Abstract
Trees will have to cope with increasing levels of CO2 and ozone in the atmosphere. The purpose of this work was to assess whether the lignification process could be altered in the wood of poplars under elevated CO2 and/or ozone. Young poplars were exposed either to charcoal-filtered air (control), to elevated CO2 (800 μl l−1), to ozone (200 nl l−1) or to a combination of elevated CO2 and ozone in controlled chambers. Lignification was analysed at different levels: biosynthesis pathway activities (enzyme and transcript), lignin content, and capacity to incorporate new assimilates by using 13C labelling. Elevated CO2 and ozone had opposite effects on many parameters (growth, biomass, cambial activity, wood cell wall thickness) except on lignin content which was increased by elevated CO2 and/or ozone. However, this increased lignification was due to different response mechanisms. Under elevated CO2, carbon supply to the stem and effective lignin synthesis were enhanced, leading to increased lignin content, although there was a reduction in the level of some enzyme and transcript involved in the lignin pathway. Ozone treatment induced a reduction in carbon supply and effective lignin synthesis as well as transcripts from all steps of the lignin pathway and some corresponding enzyme activities. However, lignin content was increased under ozone probably due to variations in other major components of the cell wall. Both mechanisms seemed to coexist under combined treatment and resulted in a high increase in lignin content.
Keywords: 13C labelling, elevated CO2, lignin, ozone, poplar, wood
Introduction
Climate change has been attributed to greenhouse gas emissions due to human activities since the industrial era. CO2 and tropospheric ozone are two of the most important greenhouse gases in our atmosphere that are predicted to continue increasing during this century (IPCC, 2007).
CO2 is the first greenhouse gas of anthropogenic origin involved in global warming. The concentration of CO2 has increased from 280 μl l−1 before the industrial era to 380 μl l−1 at the present time and could double in 2100 according to IPCC scenarios (IPCC, 2007). The effects of elevated CO2 on forest trees have been studied extensively. Usually, an increase in growth and biomass has been observed in tree species (Ainsworth and Long, 2005). Stimulation of growth and biomass is often due to higher carbon assimilation (Curtis and Wang, 1998; Ainsworth and Rogers, 2007).
The average concentration of ozone in the troposphere before the industrial era was about 10 nl l−1. Since the intensification of industrial activities and transport in the 20th century, the ozone content in the atmosphere has reached 20 nl l−1 to 45 nl l−1 in different regions of the world (Vingarzan, 2004). Models predict an average ozone concentration of up to 80 nl l−1 in 2100 (IPCC, 2007). The increase in ground-level ozone can be accompanied by episodes of ozone peaks, sometimes over 200 nl l-1. Tropospheric ozone, as well as being a greenhouse gas, is now considered to be the most important air pollutant affecting vegetation (Karnosky et al., 2007). Ozone affects tree growth and biomass (Wittig et al., 2009) by decreasing photosynthesis and stomatal conductance (Wittig et al., 2007).
The opposite effects of elevated CO2 and ozone as single factors on plant growth and biomass have often been investigated, whereas their combined effects are less well documented. Previous studies showed that elevated CO2 compensated the negative effects of ozone (Isebrands et al., 2001; Karnosky, 2003; Riikonen et al., 2004; King et al., 2005). The protection provided by CO2 enrichment could result from the stimulation of photosynthesis, which would support the detoxification process (Gillespie et al., 2011). However, other results reported negative effects of the interaction between the two gases (Wustman et al., 2001). The beneficial effect of high CO2 is dependent on species, soil fertility, and forest age (Karnosky et al., 2003).
Wood is of primary importance in various industries such as paper manufacture and construction or as a renewal energy source for biofuel (Carroll and Somerville, 2009). Changes in tree growth and biomass resulting from modifications of atmospheric composition, could affect biofuel yields from lignocellulosic biomass. Besides the amount of biomass produced, wood composition is important in bioenergy production. The saccharification process used in biofuel production, is strongly inhibited by the presence of lignins (Weng et al., 2008). A current challenge is thus to reduce significantly the presence of lignins in the lignocellulosic biomass or to remove lignin by physical treatment with acid or steam (Hamelinck et al., 2005). Lignins are the second most abundant component of wood after cellulose and account for 17–30% of the cell wall. Lignins provide the rigidity and structural support that allow water transport in the vascular system. These polymers are mostly derived from three hydroxycinnamyl alcohol monomer precursors (monolignols) which differ in their degree of methoxylation, namely p-coumaryl, coniferyl, and sinapyl alcohols (Boerjan et al., 2003). When incorporated into the lignin polymer, these monolignols produce p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units, respectively. However, lignin content and composition vary between species, cell types, and cell wall layers, and may be influenced by developmental and environmental factors (Campbell and Sederoff, 1996).
Although wood composition is important for the production of bioenergy, our knowledge of the effects of elevated CO2 and ozone on lignification is still limited. Most studies addressed lignin content and various responses were reported. Elevated CO2 decreased lignin content in beech (Blaschke et al., 2002), birch (Kostiainen et al., 2006), and Scots pine (Overdieck and Fenselau, 2009). Lignin content was increased in poplar subjected to elevated CO2 (Luo et al., 2008; Luo and Polle, 2009). Other studies showed that lignin content remained unchanged under elevated CO2 (Runion et al., 1999; Atwell et al., 2003; Kilpelainen et al., 2003; Kaakinen et al., 2004; Kostiainen et al., 2008, 2009). The results varied greatly depending on the species and experimental devices used. In particular, soil nitrogen content seemed to affect the response of lignification to elevated CO2 (Luo and Polle, 2009). Tropospheric ozone increased lignin content (Kaakinen et al., 2004) in trembling aspen and silver birch after exposure for three years, but this was no longer true after five years (Kostiainen et al., 2008). Increased lignin content was found in young poplars subjected to ozone (Richet et al., 2011). Finally, the combined effects of elevated CO2 and ozone on wood composition are not well documented. The increase in lignin concentration in response to ozone in aspen and birch was counteracted by elevated CO2 (Kaakinen et al., 2004), but this was no longer observed at the same site two years later (Kostiainen et al., 2008).
