the deep crypts of tonsils may be immune privileged sites which can inhibit the effector function of HPV-specific T cells and thereby facilitate immune evasion at the time of initial HPV infection. This provides a biologic explanation of how a virus can infect a lymphoid organ, such as the tonsil and base of tongue, yet still evade immune recognition and clearance (Lyford-Pike S. et al, in press).
Inhibitory effects of viral proteins
In addition to the local immunosuppressive microenvironment that HPV infects, the viral proteins also have local inhibitory effects on inflammatory cytokines to dampen both innate and adaptive immune responses. The HPV E5 and E7 proteins down-regulate expression of the major histocompatibility complex (MHC) class I molecules, which inhibits viral antigen processing and presentation to the immune system [34, 35]. E6 and E7 have also been shown to reduce expression of Toll-like Receptor 9 [36] and cytokines, such as IL-8 [37], and IL-18 [38], which are all potent pro-inflammatory molecules. A blunted response to interferon (IFN)-α and IFN-γ has also observed in HPV infections [39, 40]. One mechanism for the blunted response is a reduction in the expression of Interferon Regulatory Factor (IRF-1), which is a transcription factor that mediates interferon responses [41]. Because interferon signaling is a critical component in the activation of many aspects of both the innate and adaptive immune responses, as well as a potent anti-proliferative agent, HPV thus disables a major mechanism of immune surveillance to oncogenic transformation.
Prevention of HPV Infection through Vaccination
Since the immune system is so important in controlling HPV infections and the lesions associated with these viruses, in the past decade vaccination programs against HPV have been initiated in the United States and other parts of the world.
Vaccines for the Prevention of Cancer
The discovery that the L1 viral proteins self assemble into viral like proteins (VLPs) in the absence of viral DNA was the critical first step in developing preventative vaccines [42]. Recombinant techniques could then be used to produce hollow VLPs which could induce protective L1 antibody levels that can prevent against new HPV infection without the risk of being exposed to an infectious virus [43] (Figure 3).
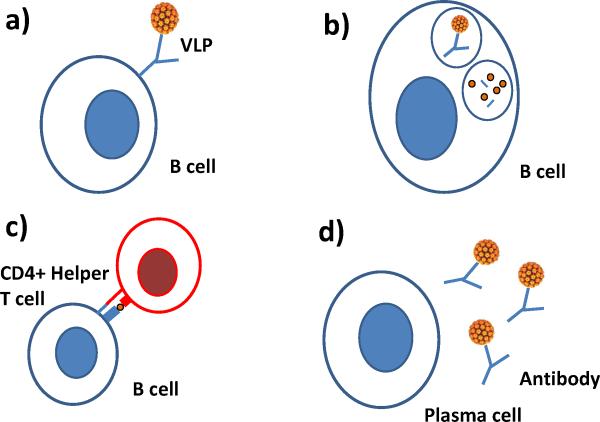
Figure 3. Mechanism of Preventative Vaccines in Inducing Humoral Immune Responses After Vaccination.
The quadrivalent and bivalent HPV vaccines consist of the L1 capsid protein which self-assembles into virus-like particles (VLP). The humoral immune system is activated by the A) recognition of the virus-like particles (VLP) by a B cell surface immunoglobulin. B) The VLP is internalized and degraded into peptides, which C) are then presented to CD4+ Helper T cells via MHC class II molecules. D) An activated B cell then proliferates and differentiates into an antibody-secreting plasma cell. These circulating antibodies then recognize and bind HPV to prevent viral infection of an epithelial cell.[78]
Quadrivalent VLP vaccine for girls and women
Large scale trials to test the efficacy of this vaccination strategy were carried out in the early 2000s, and have led to the approval of two vaccines for the prevention of HPV-related diseases and cancers. A quadrivalent (HPV types 6/11/16/18) VLP vaccine was approved in June 2006 for administration to women aged 9 to 26. Randomized, double-blinded placebo controlled trials evaluated the ability of the quadrivalent vaccine to prevent HPV-related anogenital diseases in women [44, 45]: genital warts, vulvar, vaginal, cervical neoplasia (Future I); also: high-grade cervical intraepithelial neoplasia (FUTURE II). The trials demonstrated 100% protection against the development of anogenital lesions related to the four viral types. As a result, the Centers for Disease Control (CDC) Advisory Committee on Immunization Practices (ACIP) in 2007 recommended vaccination for all girls ages 11 to 12, before the age of sexual debut and the risk of exposure to HPV.
