Abstract
Reduced levels of trienoic fatty acids (TAs) in chloroplast membranes induce thermotolerance in several plant species, but the underlying mechanisms remain unclear. TA peroxidation in plant cell membranes generates cytotoxic, TA-derived compounds containing α,β-unsaturated carbonyl groups. The relationship between low TA levels and the amounts of cytotoxic TA-derived compounds was examined using thermotolerant transgenic cyclamen (Cyclamen persicum Mill.) with low TA contents. Changes in the levels of the cytotoxic TA-derived acrolein (ACR), methyl vinyl ketone (MVK), (E)-2-hexenal, 4-hydroxy-2-nonenal, and malondialdehyde were analysed in the leaf tissues of wild-type (WT) and thermotolerant transgenic cyclamen under heat stress. Levels of ACR and MVK in the WT increased in parallel with the occurrence of heat-induced tissue damage, whereas no such changes were observed in the thermotolerant transgenic lines. Furthermore, exogenous ACR and MVK infiltrated into leaves to concentrations similar to those observed in heat-stressed WT leaves caused similar disease symptoms. These results suggest that thermotolerance in transgenic cyclamen depends on reduced production rates of ACR and MVK under heat stress, due to the low level of TAs in these plants.
Keywords: Acrolein, Cyclamen persicum Mill., methyl vinyl ketone, thermotolerance, trienoic fatty acids
Introduction
Global warming may greatly affect plant ecosystems and agricultural production. In addition to droughts and other indirect effects, higher temperatures may have direct negative effects on productivity and crop quality. Active research on responses to global warming is proceeding (Iba, 2002; Sharkey, 2005; Sharkey and Zhang, 2010). One approach focuses on the fact that heat tolerance can be increased by reducing the trienoic fatty acid (TA) content of chloroplast membranes via suppression of the ω-3 fatty acid desaturase (FAD; Murakami et al., 2000; Falcone et al., 2004; Liu et al., 2006; Wang et al., 2006). However, the mechanism by which reduced TA levels lead to increased thermotolerance has not been clarified.
In vivo, TA peroxidation via enzymatic and non-enzymatic pathways generates a wide variety of metabolites (Esterbauer et al., 1991; Rustérucci et al., 1999; Alméras et al., 2003). Some of these metabolites contain α,β-unsaturated carbonyl groups and are strongly cytotoxic (Esterbauer et al., 1991; Vollenweider et al., 2000; Alméras et al., 2003; Mano et al., 2009). It was hypothesized that the reduced TA content may lead to decreases in the production of cytotoxic compounds in thermotolerant transgenic plants. Among the products of TA peroxidation, acrolein (ACR), methyl vinyl ketone (MVK), and 4-hydroxy-2-nonenal (HNE) contribute to significant reductions in photosystem II fluorescence (Alméras et al., 2003). ACR, (E)-2-hexenal, and HNE inhibit photosynthesis and inactivate multiple enzymes in the Calvin cycle (Mano et al., 2009). Furthermore, ACR and MVK activate defence-related genes that are associated with cell damage. In addition to ACR and MVK, malondialdehyde (MDA) can also act as a powerful activator of defence-related genes, and prolonged exposure to these compounds results in damage to leaf tissues (Alméras et al., 2003).
For these reasons, the potential contribution of ACR, MVK, (E)-2-hexenal, HNE, and MDA to the acquisition of thermotolerance in higher plants was studied. In this study, the induction of thermotolerance in cyclamen (Cyclamen persicum Mill.), which develops leaf damage under heat conditions (>38 °C), was demonstrated. This commercially important plant was employed rather than one of the standard experimental models because, due to global warming, cultivars derived from C. persicum may be difficult to produce commercially in the future, while wild cyclamen species will face an increased risk of extinction (Yesson and Culham, 2006). Furthermore, the changes in the amounts of ACR, MVK, (E)-2-hexenal, HNE, and MDA in leaf tissues of wild-type (WT) and thermotolerant transgenic cyclamen with low TA content under heat stress were examined.
Materials and methods
Plant materials and growth conditions
The fixed diploid cyclamen (C. persicum Mill.) cultivar ‘Victoria’ was employed as the WT to produce transgenic lines. The tetraploid ‘Victoria’ cyclamen cultivar was used to extract genomic DNA and identify C. persicum FAD7 (CpFAD7). These lines were obtained from the Kage Shinkoen Nursery (Japan). Commercial cultivars were obtained from Morel Diffusion S.A.S, Goldsmith, Varinova, Schoneveld Twello B.V., and Syngenta Seeds. Seeds were sterilized and sown on half-strength Murashige and Skoog (MS) medium without growth regulators in plant boxes (Magenta vessel GA-7; Sigma-Aldrich, USA), and kept in the dark at 20 °C until germination. Then, seedlings in plant boxes were grown at 20 °C under 16 h light conditions (70 μmol m−2 s−1) until utilization. To evaluate drought stress in seedlings and heat stress in mature plants, seedlings were transferred to horticultural soil (ZEN-NOH, Japan Agricultural Cooperatives, Japan) and grown under otherwise identical conditions.
Determination of the genomic CpFAD7 sequence
Genomic DNA was extracted from leaves (200 mg) using a modified cetyltrimethylammonium bromide (CTAB) method. To identify CpFAD7, the degenerate primers, iF1 (5′-ACDCAYCAYCARAACCAYGG-3′) and iR2 (5′-CTCCAYKCCTYKCCDCKRTACCA-3′) derived from the sequence of FAD7 in Arabidopsis thaliana were used. The sequence of CpFAD7 was submitted to the DDBJ/EMBL/GenBank databases (accession number AB250917).