Most studies were performed in free air experiments which have certain ecological relevance (Matyssek et al., 2010). Nevertheless, they provide limited information about the underlying mechanisms because of interactions with other stresses (biotic or abiotic) or specific site conditions. Indeed, very few reports have dealt with the response of lignin metabolism. One study performed in controlled chambers showed that lignin biosynthesis activities were decreased by ozone in poplar whereas the lignin content increased in the cell wall (Richet et al., 2011). This increase was explained by a strong decrease in cellulose content, the major component of wood.
The purpose of this work was to determine the effect of elevated CO2 and/or ozone on the lignification process and carbon allocation to the wood. Young poplars (Populus tremula×alba clone INRA 717-1-B4) were grown for 60 d under different conditions, charcoal-filtered air (control), elevated CO2 (800 μl l−1), ozone (200 nl l−1) or a combination of elevated CO2 and ozone. The experiment was performed in controlled chambers in order to control various parameters (light intensity, photoperiod, humidity, gas concentration, and soil fertility). Previous work had demonstrated that upright poplars (Populus tremula×alba clone INRA 717-1-B4) growing in controlled chambers produced tension wood (TW) which was not distributed in any set pattern (Richet et al., 2011). The trees were therefore bent to limit TW formation to the upper side of the stem and normal or opposite wood (OW) to the lower side. Since these two kinds of wood have different lignin contents, their responses were analysed separately. The activities and transcript levels of enzymes of lignin metabolism were monitored in both woods for each culture condition. Lignin content was also analysed. Finally, 13C labelling was used to measure carbon allocation of new assimilates to wood and lignin.
Materials and methods
Plant material and growth conditions
Plants of hybrid poplar (Populus tremula×alba, clone INRA 717-1-B4) obtained by micropropagation were potted in 5.0 l containers filled with compost (N/P/K 14/16/18, 1.2 kg m−3, Gramoflor SP1 Universel) and 20 g of slow release 13:13:13 N:P:K fertilizer (Nutricot T 100, Fertil, Boulogne-Billancourt, France). Plants were grown in controlled chambers under the following climatic conditions: 14 h light period, PAR (photosynthetically active radiation) 250–300 μmol m−2 s−1 supplied by high pressure sodium vapour lamps (SON-T AGRO, 400W, Philips, Eindhoven, The Netherlands), relative humidity 75/85% (day/night) and temperature 22/18 °C (day/night). The young tree shoots were artificially stacked and tilted at 42° from the vertical in order to limit TW formation to the upper side of the stem (Richet et al., 2011).
Elevated CO2 and ozone treatments
Elevated CO2 and ozone fumigation experiments were performed in the controlled chambers used for plant acclimation under the same climatic conditions as described before. The young trees (seven fully expanded leaves, 3 months old) were exposed either to charcoal-filtered air (control), to elevated CO2 (800 μl l−1) (24 h d−1), to ozone fumigation (200±20 nl l−1) during the light period or to a combination of elevated CO2 and ozone. Eight chambers were used, that is two chambers per condition. The fumigation experiment ran for 60 d. Additional CO2 was provided directly by CO2 gas cylinder (CO2 B50, >99.5%; AIR LIQUIDE, SA, Paris, France). Ozone was generated by electrical discharge (CMG3-3; Innovatec II, Rheinbach, Germany) using pure oxygen. Air from all controlled chambers was continuously monitored for CO2 by infrared spectrometer (Beryl 100 Cosma®, Cosma®, Igny, France) and for ozone by UV analyser (O341M, Environment SA, Paris, France).
Growth and biomass measurements
The time–course of growth was monitored throughout the experiment. Growth (height and diameter) was measured on six plants per treatment (randomly selected in two chambers per treatment). Radial growth was measured 10 cm above the root collar with a Vernier calliper. At the end of the experiment (day 60), six plants per treatment were harvested and dried at 60 °C for shoot (leaves and stems) biomass analysis. The number of leaves lost during the experiment was recorded.
Leaf gas-exchange
Net CO2 assimilation (μmol CO2 m−2 s−1) and stomatal conductance to water vapour (g w, mol H2O m−2 s−1) were monitored using a portable gas-exchange system Li-6200 (Li-Cor, Inc., Lincoln, NE, USA) after 35 d of fumigation as described in Bagard et al. (2008). Measurements were performed inside the fumigation chambers on the first fully developed leaf 2 h after the beginning of the light period at 22 °C, 75% of relative air humidity, and under 200±20 μmol PAR m−2 s−1. Measurements were performed on four trees randomly selected in two chambers per treatment.
Cell wall thickness and cambial activity
Three poplars were randomly selected in two chambers per condition after 60 d of treatment for measurements of cell wall thickness and cambial activity. Samples for cell wall thickness were saturated in distilled water. Images were obtained by an Environmental Scanning Electron Microscopy (ESEM) (FEI Quanta 200 ESEM). The wood samples in the ESEM chamber were kept at a pressure higher than 8 Torr (several purge cycles ensured that the gas in the chamber was mostly water vapour), to maintain full hydration of the cell walls. Cell wall thickness was measured on approximately 100 fibres per image and six images per plant (3 OW and 3 TW) using the custom software MeshPore (Perré, 2005).
Cambial activity was assessed as previously described by Richet et al. (2011). The number of cambium cell layers was recorded on three trees per treatment.
Enzyme extraction
Three trees were randomly selected in two chambers per condition for each enzyme activity measurement. Samples were harvested in the middle of the photoperiod, frozen in liquid nitrogen, and stored at –80 °C until analysis. Two stem levels, corresponding to two different developmental stages, were sampled. The lower stem was located 10 cm above the root collar and corresponded to wood which had developed before and during the exposure experiment. The middle stem had developed only during the fumigation experiment. The bark was removed from each sample and the opposite wood (OW) on the lower quarter side was kept separate from the tension wood (TW) on the upper quarter side. Frozen stems (about 300 mg) were ground in a mortar chilled with liquid nitrogen. Extracts were obtained from the powdered stems, as described by Cabané et al. (2004).