Bivalent VLP vaccine for girls and women
A second prophylactic bivalent (HPV types 16/18) VLP vaccine was approved in October of 2009 for use in women aged 10 to 25. Although this vaccine does not include HPV types 6 and 11, a head-to-head clinical trial demonstrated that the bivalent vaccine induced higher antibody titers against the high risk viral types as compared to the quadrivalent vaccine [46]. Both of these vaccines are given as three doses administered over a 6-month period. The length of protection against HPV infection achieved through these vaccines is currently being studied in order to determine whether booster vaccinations would be required to maintain immune protection against HPV.
Quadrivalent vaccine for boys and men
In October of 2009, the quadrivalent vaccine was approved for males aged 9 to 26. A randomized, placebo-controlled double-blinded trial reported on the safety of the quadrivalent vaccine and on its efficacy in preventing the development of HPV-related external anogenital lesions in boys and men [47]. The study enrolled 4,065 healthy boys and men between 16 to 26 years of age across 18 countries. There was a per-protocol population in which subjects were documented to be negative for exposure to the HPV types at the time of enrollment and these subjected received all three vaccinations. The intention-to-treat population included subjects whose baseline HPV exposure history was unknown and these subjects received either the vaccine or placebo. The study reported that the quadrivalent vaccine efficacy was 90.4% against the development of genital lesions related to the HPV-6, 11, 16, or 18 viral types in HPV naïve patients and the efficacy dropped to 65.5% in patients with an unknown HPV exposure history. Based on these study results, in October 2011, the CDC ACIP recommended that boys aged 11 to 12 receive the vaccine. These studies demonstrate that there is benefit to vaccinating both boys and girls. However, the benefits of the HPV vaccine will not be appreciated for several decades, and it is unclear how the vaccine will impact oral mucosal immunity or oral HPV infection and, thus, the development of HPV-related head and neck cancers.
Implementation of Vaccination and the Elimination of Population Risk
In order to eradicate HPV, a herd immunization strategy would need to be implemented. However, even with strong governmental recommendations for HPV vaccination, there are many sociologic and logistical factors that make this goal challenging. A nationwide survey of 13- to 17-year old girls by the Centers for Disease Control and Prevention in 2010 found that less than half (48.7%) had received at least one dose of the three-part HPV vaccination series and, of the teenagers who commenced the vaccination series, 30% did not complete the series [48]. There are several reasons that are believed to account for the low vaccination rates, including some parent's misconception that giving the vaccine promotes sexual activity, associated high vaccine costs ($130 for the series) as well as delivery costs, lack of insurance coverage for vaccination, the requirement for obtaining three doses of vaccine over a 6-month period, inability to reach out to this target population since it is one not previously served by immunization programs in the past, and/or not receiving any routine primary preventive care.
As increasing numbers of boys and girls receive the vaccine, the overall prevalence of HPV and its associated diseases in the population should decrease, indirectly benefiting those who may not have been vaccinated. Using disease modeling of the 14 high-risk HPV types, it is estimated that if 50% of women are vaccinated against HPV-16/18, there would be a 47% reduction in cervical cancer, with 25% of the prevented cases occurring in women who never received the vaccine [49]. With over 20 million Americans currently infected with HPV in the United States, and an estimated 6 million new infections to occur each year [50], vaccination programs when implemented have great potential to reduce the burden of HPV disease and to prevent its related cancers.
As stated previously, the impact of current prophylactic vaccines on HPV-associated oropharyngeal cancer is unknown. As vaccination programs decrease the overall prevalence of HPV infection in the population, the trends of increasing oropharyngeal cancer [51] may show signs of slowing or even reversing. However, none of the prophylactic vaccine studies performed thus far have evaluated oral HPV infection or oral immunity to HPV, so it remains an open question regarding its impact on the incidence of HPV-associated oropharyngeal cancer.