Production of transgenic cyclamen with low TA content by RNAi
To reduce the TA contents in cyclamen plants, RNA interference (RNAi) using an RNAi vector containing parts of the CpFAD7 sequence was employed. A fragment containing the seventh exon and part of the fourth intron of CpFAD7 was isolated from genomic DNA using the primers Xba-Ex7S (5′-CCCTCTAGAGAATGGAGTTATTTGCGAGGA-3′; XbaI site underlined), and Ex7AS-4thint5′ (5′-GAGTAATAGGCTCACTGCTTCAATTAAGT-3′). The resulting amplified fragment served as a template and was amplified again using Xba-Ex7S and intron-EcoRIAS (5′-TTAGAATTCGGAAAACTAAAATGTTAACAAACTTGTGGATCAATTCAGCAAGGGGATGGAGTAATAGGCTCAC-3′; EcoRI site underlined). This XbaI–seventh exon–fourth intron–EcoRI fragment was digested with XbaI and EcoRI (TaKaRa, Japan), and ligated into the pGEM-7Zf (+) vector (Promega, USA). Similarly, a SacI–seventh exon antisense–fourth intron–EcoRI fragment was amplified and subcloned into pGEM-7Zf (+) using Sac-Ex7S (5′-GGGGAGCTCGAATGGAGTTATTTGCGAGG-3′; SacI site underlined), Ex7AS-4thint3′ (5′-CTGTTTATTCCTCATGCTTCAATTAAGTGA-3′), and EcoRI-intron (5′-TTCCGAATTCTAATGATTTTTTACGTTTTTTCTGTTTATTCCTCAG-3′; EcoRI site underlined). Finally, an XbaI–seventh exon–fourth intron–seventh exon antisense–SacI fragment was constructed by ligation at the EcoRI site, and then inserted into the XbaI–SacI site of the binary Ti vector pBI121. For more effective gene expression and antibiotic selection in cyclamen, the Cauliflower mosaic virus (CaMV) 35S promoter and the NPTII gene in the pBI121 vectors were replaced with the soybean Cucumber mosiac virus (CMV) non-coding region promoter and HPT, respectively. The resulting RNAi vector was introduced into the Agrobacterium tumefaciens strain EHA105 which was then used to transform C. persicum diploid ‘Victoria’ using etiolated petiole explants (Aida et al., 1999). Cyclamen seedlings were grown in the dark to obtain the etiolated petioles for transformation. Sixty days after sowing without subculture, etiolated petioles were cut into 1 cm segments and used as explants to introduce the constructed vector. The explants were incubated in the EHA105 suspension for 30 min, then transferred to solidified MS co-culture medium containing 1.0 mg l−1 thidiazuron, 0.1 mg l−1 2,4-dichlorophenoxyacetic acid, and 100 μM acetosyringone. The explants and bacteria were co-cultured in the dark at 20 °C for 7 d, then the explants were transferred to solidified MS selective medium containing 10 mg l−1 hygromycin and 10 mg l−1 meropenem. The explants were incubated in the dark for 60 d with subculturing every 2 weeks, then the hygromycin-resistant calli were transferred to half-strength MS solidified medium without growth regulators, and incubated at 20 °C under 16 h light (70 μmol m−2 s−1). Transgenic plants were transferred to pots with horticultural soil and grown at 20 °C under 16 h light (70 μmol m−2 s−1) to the reproductive stage. To investigate thermotolerance, drought tolerance, and levels of TA-derived compounds in the transgenic plants, they were pollinated with pollen from WT plants. From the segregating offspring, individuals with low TA contents as established by gas chromatography were selected, and these plants, named T15 and T31, were used for further analyses.
Northern blot analysis
Total RNA was extracted from leaves, tubers, and roots of cyclamen; 10 μg samples were used for northern blot analysis. The RNA was separated on a denaturing 1% agarose gel and then transferred to a positively charged nylon membrane (Roche Applied Science, Germany) in 20× SSC. To synthesize the CpFAD7 probe, mRNA was purified from total leaf RNA using the Oligotex™-dT30 mRNA purification kit (TaKaRa, Japan). cDNA was synthesized using the SuperScript™ Choice System for cDNA Synthesis (Invitrogen, USA) with an oligo(dT)12–18 primer. The CpFAD7 cDNA was amplified using the primers 5′-GCCCCTCTCCAGAATCTACC-3′ and 5′-GGGATCTGAGGGAATAGATGG-3′, ligated into the pGEM-T vector (Promega, USA), and then a digoxigenin-labelled CpFAD7 probe was synthesized using the PCR DIG Probe Synthesis kit (Roche Applied Science, Germany).
Fatty acid analysis
The fatty acid compositions of leaf tissues were analysed by gas chromatography (Kodama et al., 1994). The analyses were conducted with three replications from three independent plants, using 4.0×4.0 cm leaf sections.
Heat and drought stress treatments
For heat stress treatments, plants on MS medium or in soil were exposed to 38 °C under constant light (70 μmol m−2 s−1), according to the method of Murakami et al. (2000), with some modifications. For statistical analysis of leaf damage, leaves with a few wilting or browning parts were counted as injured leaves, and seedlings with at least two injured leaves were scored as damaged seedlings according to Zhang et al. (2005). Each line was analysed using six individuals and three replications.