Enzyme activities
The time-course of enzyme activities involved in lignin biosynthesis was analysed in OW and TW from the lower and middle stems from each treatment. Shikimate dehydrogenase (SHDH) (EC 1.1.1.25), phenylalanine ammonia-lyase (PAL) (EC 4.3.1.5), and cinnamyl alcohol dehydrogenase (CAD) (EC 1.1.1.195) activities were assayed as previously described by Richet et al. (2011). Enzyme activities were reported as the mean value for three trees per time and per treatment. The protein content of the enzyme source was determined with Bio-Rad protein reagent dye (Bio-Rad) using bovine serum albumin as standard.
RNA extraction and cDNA synthesis
Three trees were randomly chosen in two chambers per treatment for RNA analysis. Samples (about 100 mg) of OW and TW from lower stem were placed in teflon jars chilled with liquid nitrogen and ground to a fine powder for 2 min using a mixer mill MM301 (Retsch, France). Total RNA was extracted with TRIzol (Invitrogen) following the manufacturer’s instructions, treated with DNAse I, Amp Grade (Invitrogen), and cleaned with RNeasy MinElute CleanUp columns (Qiagen). cDNA was generated from 1 μg of total RNA using the iScriptTM cDNA Synthesis Kit (Bio-Rad).
Quantitative real-time PCR
Quantitative real-time PCR reactions were prepared using iQTM SYBR® Green Supermix (Biorad) and performed in a MyiQ Single-Color Real-Time PCR Detection System ICycler (Biorad). Conditions for real-time PCR were as follows: denaturation by hot start at 95 °C for 3 min, followed by 40 cycles of a two-step programme consisting of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 45 s. Dissociation curves were verified for each reaction. The relative transcript abundance of genes involved in lignin synthesis was quantified. All genes from the phenylpropanoid and monolignol pathways showing high expression levels in poplar xylem were selected (Shi et al., 2010), namely, phenylalanine ammonia-lyase (PAL), PtrPAL 1/3, PtrPAL2, PtrPAL4, PtrPAL5; cinnamate-4-hydroxylase (C4H), PtrC4H1, PtrC4H2, 4-coumarate:CoA ligase (4CL), Ptr4CL3, Ptr4CL5; cinnamoyl-CoA reductase (CCR), PtrCCR2; cinnamyl alcohol dehydrogenase (CAD), PtrCAD1; p-hydroxycinnamoyl- CoA:quinate shikimate p-hydroxycinnamoyltransferase (HCT), PtrHCT1; 4-coumarate 3-hydroxylase (C3H), PtrC3H3; caffeoyl-CoA O-methyltransferase (CCoAOMT), PtrCCoAOMT1, PtrCCoAOMT2; coniferyl aldehyde 5-hydroxylase/ferulate 5-hydroxylase (CAld5H), PtrCAld5H1, PtrCAld5H2; caffeic acid/5-hydroxyconiferaldehyde O-methyltransferase (COMT), PtrCOMT2. Primers used for amplification are listed in Supplementary Table S1 at JXB online.
Critical thresholds (Ct) for all genes were quantified in triplicate and normalized with GeNorm software (Vandesompele et al., 2002). Four genes were used as endogenous controls: Ubiquitin-conjugated enzyme E2-17 kDa 10/12 (UBC10) (primers according to Richet et al. (2011), polyubiquitin (UBQ11), actin 2/7 (ACT2), and cyclophylin (CYP) (primers according to Brunner et al. (2004)). Transcript relative abundance was calculated as the mean of three biological replicates (three trees per condition) and three analytical replicates.
Lignin determination
Dry wood samples (300 mg) were ground and subjected to an exhaustive solvent extraction in a Soxhlet apparatus (toluene:ethanol, 1:1 v:v, then ethanol, and finally water) to obtain extractive-free samples namely cell wall residues. The lignin content was determined by the Klason method from extractive-free powder according to the standard procedure (Dence, 1992). The Klason lignin content was expressed as a percentage of cell wall residue (extractive-free material) or dry mass. All Klason analyses were run in duplicate (technical repeat) with three trees randomly chosen in two chambers per treatment. In addition, the acid-soluble lignin was determined according to the standard procedure and showed no differences between treatments.
13C labelling and sampling
After 35 d of the experiment, five hybrid poplars were randomly selected in two chambers per treatment and placed in a VTPH 5/1000 controlled environment chamber (Vötsch Industrietechnik GmbH, Reiskirchen-Lindenstruth, Germany) operated as a semi-closed system designed for 13C labelling (Maillard et al., 2001). Plants were then exposed for 4 h during the light period to 13C-enriched air (4.4 atom% 13C) with a CO2 concentration of 400 μl l−1 or 800 μl l−1. Climatic conditions (light intensity, humidity, and temperature) were similar to those of the controlled chambers during the exposure treatments. At the end of the labelling period, trees were replaced in the appropriate controlled chambers for 72 h and then harvested in the middle of the light period. The plant material was quickly frozen in liquid nitrogen, freeze-dried, weighed, and ground to a fine powder with a laboratory mill (Tecator, Cyclotec 1093, Höganäs, Sweden) for 13C analyses in wood and lignin. At the same time, two unlabelled plants from each treatment were harvested for measurement of natural 13C abundance.
13C analyses and calculations
The 13C/12C isotopic ratio of wood and lignin was measured with an elemental analyser (NA 1500 NCS, Carlo Erba, Milan, Italy) coupled to a Delta-S isotopic ratio mass spectrometer (Finnigan, Mat, Thermoquest Corp., San Jose, CA).