Immunotherapy for the Treatment of Established HPV-associated Disease
The vaccination strategies discussed have been focused to prevent virus infection of epithelial cells through the generation of antibody responses that recognize the viral capsid proteins. However, this strategy is not effective for treating existing infections or established HPV-related diseases. Treatment of established disease requires activation of the cellular immune system, both CD4+ and CD8+ T cells, which can recognize and eliminate virus-infected cells. The differences between preventative and therapeutic vaccine strategies are outlined in Table 1.
Table I.
Differences between Prophylactic and Therapeutic Vaccines
| Prophylactic Vaccine | Therapeutic Vaccine | |
|---|---|---|
| Prevent new infection | Yes | No |
| Treat existing infection | No | Yes |
| Effector Cells | B cells | CD4+ T cells, CD8+ T cells |
| Humoral (Antibody) Response | High | Low |
| Cell Mediated (T-cell) Response | Low | High |
| Vaccine construction | Whole organism – live / attenuated / killed; Protein subunits | DNA plasmid; Peptides/proteins; Dendritic cell |
| Targeted antigen | Cell surface viral capsid protein | Intracellular viral protein |
Immunotherapy for the Treatment of Cancer
In developing therapeutic vaccination strategies, the identification of tumor-specific antigens determines the specificity of the targeted immune response. The HPV E6 and E7 proteins represent such model tumor antigens for several reasons. These oncoproteins are foreign viral proteins and, thus, are more immunogenic as compared to a self protein which may be mutated in the cancer cell, such as p53, or aberrantly upregulated, such as Mage-A3. The proteins are encoded by the viral genome and are uniquely expressed by all virus infected cell and, thus, every virus-related cancer cell. Since the E6 and E7 oncoproteins are required for the induction and maintenance of the malignant phenotype, these proteins are constitutively expressed within the cancer cells and it is unlikely the cancer cell would downregulate expression of these proteins in order to evade an immunologic response.
Therapeutic Vaccines for HPV
One group developed a therapeutic HPV vaccine which consisted of synthetic long peptides which spanned both the HPV-16 E6 and E7 proteins. A phase II clinical trial was completed in 20 women with HPV-16 associated grade III vulvar intraepithelial neoplasia (VIN) [52]. The patients were vaccinated with these overlapping peptides every 3 weeks for a total for four vaccinations. Biopsies were performed at 3 and 12 months after the last vaccination. All patients mounted vaccine-induced immune responses and clinical responses correlated with the induction of HPV-16 specific CD8+ T cells. The majority of the immune responses were specific to the HPV-16 E6 protein. A complete response was observed in 25% (5/20) of patients 3 months after the last vaccination, and this increased to a 47% (9/19) complete response rate 12 months after the last vaccination. This study demonstrated for the first time that, with vaccination, complete response rates could be achieved for established HPV disease.
In addition to peptide and protein based vaccines, several groups have evaluated DNA vaccines which allow for continued, high levels of target gene expression in transfected cells and, thus, sustained immunologic responses. HPV DNA vaccines have been evaluated in clinical trials for HPV-associated diseases such as cervical intraepithelial neoplasia (CIN) [53] and also are being evaluated in HPV-related head and neck cancers in an ongoing clinical trial [54].
Strategies to Enhance Therapeutic Vaccines
One of the challenges with immunotherapy and therapeutic vaccination is generating a robust and relevant T cell response specific to the antigen of interest. Therefore, various strategies for increasing the immunogenicity of vaccines have been evaluated in the development of DNA vaccines. For example:
The target antigen can be linked to chaperone proteins which target the tumor antigen to cellular pathways which enhances its presentation to the immune system [55].
The DNA vaccine can also be administered using electroporation, or via gene gun, rather than traditional intramuscular needle injection, which can significantly enhance the levels of antigen expression within the cell and/or increase the transfection rate of dendritic cells within the skin milieu [20].
Combining vaccination with chemotherapeutic agents can also enhance the immune response by inducing tumor cell apoptosis and inflammation that broadens tumor antigen presentation to the immune system [56].
All of these strategies to enhance the efficacy of HPV DNA vaccines in addition to others are currently under investigation in the treatment of HPV-associated head and neck cancers [54].
Other Molecular Targets for the Treatment of HPV-associated Disease
Although the E6 and E7 oncoproteins are the most common immunotherapeutic targets in HPV-associated cancers, the virus alters other cellular pathways which can be targeted by non-immunologic methods.