For evaluation of drought tolerance, seedlings were grown in soil for 6 months (to the 4–5 leaf stage) and irrigated every 3 d. Irrigation was then withheld for 18 d, after which irrigation was resumed. Drought tolerance was evaluated at 12 d after the beginning of the drought treatment as the ratio of withered leaves to all leaves. Each line was analysed using six individuals and three replications. The plants were photographed at the beginning of the experiment, on the 18th day of drought treatment, and 14 d after resuming irrigation.
Measurement of TA-derived compounds
TA-derived compounds were extracted from leaves, and derivatized (Deighton et al., 1999). Leaves detached from treated seedlings or detached leaves that had been infiltrated with ACR or MVK were analysed. The tissue sample (1 g) was homogenized in liquid nitrogen, and 5 ml of methanol containing 0.005% butylated hydroxytoluene were added. After centrifugation (10 min, 500 g, 4 °C), 3 ml of the supernatant were transferred to a new tube containing 5 ml of 350 mg l−1 2,4-dinitrophenylhydrazine (DNPH) in 1 M HCl. The reaction was incubated for 30 min at 20 °C. The mixture was extracted twice with 5 ml of dichloromethane, then dried at room temperature. The solid residue was dissolved in 400 μl of acetonitrile. ACR–DNPH was determined using an liquid chromatography–mass spectrometry (LC-MS) system (Grosjean et al., 1999). The molecular mass of the ACR–DNPH derivative was 234.9, as determined using a standard ACR (Sigma-Aldrich, USA). The amount of ACR in leaf samples was determined by comparison with the standard ACR. MVK–DNPH, (E)-2-hexenal–DNPH, and HNE–DNPH were assayed using reversed-phase high-performance liquid chromatography (HPLC) by measuring the absorbance at 360 nm, while MDA–DNPH was assayed by its absorbance at 307 nm (Korchazhkina et al., 2003). The retention times and amounts of MVK–DNPH, (E)-2-hexenal–DNPH, and HNE–DNPH were established using standard chemicals (Sigma-Aldrich), and those of MDA–DNPH were determined with standard MDA prepared from 1,1,3,3-tetramethoxypropane (Sigma-Aldrich). Each experiment was carried out with three replications using independent plants.
Effects of TA-derived compounds on infiltrated leaves
To investigate whether ACR and MVK in leaves contributed to damage resulting from heat stress, infiltration tests were conducted with ACR and MVK in WT leaves. Detached whole leaves (∼4.0×4.0 cm) of WT seedlings (4–5 leaf stage) were floated with their abaxial side on the surface of water (water-infiltrated) or on freshly prepared solutions of 5 ppm ACR or 50 ppm MVK in a sealed container at 20 °C under 16 h light (70 μmol m−2 s−1). The treatment periods were 3 d for ACR infiltration tests and 2 d for MVK infiltration tests. The infiltrated samples were washed three times with 1 litre of distilled water and then were analysed for their ACR and MVK contents as described above. Each experiment was carried out with three replications.
Results
Production of transgenic cyclamen with low TA content
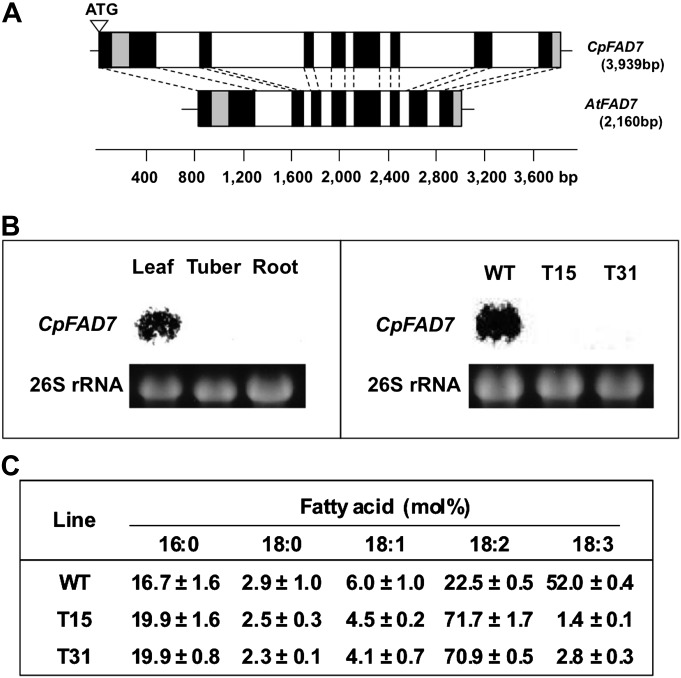
The cyclamen gene encoding the plastid-localized CpFAD7 enzyme (accession number AB250917) was identified using the degenerate primers based on the FAD7 gene sequence from A. thaliana (Iba et al., 1993) (Fig. 1A). The sequence of CpFAD7 included 3939 bp with a 1305 bp open reading frame encoding 435 amino acid residues. Its first exon showed high homology with the transit peptide region of FAD7 in A. thaliana. Northern blot analysis supported the idea that CpFAD7 encodes the plastid-located ω-3 FAD, since the expression of endogenous CpFAD7 mRNA was detected in leaves but not in tuber and root tissues which lack chloroplasts (Fig. 1B, left). Two transgenic cyclamen lines, T15 and T31, with decreased TAs were produced using RNAi vectors to inhibit the expression of CpFAD7. No endogenous CpFAD7 mRNA was detectable in leaves of these lines (Fig. 1B, right). The levels of 18:3 fatty acid, the only TA in cyclamen, were dramatically reduced in leaves of the transgenic plants, while 18:2 dienoic fatty acid (DA), showed a corresponding increase (Fig. 1C).