Carbon isotopic abundance (A C%) was computed for labelled (A C labelled%) and unlabelled (A C unlabelled%) plant components as:
Relative specific allocation of C (RSA C%) is defined as the proportion of newly incorporated C atoms relative to the total C atoms in the plant component:
with A C labelled atmosphere of 4.4%.
To calculate A C unlabelled atmosphere for each treatment, the time-integrated δ13C of air in the chambers was assessed by measuring δ of leaf dry matter of Zea mays L. according to Marino and McElroy (1991).
Statistical analysis
Student’s t test or the non-parametric Mann–Whitney U-test for independent samples, in accordance with the preliminary test for equality of variances (P <0.05), were used to test the trends between the replicate chambers for each treatment. No significant effect was observed for height and radial growth of trees in the replicate chambers. The experiments were then considered to be completely randomized designs for tree selection and sampling. An analysis of variance (ANOVA) followed by Fisher’s LSD test was used to reveal any significant differences (P <0.05 or P <0.1) between the control and other treatments. In all figures and tables, values are mean ±SE.
Results
Young poplars (Populus tremula×alba, clone INRA 717-1-B4) growing upright (staked or not) in controlled chambers developed TW without any defined pattern (Richet et al., 2011) so the trees were bent to limit the development of TW to the upper side of the stem and OW to the lower side. Young poplar trees were grown for 60 d in controlled chambers under four different conditions, control (filtered air), elevated CO2 (800 μl l−1), ozone (200 nl l−1), and a combination of elevated CO2 and ozone.
Growth and biomass
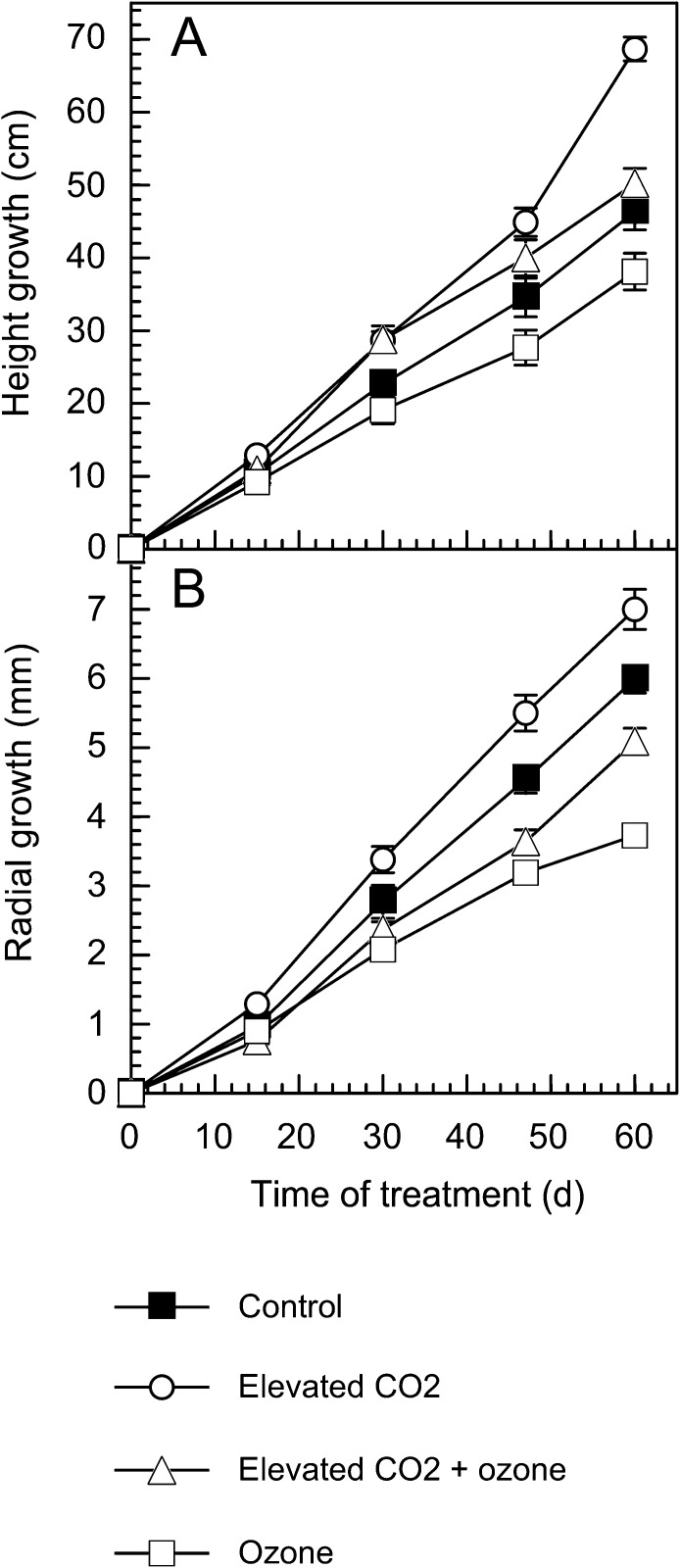
Growth (height and diameter) was increased under elevated CO2 treatment and decreased under ozone exposure (Fig. 1). The profiles of height growth for the elevated CO2/ozone combination and the control were similar and the profile of radial growth was intermediate between the control and ozone treatments.
Fig. 1.
Height (A) and radial (B) growth of poplars since the beginning of the treatment, i.e. height and diameter differences compared with the height and diameter at time zero. Trees were grown for 60 d under control (filtered air), ozone (200 nl l−1), elevated CO2 (800 μl l−1) or a combination of ozone and elevated CO2 conditions. Values are mean ±SE (n=6).
The elevated CO2 treatment enhanced the biomass of stem and leaves by about 20% without modifying leaf loss. Under ozone treatment, shoot biomass was reduced by 33% in the stem, up to 55% in the leaves, and leaf loss was dramatically increased (Table 1). The combined treatment induced the same effects in leaves (leaf biomass and leaf loss) as the ozone treatment but these were less pronounced. However, the reduction of stem biomass was similar to that observed with the ozone treatment.