Epidermal growth factor receptor (EGFR) is highly expressed in a large percentage of head and neck cancers [57] and a monoclonal antibody against the receptor (Cetuximab) has been found to have clinical efficacy in head and neck cancer [58]. The HPV-16 viral protein E5 in particular has effects on EGFR trafficking in the cell, and enhances EGFR pathway activation [59, 60]. Increased EGFR expression is also seen in recurrent respiratory papillomatosis, a disease caused by HPV types 6 and 11 [61, 62]. There is an ongoing clinical trial for HPV-associated oropharyngeal cancer using induction chemotherapy followed by cetuximab with either reduced or standard dose radiation [63]. The goal of the study is to determine if comparable outcomes for HPV-associated cancer can be achieved with less intensive radiation by targeting EGFR. However, the interaction between these risk factors is not completely understood, as HPV positivity portends a good outcome in oropharyngeal cancer [64] whereas EGFR expression is an independent risk factor for poor prognosis in head and neck cancer [65].
Vascular endothelial growth factor (VEGF) has been implicated in many tumors as a promoter of tumor angiogenesis. There is evidence that HPV-16 E6 and E7 can upregulate VEGF expression [66, 67], and that E5 increases VEGF expression through the EGFR pathway [68]. This finding has relevance because a commercially available inhibitor of VEGF (bevacizumab) is on the market, although there have been no clinical studies to date to evaluate its use in HPV-associated oropharyngeal cancer.
Finally, there are two ongoing clinical trials evaluating Celebrex (celecoxib) to treat HPV-associated disease – cervical intraepithelial neoplasia [69] and recurrent respiratory papillomatosis (RRP) [70]. These studies are based on the observation that the COX-2 enzyme is over-expressed in HPV-related precancerous cervical lesions [71] as well as in RRP [72]. These studies highlight the fact that targeted therapy against HPV-associated oropharyngeal cancers need not be limited to the viral oncoproteins themselves but also to downstream cellular pathways which may be altered based on viral protein expression.
Challenges and Future Directions of Immunotherapy for HPV-associated Cancers
Although targeted immunotherapeutic strategies have great promise in the treatment of HPV-associated disease, significant challenges remain. The role of regulatory T cells in contributing to a local immunosuppressive microenvironment and in suppressing cytotoxic T cell function in cancers is increasingly being appreciated in the field [73]. The function of these regulatory T cells is to modulate activated T cell function to prevent autoimmunity. However, in the setting of cancer immunotherapy, the regulatory T cells can dampen the desired vaccine induced T cell responses. Both HPV-related tumors and other cancers have populations of regulatory (FOXP3+) T cells and the presence of this T cell population can predict lack of clinical response to therapeutic vaccination against HPV-16 [74]. Therefore, groups have evaluated the combination of chemotherapeutic agents, such as cyclophosphamide, and cancer vaccines to eliminate the regulatory T cell population in order to enhance vaccine specific immune responses [75].
In addition, antigen-induced activation and proliferation of T cells are regulated by the temporal expression and binding of both co-stimulatory and co-inhibitory receptors. The orchestrated signaling through these receptors in adaptive cellular immunity modulates the initiation, escalation and subsequent resolution of host immune responses. In the absence of co-inhibitory signaling, persistent T cell activation can lead to excessive tissue damage in the setting of infection as well as autoimmunity. In the context of cancer immunology, in which immune responses are directed against antigens specifically or selectively expressed by cancer cells, these immune checkpoints can represent major obstacles to overcoming tumor-specific tolerance and generating clinically meaningful tumor control. Therefore, efforts have been made in the clinical arena to investigate blockade of immune checkpoints as novel therapeutic approaches to cancer.