Fig. 1.
Production of transgenic lines with low TA contents. (A) Comparison of CpFAD7 and AtFAD7 gene structures. White and black boxes represent introns and exons, respectively. The grey boxes indicate regions of low homology between CpFAD7 and AtFAD7. (B) Expression of CpFAD7 in WT and transgenic lines. Northern blot analysis of CpFAD7 expression in WT leaves, tubers, and roots (left), and in leaves of WT, T15, and T31 plants (right). The blots were hybridized with a full-length CpFAD7 cDNA as probe. The denaturing RNA gels were stained with ethidium bromide, and 26S rRNA bands were used as loading controls. (C) Composition of fatty acids in leaves of WT and transgenic lines (T15 and T31). Values are means ±SD (n=3). Plants were grown to seedling stage (4–5 leaves) on half-strength MS medium at 20 °C under 16 h light (70 μmol m−2 s−1).
Thermotolerance in transgenic cyclamen with low TA content
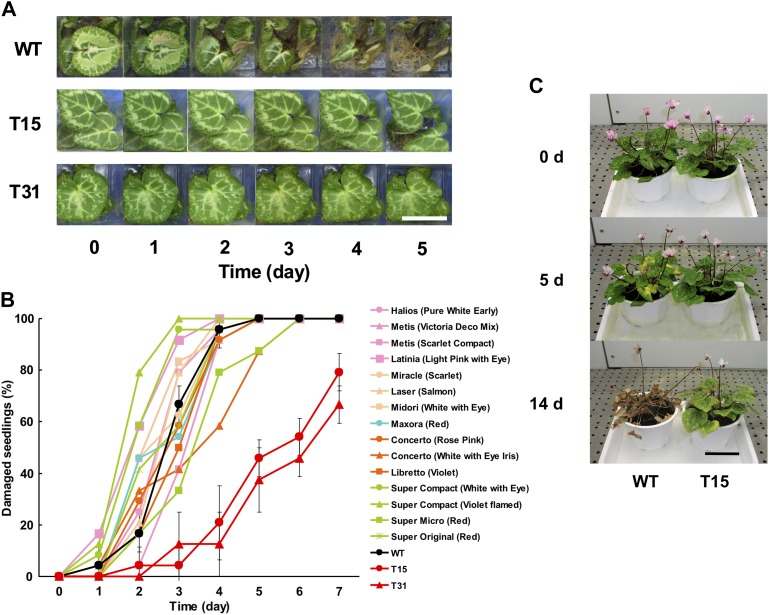
Conditions were sought which allowed for a reliable and reproductive evaluation of phenotypic signs of thermotolerance in cyclamen (Supplementary Fig. S1 available at JXB online). A treatment of 35 °C for 7 d led to quite variable results in terms of medium desiccation and leaf damage. On the other hand, a temperature of 41 °C resulted in severe browning symptoms on leaves after <2 d. Consequently, a moderately high temperature (38 °C) was applied which avoided significant medium desiccation for 5 d of treatment. At this temperature, some WT seedlings displayed symptoms of heat damage (wilting and browning of leaves) after 2 d, and all WT seedlings showed these symptoms after 5 d. The occurrence of such wilting and browning symptoms was significantly delayed in the two transgenic lines with decreased TA levels, T15 and T31 (Fig. 2A, B; Supplementary Table S1). Moreover, the thermotolerance of 15 commercial cultivars did not differ from that of the WT in spite of the varying TA contents (45.9–59.7 mol%) in these cultivars (Fig. 2B; Supplementary Tables S1, S2). These results demonstrated that the transgenic seedlings were more thermotolerant than the commercial cultivars and the WT. The increased thermotolerance of the T15 line was maintained at the flowering stage (Fig. 2C).
Fig. 2.
Thermotolerance of transgenic lines with low TA contents. Plants were grown to the 4–5 leaf stage on half-strength MS medium (A and B) or to the reproductive stage (20–25 leaves) on soil (C) at 20 °C under 16 h light (70 μmol m−2 s−1). (A) Development of heat damage symptoms in WT and transgenic seedlings under heat stress treatment (38 °C, constant light). The seedlings were photographed daily for 5 d. The bar indicates 5 cm. (B) Comparison of thermotolerance in seedlings of commercial cultivars, WT, and transgenic lines (pink, lines obtained from Morel Diffusion S.A.S.; pale orange, Goldsmith; blue, Varinova; orange, Syngenta Seeds; pale green, Schoneveld Twello B.V. black, WT; red, transgenic lines). Each line was analysed using six individuals and three replications. Leaves with wilting or browning parts were counted as injured, and seedlings with at least two injured leaves were scored as damaged. Values are means ±SD (n=3). (C) Comparison of thermotolerance in WT and T15 plants at the reproductive stage (20–25 leaves) under heat stress (38 °C, constant light). Scale bar, 10 cm.