Table 1.
Shoot biomass and leaf loss of hybrid poplar grown under different treatments for 60 d
| Treatment | Dry mass (g) | Leaf loss (% total leaves) | |
| Stem | Leaves | ||
| Control | 19.1±1.6 a | 17.5±1.5 a | 10.6±2.4 a |
| Elevated CO2 | 23.3±2.3 b | 21.9±1.6 b | 14.8±4.4 a |
| Elevated CO2 and ozone | 14.3±1.4 c | 14.1±1.1 c | 44.2±3.9 b |
| Ozone | 12.7±0.5 c | 8.0±1.0 d | 66.4±3.3 c |
Values are mean ±SE (n=6). Mean values not sharing the same letter are significantly different at P <0.05.
Gas exchange measurements
Net CO2 assimilation and stomatal conductance to water vapour (g w) were measured after 35 d of fumigation. Net CO2 assimilation was increased by 30% under elevated CO2 and decreased by 45% under ozone treatment (Table 2). However, net CO2 assimilation remained unchanged under the combined treatment. Stomatal conductance (g w) was decreased by 40–50% compared with the control, whatever the treatment (Table 2).
Table 2.
Net CO2 assimilation and stomatal conductance to water vapour (g w) expressed on a leaf area basis in hybrid poplar after 35 d of treatments
| Net CO2 assimilation (μmol CO2 m−2 s−1) | g w (mol H2O m−2 s−1) | |
| Control | 7.63±0.18 a | 0.073±0.010 a |
| Elevated CO2 | 10.03±1.02 b | 0.044±0.001 b |
| Elevated CO2 and ozone | 8.72±0.93 a,b | 0.038±0.007 b |
| Ozone | 4.19±0.05 c | 0.035±0.001 b |
Values are mean ±SE (n=4). Mean values not sharing a same letter are significantly different at P <0.05.
Wood anatomy parameters
Cambial activity (number of cell layers) and fibre cell wall thickness were measured at the end of exposure (day 60) in OW and TW of lower stems from each treatment (Table 3). Under elevated CO2, cambial activity was increased (1.4-fold) only in TW. The number of cell layers was reduced (2-fold) under ozone exposure compared with the control whatever the wood tissue. Cambial activity in the combined treatment remained unchanged compared with the control whatever the tissue.
Table 3.
Wood anatomy in lower stems of hybrid poplar grown for 60 d under different conditions
| Treatment | Cambium cell layers (number) | Fibre cell wall thickness (μm) | ||
| Opposite wood | Tension wood | Opposite wood | Tension wood | |
| Control | 6.14±0.25 a | 6.36±0.51 a | 3.59±0.16 a,b | 5.17±0.24 a |
| Elevated CO2 | 6.31±0.45 a | 8.56±0.80 b | 3.63±0.23 a | 5.35±0.10 a |
| Elevated CO2 and ozone | 5.48±0.28 a | 5.68±0.24 a | 2.99±0.22 b,c | 4.32±0.13 b |
| Ozone | 3.06±0.51 b | 3.38±0.32 c | 2.73±0.21 c | 3.74±0.10 c |
Values are mean ±SE (n=3 biological replicates). Mean values not sharing the same letter are significantly different at P <0.05.
Cell wall thickness was generally higher in TW than in OW (Table 3). Cell wall thickness of fibres from the stem of trees subjected to elevated CO2 remained unchanged whatever the wood tissue compared with the control. Ozone treatment reduced fibre cell wall thickness in both tissues compared with the control, the decrease being more pronounced in TW than in OW. After the combined treatment, cell wall thickness was not affected in OW but was reduced in TW.
Enzyme activities involved in lignification
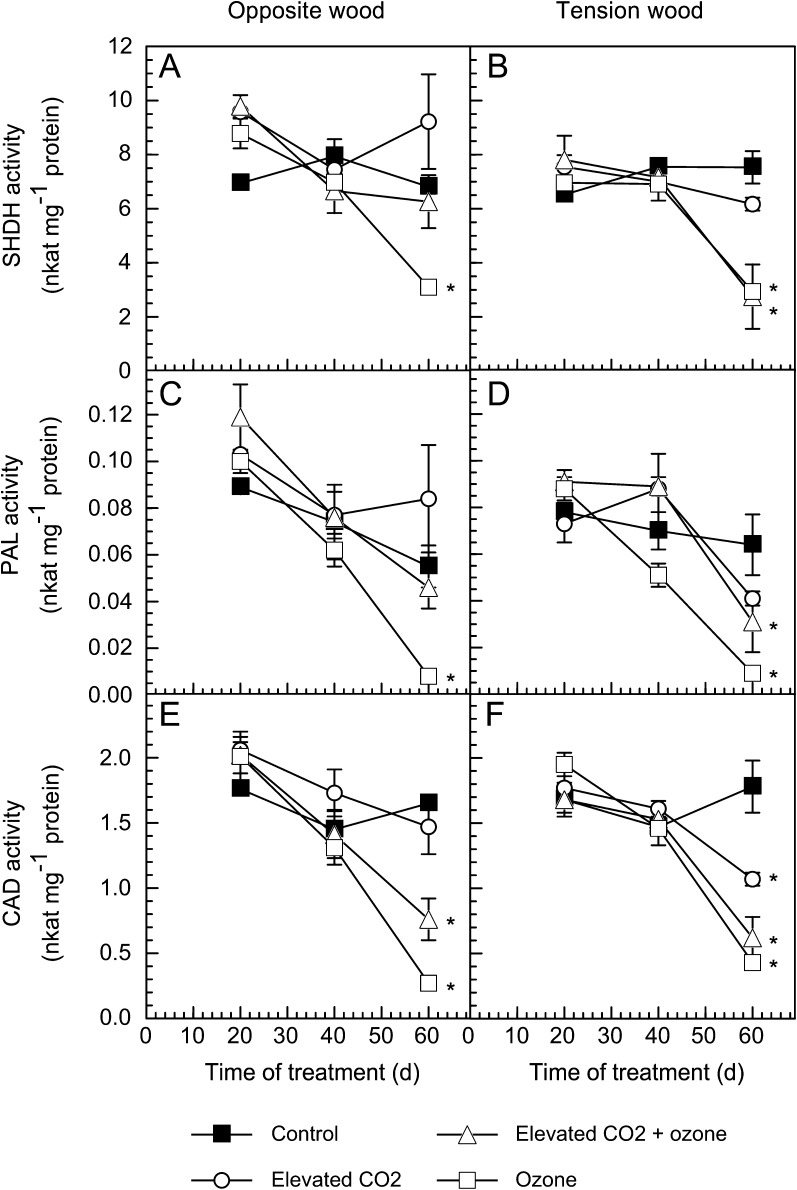
The response of the lignin pathway during the experiment was assessed by monitoring the time-course of activity of the enzymes involved in lignin biosynthesis (SHDH, PAL, CAD) in the OW and TW of lower and middle stems of young trees subjected to different treatments for 60 d (Fig. 2; see Supplementary Fig. S1 at JXB online).