Two such co-inhibitory T cell receptors which are the focus of intense current interest are:
CTLA-4
programmed cell death-1 (PD-1)
Ipilimumab, a monoclonal antibody (mAb) that blocks CTLA-4, was evaluated in patients with advanced metastatic melanoma in a randomized phase III clinical trial and demonstrated a survival benefit; however, it was associated with significant immune-related toxicities [76]. In a phase I clinical trial, a blocking mAb against PD-1 (MDX-1106) was evaluated in patients with advanced metastatic melanoma, colorectal cancer, castrate-resistant prostate cancer, non-small-cell lung cancer, and renal cell carcinoma. Blocking the PD-1 immune checkpoint seemed to be better tolerated than CTLA-4 blockade and, although the study was primarily a safety study, clinical activity was observed in all of the evaluated histologies except for prostate cancer [77]. Furthermore, tumor cell surface, or “membranous” expression of the major PD-1 ligand PD-L1 (also termed B7–H1) appeared to correlate with the likelihood of response to therapy. Future studies need to focus on characterizing the gene signatures of tumor immune infiltrates, to identify the factors responsible for inducing local immunosuppression within the tumor microenvironment.
Conclusions
HPV is a ubiquitous virus that causes a wide range of human diseases, including a growing subset of oropharyngeal carcinomas. Significant progress has been made within the past decade in designing and implementing preventative vaccination programs against HPV. However, there are a variety of societal and economic challenges in implementing these vaccination programs and the burden of HPV and its related cancers will continue until these challenges are addressed. Therefore, therapeutic vaccines need to be developed to treat those individuals already infected and/or diagnosed with established HPV-related disease. However, a different set of challenges are uncovered with therapeutic vaccines based on the biology of these tumors. As the field of cancer immunology matures, the obstacles to achieving successful immunotherapy are being revealed and strategies are being applied to address these barriers. The unharnessed potential of immunotherapy in treating virus-related cancers undoubtedly will be realized in the near future.
Synopsis.
Human papillomaviruses (HPV) are a ubiquitous family of DNA viruses. The lifetime exposure risk for HPV infection is high; however, the majority of immune competent individuals are able to clear the infection naturally within 2 years. In men, HPV type 16 (HPV-16) has been identified as one of the viral types that is cleared from the body most slowly. HPV-16 causes over 90% of HPV-related head and neck cancers in the United States and is diagnosed more often in men than in women. Given the viral etiology of HPV-related cancers, vaccination programs to prevent infection have been implemented worldwide. However, it is unclear how these prophylactic vaccines will impact oral mucosal immunity and, thus, the development of HPV-related oropharyngeal cancers. This article outlines the biology of HPV infection of human mucosa and the cellular pathways that are altered through viral infection. The human biologic and immunologic responses to HPV infection are discussed, and questions are raised as to why some patients are able to clear the viral infection while others develop persistent infection and subsequently associated cancers. This lays out the conceptual framework with which to understand the two major immunologic strategies to address HPV-related diseases: 1) prevention of primary HPV infection through the use of prophylactic vaccines and 2) treatment of established infection and diseases through therapeutic vaccines. Non-immunologic therapy that targets cellular dysregulation induced by HPV infection will also be discussed. Finally, the challenges that exist in actualizing these conceptually attractive therapies on both a societal and biologic level will be examined.
Key Points
HPV-related cancers result from the failure of the immune system to recognize and eliminate virus infected cells.
The lifetime exposure risk of HPV infection is high, yet the majority of individuals are able to clear the infection within 2 years.
HPV-16 is one of the viral types to be cleared the slowest in men and is responsible for over 90% of HPV-related head and neck cancers in the United States.
Established HPV infections are naturally cleared through T cell mediated immune responses (CD4+ and CD8+ T cells) rather than humoral antibody responses.
Therapeutic DNA vaccines induce T cell responses against HPV-infected cells, and, therefore, may play an important role in treating patients with established HPV-related diseases.