Changes in TA-derived compounds in heat-stressed leaves
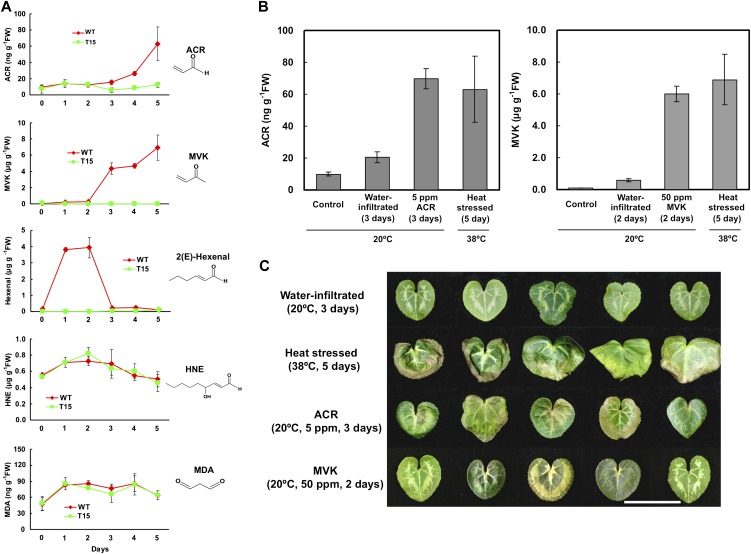
TA peroxidation via enzymatic and non-enzymatic pathways generates a wide variety of metabolites that contain toxic α,β-unsaturated carbonyl groups. Among these metabolites, ACR, MVK, (E)-2-hexenal, HNE, and MDA are known to inhibit photosynthesis, inactivate multiple enzymes in the Calvin cycle, and result in damage to leaf tissue following prolonged exposure. Changes in the levels of ACR, MVK, (E)-2-hexenal, HNE, and MDA in leaf tissues of WT and transgenic cyclamen under heat stress were evaluated (Fig. 3A). ACR levels remained similar in WT and T15 leaves for 2 d, but then increased in the WT while remaining low in T15 (Fig. 3A). MVK showed a similar trend, although its concentrations generally were two orders of magnitude higher than those of ACR (Fig. 3A). Evidently, the increases in ACR and MVK in the WT seedlings coincided with the development of heat damage in leaves (compare Fig. 2B). In contrast, (E)-2-hexenal levels were dramatically but transiently elevated after 1 d and 2 d in WT but not in T15 seedlings (Fig. 3A). The amounts of HNE and MDA remained fairly stable and similar in WT and T15 seedlings throughout the duration of the experiment (Fig. 3A). It was concluded that ACR and MVK were likely to be involved in the causation of leaf damage during the heat exposure.
Fig. 3.
Changes in the levels of TA-derived compounds during heat treatment, and leaf damage caused by these compounds. Plants were grown to the seedling stage (4–5 leaves) at 20 °C under 16 h light (70 μmol m−2 s−1). (A) Changes of candidate cytotoxic compounds in the WT and T15 line under heat stress (38 °C, constant light). ACR, acrolein; MVK, methyl vinyl ketone; HNE, 4-hydroxy-2-nonenal; MDA, malondialdehyde. Values are means ±SD (n=3). (B) Amounts of ACR and MVK in control, water-infiltrated, heat-stressed (38 °C, 5 d, constant light), and ACR-/MVK-infiltrated leaves excised from WT seedlings. The infiltrated leaves were treated with 5 ppm ACR for 3 d (ACR) or 50 ppm MVK for 2 d (MVK) at 20 °C under 16 h light. Values shown are means ±SD (n=3). (C) Damage symptoms displayed by excised WT leaves after heat stress treatment or infiltration with water (water-infiltrated), ACR, or MVK. The treatment conditions were as described for B. Scale bar, 5 cm.
Effects of exogenous ACR and MVK on leaves
The above findings suggested that ACR and MVK were involved in heat stress-induced leaf damage. To test this idea, detached WT leaves were infiltrated with ACR and MVK. Under normal growth conditions, the amounts of ACR and MVK in WT leaves (Control) were 9.9 ng g−1 FW and 0.1 μg g−1 FW, respectively (Fig. 3B). In contrast, WT leaves showed wilting or browning at least over parts of the leaf area under heat stress treatment, and ACR and MVK accumulated to 63.0 ng g−1 FW and 6.9 μg g−1 FW, respectively (Fig. 3B). Infiltration conditions that resulted in similar levels of ACR and MVK were identified. For ACR, the optimum infiltration conditions were 5 ppm ACR for 3 d, resulting in 69.7 ng g−1 FW (Fig. 3B, left). For MVK, the optimum conditions were 50 ppm MVK for 2 d, resulting in 6.0 μg g−1 FW (Fig. 3B, right). On the other hand, the amounts of ACR and MVK in water-infiltrated leaves reached 20.5 ng g−1 FW after 3 d of treatment, and 0.6 μg g−1 FW after 2 d of treatment, respectively (Fig. 3B). Under these conditions, ACR- and MVK-infiltrated leaves developed browning and wilting symptoms that resembled those of heat-stressed leaves, but water-infiltrated leaves did not after 3 d of treatment (Fig. 3C). These results suggested that the enhanced amounts of ACR and MVK in leaves under heat stress could be causing the browning and wilting symptoms.