Fig. 2.
Time-course of activities of SHDH (A, B), PAL (C, D), and CAD (E, F) enzymes in opposite wood (OW) (A, C, E) and tension wood (TW) (B, D, F) of the lower stem of hybrid poplar subjected to different treatments. Values are mean ±SE (n=3). Difference between control and treatments at day 60 was statistically significant at P <0.05 (*).
Under elevated CO2 exposure, SHDH, PAL, and CAD activities remained unchanged in the OW of lower stems. These activities were not affected in TW before 40 d of fumigation. However a reduction, compared with the control, was observed after 60 d. CAD activity was reduced to a greater extent than SHDH and PAL (Fig. 2). The enzyme activities in middle stems were similar to those in the control treatment (see Supplementary Fig. S1 at JXB online).
The activities of SHDH, PAL, and CAD were decreased in plants exposed to ozone compared with control plants, whatever the wood tissues (OW and TW) in both lower and middle stems (Fig. 2; see Supplementary Fig. S1 at JXB online). The decline of PAL and CAD activities was more pronounced (4–6-fold), than that of SHDH in the lower stems after 60 d of treatment. By comparison, the effects were less pronounced in the middle stems in both wood tissues (see Supplementary Fig. S1 at JXB online).
Enzyme activities in the combined treatment, compared with the control, were decreased in both wood tissues from the lower stems (Fig. 2). The reduction was moderate in OW and similar to the ozone-induced reduction in TW. CAD activity was generally more affected than SHDH and PAL activities. The effects were similar but less pronounced in both wood tissues of middle stems than in those of lower stems (see Supplementary Fig. S1 at JXB online).
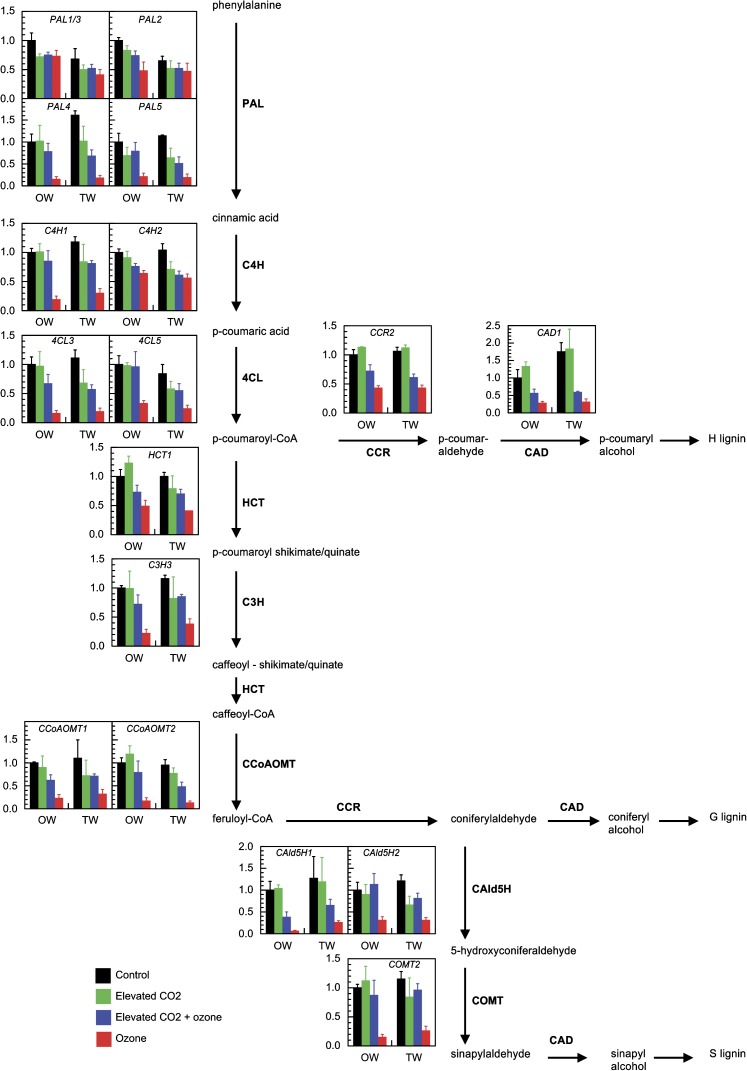
Transcript levels in the lignin pathway
Transcripts were analysed at the end of the experiment (day 60) in OW and TW of lower stems. All genes from the lignin pathway showing high expression levels in poplar xylem were examined (Shi et al., 2010). Under control conditions, the lignin-related gene levels were similar in OW and TW except for PAL1/3, PAL2 (lower in TW), and CAD1 (higher in TW) (Fig. 3; see Supplementary Table S2 at JXB online).
Fig. 3.