Abbreviations: Biology of HPV Infection
- CIN
Cervical intraepithelial neoplasia
- CTL
Cytotoxic T lymphocyte
- IFN
Interferon
- IRF
Interferon Regulatory Factor
- mAb
Monoclonal antibody
- MHC
Major histocompatibility complex
- VLP
Viral like proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunne EF, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 377(9769):932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castle PE, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 4.Munoz N, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, et al. Prevalence of Oral HPV Infection in the United States, 2009–2010. JAMA. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Souza G, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263–9. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Souza G, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121(1):143–50. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 8.Buck CB, et al. Arrangement of L2 within the papillomavirus capsid. J Virol. 2008;82(11):5190–7. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck CB, et al. Maturation of papillomavirus capsids. J Virol. 2005;79(5):2839–46. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kines RC, et al. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106(48):20458–63. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peitsaro P, Johansson B, Syrjanen S. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J Clin Microbiol. 2002;40(3):886–91. doi: 10.1128/JCM.40.3.886-891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazo PA. The molecular genetics of cervical carcinoma. Br J Cancer. 1999;80(12):2008–18. doi: 10.1038/sj.bjc.6690635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pett M, Coleman N. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J Pathol. 2007;212(4):356–67. doi: 10.1002/path.2192. [DOI] [PubMed] [Google Scholar]
- 14.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86(2):104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 15.Howley PM. Role of the human papillomaviruses in human cancer. Cancer Res. 1991;51(18 Suppl):5019s–5022s. [PubMed] [Google Scholar]
- 16.Bourgault Villada I, et al. Spontaneous regression of grade 3 vulvar intraepithelial neoplasia associated with human papillomavirus-16-specific CD4(+) and CD8(+) T-cell responses. Cancer Res. 2004;64(23):8761–6. doi: 10.1158/0008-5472.CAN-04-2455. [DOI] [PubMed] [Google Scholar]
- 17.Paternoster DM, et al. Human papilloma virus infection and cervical intraepithelial neoplasia in transplanted patients. Transplant Proc. 2008;40(6):1877–80. doi: 10.1016/j.transproceed.2008.05.074. [DOI] [PubMed] [Google Scholar]
- 18.Fruchter RG, et al. Multiple recurrences of cervical intraepithelial neoplasia in women with the human immunodeficiency virus. Obstet Gynecol. 1996;87(3):338–44. doi: 10.1016/0029-7844(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 19.Moscicki AB, et al. Persistence of human papillomavirus infection in HIV-infected and - uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190(1):37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 20.Best SR, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27(40):5450–9. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng WF, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108(5):669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384(2):410–4. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Carter JJ, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 24.Carter JJ, et al. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61(5):1934–40. [PubMed] [Google Scholar]
- 25.Jochmus-Kudielka I, et al. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst. 1989;81(22):1698–704. doi: 10.1093/jnci/81.22.1698. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KS, et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br J Cancer. 104(12):1896–905. doi: 10.1038/bjc.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68(12):8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welters MJ, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63(3):636–41. [PubMed] [Google Scholar]
- 29.de Jong A, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64(15):5449–55. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa M, et al. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175(4):927–31. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 31.Hemminki K, Chen B. Familial risks for cervical tumors in full and half siblings: etiologic apportioning. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1413–4. doi: 10.1158/1055-9965.EPI-05-0933. [DOI] [PubMed] [Google Scholar]
- 32.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2(1):59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 33.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Woodworth CD. HPV innate immunity. Front Biosci. 2002;7:d2058–71. doi: 10.2741/A898. [DOI] [PubMed] [Google Scholar]
- 35.Ashrafi GH, et al. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113(2):276–83. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 36.Hasan UA, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178(5):3186–97. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 37.Huang SM, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J Virol. 2002;76(17):8710–21. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, et al. Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-gamma production in human peripheral blood mononuclear and NK cells. J Immunol. 2001;167(1):497–504. doi: 10.4049/jimmunol.167.1.497. [DOI] [PubMed] [Google Scholar]
- 39.Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999;259(2):305–13. doi: 10.1006/viro.1999.9771. [DOI] [PubMed] [Google Scholar]
- 40.Li S, et al. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18(42):5727–37. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- 41.Nees M, et al. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J Virol. 2001;75(9):4283–96. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, et al. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185(1):251–7. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 43.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116(5):1167–73. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garland SM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 45.I F. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 46.GlaxoSmithKline Clinical Trial NCT00423046. cited 2012; Available from: http://clinicaltrials.gov/ct2/show/NCT00423046.
- 47.Giuliano AR, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 364(5):401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CDC National and State Vaccination Coverage Among Adolescents Aged 13 Through 17 Years --- United States, 2010. Morb Mortal Wekly Rep. 2011;117(60):1117–1123. [PubMed] [Google Scholar]
- 49.Bogaards JA, et al. Long-term impact of human papillomavirus vaccination on infection rates, cervical abnormalities, and cancer incidence. Epidemiology. 22(4):505–15. doi: 10.1097/EDE.0b013e31821d107b. [DOI] [PubMed] [Google Scholar]
- 50.CDC Genital HPV Infection - Fact Sheet. 2011 Available from: http://www.cdc.gov/std/hpv/stdfact-hpv.htm.