Drought tolerance in transgenic cyclamen
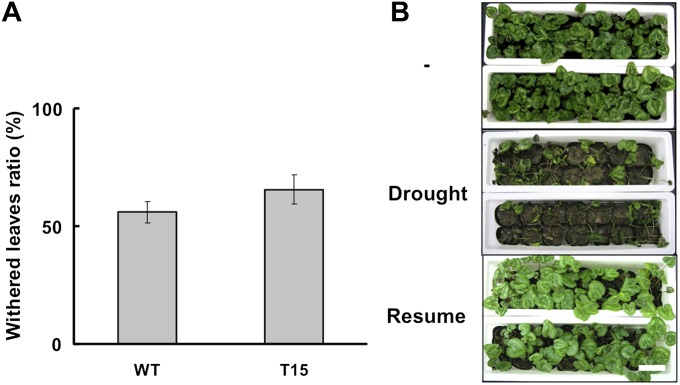
Decreases in TAs of chloroplast membranes seemed to correlate with reduced drought tolerance in tobacco (Im et al., 2002). To see whether this also is true for cyclamen, irrigation of WT and T15 plants was stopped for 12 d. As a consequence, slightly more than half of all leaves showed signs of withering, but the results for the two lines did not differ significantly (Fig. 4A). Furthermore, when irrigation of the plants was resumed after 18 d of drought stress, both WT and T15 plants recovered similarly (Fig. 4B). These results suggested that transgenic cyclamen plants with decreased TA contents did not suffer from reduced drought tolerance.
Fig. 4.
Evaluation of drought tolerance in the WT and T15 line. After initial growth for 6 months in soil at 20 °C under 16 h light (70 μmol m−2 s−1), plants with 4–5 leaves were evaluated for drought tolerance. (A) The statistical evaluation of drought tolerance was conducted after withholding irrigation for 12 d. The numbers of withered leaves were counted and expressed as the percentage of all leaves. The values are means ±SD (n=3). (B) WT (upper container) and T15 (lower container) seedlings initially were irrigated every 3 d for 6 months (–). Irrigation was withheld for 18 d (Drought), and then resumed for 14 d (Resume). Scale bar, 10 cm.
Discussion
Thermotolerance of transgenic cyclamen with low TA content
To reveal the relationship between low TA levels and thermotolerance in higher plants, transgenic cyclamen with decreased TA content were produced and the amounts of cytotoxic TA-derived compounds under heat stress were monitored. The suppression of the activity of CpFAD7 resulted in a reduction of TA from ∼50 mol% to <3 mol%, and a corresponding increase of DA from 20 mol% to 70 mol% (Fig. 1). Thus, the DA/TA ratio changed dramatically, from <0.5 in the WT to 25 in T31 and to ∼50 in T15. In tobacco, two transgenic lines with decreased TA content in leaf tissue produced by gene silencing of AtFAD7 showed DA/TA ratios of 1.5 and 2.2, compared with ∼0.1 in the WT (Murakami et al., 2000). Similarly, the DA/TA ratio in two transgenic tomato lines with low TA contents that had been generated through antisense LeFAD7 and the WT were 2.8, 1.2, and 0.5, respectively (Liu et al., 2006). The significantly higher DA/TA ratios that were found in this study in transgenic cyclamen as compared with other species may be due to peculiarities of the lipid biosynthesis pathway in cyclamen and/or a particularly efficient suppression of endogenous CpFAD7. In the A. thaliana fad3fad7fad8 mutant which lacks plastidial TAs altogether, a reduction of photosynthetic activity and retardation of growth under heat stress have been observed. The growth inhibition was ascribed to a deficiency of TA-derived jasmonates and their derivatives (Routaboul and Browse, 2002; Wallis and Browse, 2002). In contrast, transgenic cyclamen containing 1.4–2.8 mol% of TA may still be able to produce sufficient essential TA derivatives to survive under heat stress and acquire thermotolerance. Furthermore, TA-derived jasmonates contribute to plant responses to abiotic and biotic stresses (McConn et al., 1997; Tuteja and Sopory, 2008) as well as anther development and pollen maturation (McConn and Browse, 1996). In the present study, transgenic cyclamen produced viable pollen and grew normally under appropriate conditions. It would be interesting to determine changes in jasmonate levels in response to biotic and abiotic stresses in transgenic cyclamen with low TA contents.
On the other hand, heat and drought stress responses are closely related in higher plants. In a previous study, decreased TA levels in chloroplast membranes seemed to induce thermotolerance but reduced drought tolerance (Im et al., 2002). In the present study, the performance of transgenic cyclamen in a drought stress test resembled that of the WT.