Transcript abundance of genes involved in the lignin biosynthesis pathway. Analyses were performed on opposite wood (OW) and tension wood (TW) of lower stem of hybrid poplar subjected for 60 d to different treatments. Transcript levels were normalized and expressed as fold changes compared with values of opposite wood in control conditions. y axis is in arbitrary units. Values are mean ±SE (n=3 biological replicates). For statistical differences refer to Supplementary Table S2 at JXB online. The most favoured route in angiosperms lignin pathway is shown. Phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate:CoA ligase (4CL), p-hydroxycinnamoyl-CoA:quinate/shikimate p-hydroxycinnamoyltransferase (HCT), 4-coumarate 3-hydroxylase (C3H), caffeoyl-CoA O-methyltransferase (CCoAOMT), cinnamoyl-CoA reductase (CCR), coniferyl aldehyde 5-hydroxylase (CAld5H), caffeic acid/5-hydroxyconiferaldehyde O-methyltransferase (COMT), cinnamyl alcohol dehydrogenase (CAD).
Under elevated CO2, the RNA levels of all genes remained unchanged in OW compared with the control treatment. A similar result was observed in TW except for PAL4, PAL5, C4H2, 4CL3, and CAld5H2 which decreased (Fig. 3; see Supplementary Table S2 at JXB online). Ozone treatment induced a decrease in transcript levels of all genes involved in the lignin pathway whatever the tissue, OW or TW. In the combined treatment, many genes showed a reduction in transcript levels particularly in TW. The profile of the reduction was intermediate between the reductions observed in the ozone and control treatments.
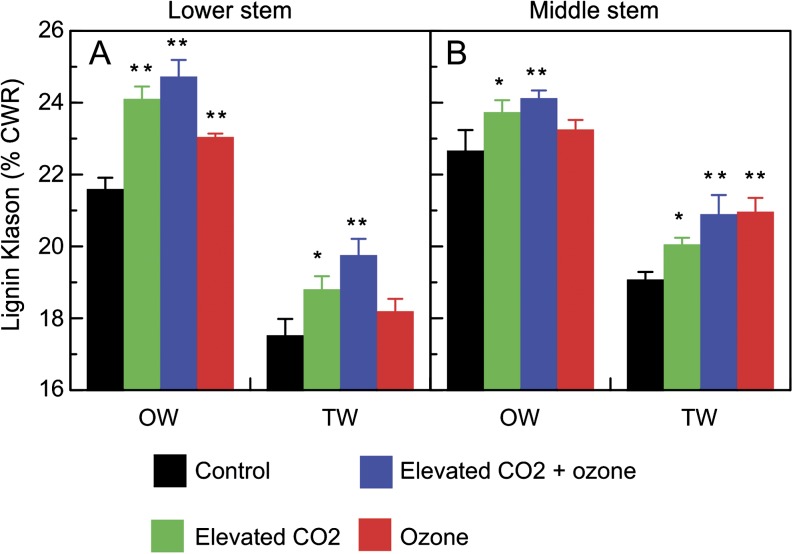
Lignin content
Lignin content in relation to cell wall residue (CWR) was determined at day 60 in the OW and TW of lower and middle stems from each treatment (Fig. 4). The lignin content was higher in OW than in TW whatever the treatment or stem level. Lignin content was increased in elevated CO2, ozone, and the combined treatment whatever the wood tissue or stem level. The highest increase was observed under the combined ozone and elevated CO2 treatment. Lignin content was enhanced by 14.5% compared with the control.
Fig. 4.
Lignin content in opposite wood (OW) and tension wood (TW) of lower (A) and middle (B) stems of hybrid poplar after 60 d of growth under different conditions control (filtered air), ozone (200 nl l−1) , elevated CO2 (800 μl l−1) or the combination of ozone and elevated CO2. Values are mean ±SE (n=3). Difference between control and treatments was statistically significant at P <0.05 (**) or P <0.1 (*).
Relative specific allocation of carbon (RSA C) in wood and lignin
The capacity of wood and lignin to concentrate newly acquired C was assessed by performing a 13C pulse–chase experiment on young poplar trees grown under different conditions and estimating RSA C in the OW and TW of lower and middle stems.
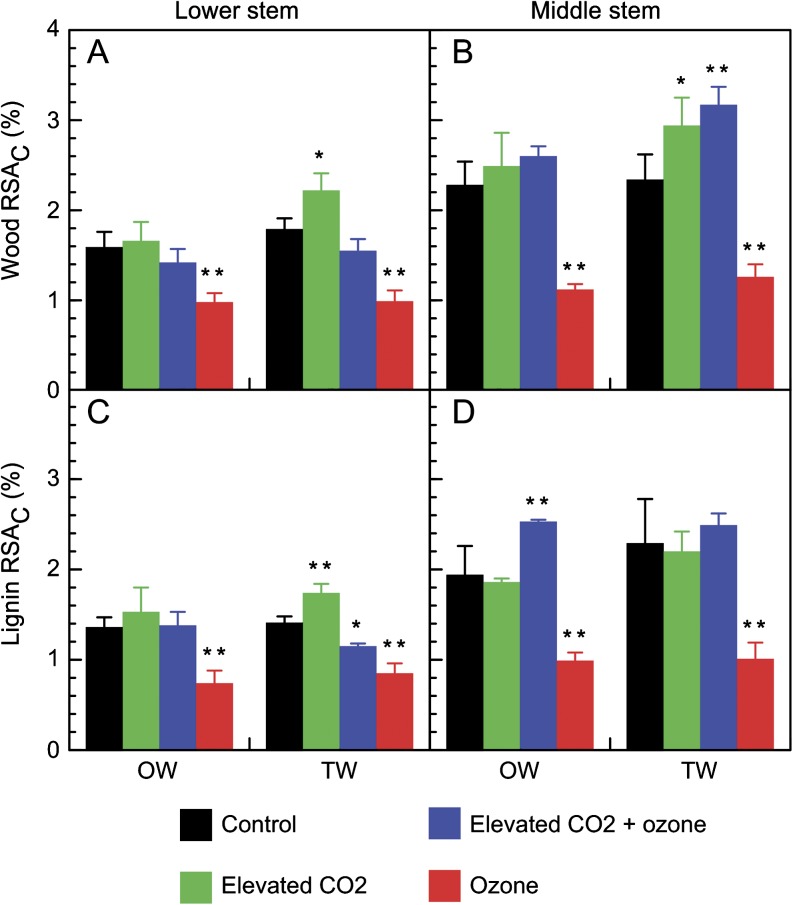
The carbon newly incorporated in wood (wood RSA C) was higher in the middle stem than in the lower stem under control conditions (Fig. 5A, B). Plants subjected to elevated CO2 showed a significant increase in the allocation of newly incorporated carbon only in the TW of lower and middle stems. Under ozone treatment, RSA C was significantly decreased (up to 2-fold) whatever the wood tissue or stem level. No changes in carbon allocation were observed in the combined treatment except in TW of the middle stem (Fig. 5B).