- 51.Chaturvedi AK, et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J Clin Oncol. doi: 10.1200/JCO.22.02625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 53.Trimble CL, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15(1):361–7. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Center SKCC. Clinical Trial NCT01493154 - Safety Study of HPV DNA Vaccine to Treat Head and Neck Cancer Patients. Available from: http://clinicaltrials.gov/ct2/show/NCT01493154. [Google Scholar]
- 55.Cheng WF, et al. Characterization of DNA vaccines encoding the domains of calreticulin for their ability to elicit tumor-specific immunity and antiangiogenesis. Vaccine. 2005;23(29):3864–74. doi: 10.1016/j.vaccine.2004.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng CW, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14(10):3185–92. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modjtahedi H. Molecular therapy of head and neck cancer. Cancer Metastasis Rev. 2005;24(1):129–46. doi: 10.1007/s10555-005-5052-4. [DOI] [PubMed] [Google Scholar]
- 58.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 59.Pim D, Collins M, Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992;7(1):27–32. [PubMed] [Google Scholar]
- 60.Crusius K, et al. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp Cell Res. 1998;241(1):76–83. doi: 10.1006/excr.1998.4024. [DOI] [PubMed] [Google Scholar]
- 61.Lyford-Pike S, et al. Differential expression of epidermal growth factor receptor in juvenile and adult-onset recurrent respiratory papillomatosis. Histopathology. 57(5):768–70. doi: 10.1111/j.1365-2559.2010.03691.x. [DOI] [PubMed] [Google Scholar]
- 62.Johnston D, et al. Elevation of the epidermal growth factor receptor and dependent signaling in human papillomavirus-infected laryngeal papillomas. Cancer Res. 1999;59(4):968–74. [PubMed] [Google Scholar]
- 63.NCI Clinical Trial NCT01084083 - Paclitaxel, Cisplatin, and Cetuximab Followed By Cetuximab and Intensity-Modulated Radiation Therapy in Treating Patients With HPV-Associated Stage III or Stage IV Cancer of the Oropharynx That Can Be Removed By Surgery. Available from: http://clinicaltrials.gov/ct2/show/NCT01084083.
- 64.Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong A, et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer. 46(11):2088–96. doi: 10.1016/j.ejca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 66.Lopez-Ocejo O, et al. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19(40):4611–20. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 67.Walker J, et al. Expression of human papillomavirus type 16 E7 is sufficient to significantly increase expression of angiogenic factors but is not sufficient to induce endothelial cell migration. Virology. 410(2):283–90. doi: 10.1016/j.virol.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SH, et al. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ ERK1,2 and PI3K/Akt. Cell Mol Life Sci. 2006;63(7–8):930–8. doi: 10.1007/s00018-005-5561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NCI Clinical Trial NCT00081263 - Celecoxib in Treating Patients With Cervical Intraepithelial Neoplasia. Available from: http://clinicaltrials.gov/ct2/show/NCT00081263.
- 70.System NSLIJH. Clinical Trial NCT00571701 - Study of Celebrex (Celecoxib) in Patients With Recurrent Respiratory Papillomatosis. Available from: http://clinicaltrials.gov/ct2/show/NCT00571701.
- 71.Saldivar JS, et al. COX-2 overexpression as a biomarker of early cervical carcinogenesis: a pilot study. Gynecol Oncol. 2007;107(1 Suppl 1):S155–62. doi: 10.1016/j.ygyno.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 72.Wu R, et al. Up-regulation of Rac1 by epidermal growth factor mediates COX-2 expression in recurrent respiratory papillomas. Mol Med. 2007;13(3–4):143–50. doi: 10.2119/2007-00005.Wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loddenkemper C, et al. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci. 2009;100(6):1112–7. doi: 10.1111/j.1349-7006.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Welters MJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 107(26):11895–9. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emens LA, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27(35):5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janeway C. Immunobiology. 5th ed. Garland Publishing; New York: 2001. [Google Scholar]