Changes in TA-derived compounds under heat stress
TA is the substrate for the synthesis of various compounds including fatty acid hydroperoxides, fatty acid ketodienes, 4-hydroxynonenal, smaller aldehydes, and numerous other oxygenated fatty acid derivatives (Esterbauer et al., 1991; Rustérucci et al., 1999; Howe and Schilmiller, 2002; Alméras et al., 2003). These compounds are produced by enzymatic and non-enzymatic oxygenation in diseased, wounded, and stressed plant tissues, and some of these substances appear to be involved in stress responses (Deighton et al., 1999; Imbusch and Mueller, 2000; Vollenweider et al., 2000; Jalloul et al., 2002; Weber et al., 2004). On the other hand, compounds containing α,β-unsaturated carbonyl groups may induce programmed cell death, and may function as genotoxic agents because of their potential to form Michael adducts. Among these compounds, the focus of the present study was on ACR, MVK, (E)-2-hexenal, HNE, and MDA because their cytotoxicity has been characterized in previous studies. Increasing accumulation of ACR and MVK was found in WT plants from 3 d after the start of the heat treatment, whereas the concentration in T15 plants remained at the basal level. Since these changes correlated with the occurrence of heat stress symptoms, a role for ACR and MVK in the development of heat stress-induced leaf damage was implied. On the other hand, the amount of (E)-2-hexenal increased transiently in WT but not in T15 plants, from day 1 to 3. Moreover, to reveal the possible involvement in thermotolerance of the transiently increasing (E)-2-hexenal, heat stress treatment (38 °C) was applied for 2 d, and leaf damage at day 7 after the end of the treatment was subsequently evaluated. Heat-inducible damage did not appear in the leaves (data not shown). These results did not suggest an involvement of (E)-2-hexenal in the thermotolerance of cyclamen with low TA content. Though (E)-2-hexenal was reported as a damaging factor in plant tissues (Mano et al., 2009), it did not affect leaves in cyclamen under the present experimental conditions. The amounts of HNE in the WT and T15 increased similarly to those of (E)-2-hexenal up to day 2 of the heat treatment, and then returned to basal levels. The similarity of patterns in WT and T15 argued against an involvement of HNE in the thermotolerance observed in T15. HNE is produced mainly from DA in Capsicum annuum (Deighton et al. 1999), but the biosynthesis pathway for HNE may differ in cyclamen. Increased MDA levels in WT and T15 were maintained from day 1 throughout the duration of the experiment, suggesting that MDA plays no role in T15 thermotolerance. Large amounts of MDA originate from TAs in A. thaliana, and changes in the amount and localization of free MDA strongly affected abiotic stress-related gene transcription (Muckenschnabel et al., 2002; Weber et al., 2004). In cyclamen, MDA may be derived from unsaturated fatty acids other than 18:3. Alternatively, the 18:3 remaining in transgenic cyclamen may be sufficient for generating MDA. While it is known that MDA can strongly affect abiotic stress-related gene expression, an involvement of MDA in heat stress effects has not been demonstrated.
Effects of ACR and MVK applied to leaves
ACR and MVK levels were unaffected by heat stress until day 3 in WT and T15 plants. Afterwards, the levels of both substances increased in the WT but not in T15. In leaves infiltrated with exogenous ACR and MVK, distinctive necrotic areas appeared that resembled the damage seen in WT leaves after heat stress for 5 d. The amount of ACR in ACR-infiltrated leaves, the amount of MVK in MVK-infiltrated leaves, and the amounts of ACR and MVK in heat-stressed leaves were comparable. ACR generally causes lesions on leaf surfaces (Haagen-Smit et al., 1952; Darley et al., 1960). Furthermore, ACR and MVK are powerful cytotoxic compounds affecting photosystem II (Alméras et al., 2003; Mano et al., 2009). These studies suggested that a variety of TA-derived compounds, including ACR and MVK, may be generated under heat stress, and that some of these compounds may bring about the heat damage observed. These results may explain why transgenic plants with suppressed FAD7 expression and reduced TA content show reduced levels of cell death under heat stress. Moreover, an assay for TA-derived cytotoxic compounds such as ACR or MVK in leaves may prove to be useful for the evaluation of thermotolerance in plants. With such a tool it would be possible to produce thermotolerant plants with suppressed expression of plastid-localized FAD, and reduced TA content, by mutagenesis.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Establishment of suitable conditions for the evaluation of thermotolerance in cyclamen.
Table S1. Thermotolerance of cyclamen cultivars, WT, and transgenic lines.
Table S2. Compositions of fatty acids in leaf tissues of various cyclamen cultivars.
Acknowledgments
We thank Dr Thomas D. Sharkey for his critical review of the manuscript, and Dr Akihiko Ito, Mrs Yu Shimizu, and Michio Tajima for helpful discussions. We also thank Mr Tetsuro Kage and the companies Morel Diffusion S.A.S., Goldsmith, Varinova, Schoneveld Twello B.V., and Syngenta Seeds for supplying cyclamen seeds. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (no. 21114002) from the Ministry of Education, Science and Culture of Japan, and by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN).
References
- Aida R, Hirose Y, Kishimoto S, Shibata M. Agrobacterium tumefaciens-mediated transformation of Cyclamen persicum Mill. Plant Science. 1999;148:1–7. [Google Scholar]
- Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, Farmer EE. Reactive electrophile species activate defense gene expression in Arabidopsis . The Plant Journal. 2003;34:205–216. doi: 10.1046/j.1365-313x.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- Darley EF, Middleton JT, Garber MJ. Plant damage and eye irritation from ozone–hydrocarbon reactions. Journal of Agricultural and Food Chemistry. 1960;8:483–485. [Google Scholar]
- Deighton N, Muckenschnabel I, Goodman BA, Williamson B. Lipid peroxidation and the oxidative burst associated with infection of Capsicum annuum by Botrytis cinerea . The Plant Journal. 1999;20:485–492. doi: 10.1046/j.1365-313x.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radical Biology and Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biology. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean E, Green PG, Grosjean D. Liquid chlomatography analysis of carbonyl (2,4-Dinitrophenyl) hydrazones with detection by diode array ultraviolet spectroscopy and by atmospheric pressure negative chemical ionization mass spectrometry. Analytical Chemistry. 1999;71:1851–1861. doi: 10.1021/ac981022v. [DOI] [PubMed] [Google Scholar]
- Haagen-Smit AJ, Darley EF, Zaitlin M, Hull H, Noble W. Investigation on injury to plants from air pollution in the Los Angeles area. Plant Physiology. 1952;27:18–34. doi: 10.1104/pp.27.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Current Opinion in Plant Biology. 2002;5:230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Iba K, Gibson S, Nishiuchi T, Fuse T, Nishimura M, Arondel V, Hugly S, Somerville CR. A gene encoding a chloroplast ω-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana . Journal of Biological Chemistry. 1993;268:24099–24105. [PubMed] [Google Scholar]
- Im YJ, Han O, Chung GC, Cho BH. Antisense expression of an Arabidopsis ω-3 fatty acid desaturase gene reduces salt/drought tolerance in transgenic tobacco plants. Molecules and Cells. 2002;13:264–271. [PubMed] [Google Scholar]
- Imbusch R, Mueller MJ. Analysis of oxidative stress and wound-inducible dinor isoprostanes F1 (Phytoprostanes F1) in plants. Plant Physiology. 2000;124:1293–1303. doi: 10.1104/pp.124.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalloul A, Montillet JL, Assigbetsé K, Agnel JP, Delannoy E, Triantaphylidès C, Daniel JF, Marmey P, Geiger JP, Nicole M. Lipid peroxidation in cotton: Xanthomonas interactions and the role of lipoxygenases during the hypersensitive reaction. The Plant Journal. 2002;32:1–12. doi: 10.1046/j.1365-313x.2002.01393.x. [DOI] [PubMed] [Google Scholar]
- Kodama H, Hamada T, Horiguchi G, Nishimura M, Iba K. Genetic enhancement of cold tolerance by expression of a gene for chloroplast ω-3 fatty acid desaturase in transgenic tobacco. Plant Physiology. 1994;105:601–605. doi: 10.1104/pp.105.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchazhkina O, Exley C, Spencer SA. Measurement by reversed-phase high-performance liquid chromatography of malondialdehyde in normal human urine following derivatisation with 2,4-dinitrophenylhydrazine. Journal of Chromatography B. 2003;794:353–362. doi: 10.1016/s1570-0232(03)00495-1. [DOI] [PubMed] [Google Scholar]
- Liu XY, Yang JH, Li B, Yang XM, Meng QW. Antisense-mediated depletion of tomato chloroplast omega-3 fatty acid desaturase enhances thermal tolerance. Journal of Integrative Plant Biology. 2006;48:1096–1107. doi: 10.1111/j.1744-7909.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- Mano J, Miyatake F, Hiraoka E, Tamoi M. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta. 2009;230:639–648. doi: 10.1007/s00425-009-0964-9. [DOI] [PubMed] [Google Scholar]
- McConn M, Browse J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. The Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis . Proceedings of the National Academy of Sciences, USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenschnabel I, Goodman BA, Williamson B, Lyon GD, Deighton N. Infection of leaves of Arabidopsis thaliana by Botrytis cinerea: changes in ascorbic acid, free radicals and lipid peroxidation products. Journal of Experimental Botany. 2002;53:207–214. doi: 10.1093/jexbot/53.367.207. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K. Trienoic fatty acids and plant tolerance of high temperature. Science. 2000;287:476–479. doi: 10.1126/science.287.5452.476. [DOI] [PubMed] [Google Scholar]
- Routaboul JM, Browse J. Trienoic fatty acids and plant tolerance of temperature. OCL-OLEAGINEUX CORPS GRAS LIPIDES. 2002;9:43–47. [Google Scholar]
- Rustérucci C, Montillet JL, Agnel JP, et al. Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. Journal of Biological Chemistry. 1999;274:36446–36455. doi: 10.1074/jbc.274.51.36446. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell and Environment. 2005;28:269–277. [Google Scholar]
- Sharkey TD, Zhang R. High temperature effects on electron and proton circuits of photosynthesis. Journal of Integrative Plant Biology. 2010;52:712–722. doi: 10.1111/j.1744-7909.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Sopory SK. Chemical signaling under abiotic stress environment in plants. Plant Signaling and Behavior. 2008;3:525–536. doi: 10.4161/psb.3.8.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider S, Weber H, Stolz S, Chételat A, Farmer EE. Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. The Plant Journal. 2000;24:467–476. doi: 10.1046/j.1365-313x.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- Wallis JG, Browse J. Mutants of Arabidopsis reveal many roles for membrane lipids. Progress in Lipid Research. 2002;41:254–278. doi: 10.1016/s0163-7827(01)00027-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Ming F, Pittman J, Han Y, Hu J, Guo B, Shen D. Characterization of a rice (Oryza sativa L.) gene encoding a temperature-dependent chloroplast ω-3 fatty acid desaturase. Biochemical and Biophysical Research Communications. 2006;340:1209–1216. doi: 10.1016/j.bbrc.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Weber H, Chételat A, Reymond P, Farmer EE. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. The Plant Journal. 2004;37:877–888. doi: 10.1111/j.1365-313x.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- Yesson C, Culham A. A phyloclimatic study of Cyclamen . BMC Evolutionary Biology. 2006;6:72. doi: 10.1186/1471-2148-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Barg R, Yin M, Gueta-Dahan Y, Leikin-Frenkel A, Salts Y, Shabtai S, Ben-Hayyim G. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. The Plant Journal. 2005;44:361–371. doi: 10.1111/j.1365-313X.2005.02536.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.