Fig. 5.
Changes in relative specific allocation of carbon (RSA C %) for wood (A, B) or lignin (C, D) in opposite wood (OW) and tension wood (TW) of lower (A, C) and middle (B, D) stems of hybrid poplar grown for 35 d under different treatments. Values are mean ±SE (n=5 for wood and n=3 for lignin analyses). Difference between control and treatments was statistically significant at P <0.05 (**) or P <0.1 (*).
The carbon newly incorporated in lignin was decreased (1.5–2-fold) under ozone treatment in both wood tissues and at both stem levels (Fig. 5C, D). Few changes were observed under the other treatments. Nevertheless, carbon allocation to lignin was significantly increased under elevated CO2 in the TW of lower stem (Fig. 5C), and in OW of middle stem of plants subjected to the combined treatment (Fig. 5D).
Discussion
The effect of elevated CO2 on lignification
As soil fertility has been shown to be very important in the response of trees to elevated CO2 (Oren et al., 2001; Long et al., 2004), the young poplars in our experiment were fertilized to avoid N limitation. Poplar growth and biomass increased under elevated CO2 in our experiment as is usually found with N fertilization.
Cell wall lignin content was increased in the wood of young poplars grown in elevated CO2 (Fig. 4). A similar result was found in coppices of Populus×euramericana and Populus nigra in a free air CO2 enrichment experiment (Luo et al., 2008; Luo and Polle, 2009) and in birch (Mattson et al., 2005). However, different responses were observed in other experiments. Lignin content was reduced in beech (Blaschke et al., 2002) whereas no modification was reported in birch, maple or aspen clones in a free air CO2 enrichment experiment (Kaakinen et al., 2004; Kostiainen et al., 2008). Responses may depend on the species, the season, and N supply. Cell wall lignification was assessed by expressing the lignin content as a percentage of the cell wall residue or extractive-free material. In our experiment, the wood of poplars grown in elevated CO2 was enriched in extractives (see Supplementary Table S3 at JXB online) so the lignin content expressed on a dry mass basis remained unchanged under elevated CO2. Since lignin content is often expressed on a dry mass basis, variations in cell wall lignin content could have been masked in some cases by the accumulation of extractives, as suggested by Penuelas and Estiarte (1998).
The allocation of new assimilates to wood or lignin was assessed by performing a 13C pulse–chase experiment. The labelling experiments revealed more pronounced effects in TW than in OW (Fig. 5). TW was probably more responsive since it represents a strong sink for carbon especially under elevated CO2. Larger amounts of new assimilates were allocated to wood, as indicated by the increased wood RSA C levels in poplars under elevated CO2 (Fig. 5). This result is consistent with the increased carbon assimilation, radial growth, and cambial activity (Tables 2, 3). As a consequence, new assimilates were more utilized for lignin biosynthesis since the lignin RSA C was higher in wood of poplars grown under elevated CO2. The continuous labelling experiment in beech also showed an increased utilization of new assimilates in lignin under elevated CO2 (Dyckmans et al., 2000). All these results, together with the increased lignin content, strongly suggest that effective lignin biosynthesis was enhanced under elevated CO2. Wood cell walls were more lignified under elevated CO2 due to the increased synthesis of lignin without any modifications of cell wall thickness (Table 3).
Lignin metabolism activities (enzyme activities and RNA levels) were unchanged or slightly decreased in TW of poplars grown under elevated CO2 (Figs 2, 3). It can be hypothesized that enzyme activities decreased to limit an increase of lignin synthesis due to substrate excess under elevated CO2. This hypothesis presupposes that the substrate supply was limiting and not the enzyme activities, under control conditions. Indeed, in transgenic trees, enzyme activities had to be strongly reduced for any effect on lignin biosynthesis to be observed under control conditions (Baucher et al., 2003). Six of the 17 genes analysed in this study were significantly down-regulated under elevated CO2. The regulation by elevated CO2 seemed to affect only a few genes and only some members within a given gene family in the lignin pathway.
Ozone effect on lignification
Young poplars subjected to ozone fumigation displayed a higher wood lignin content than control poplars (Fig. 4). These findings are in accordance with our previous results obtained under controlled conditions (Richet et al., 2011).
The pathways involved in lignin biosynthesis (enzyme activities and RNA levels) were strongly reduced in wood under ozone treatment (Figs 2, 3). A decrease in PAL1, CCR2, and CAD1 had previously been recorded in poplars after 46 d of ozone fumigation. It is shown here for the first time that all genes from the phenylpropanoid and monolignol pathways highly expressed in wood (Shi et al., 2010) were reduced at transcript level under ozone treatment. These results suggest a tight co-ordination of the response to ozone and that all these genes could share regulatory elements and associated transcription factors for their regulation in the presence of ozone. Shi et al. (2010) found common putative regulatory elements in these genes. However, it is shown here that, within a gene family, some transcripts were more altered than others suggesting that the corresponding promoters differed in their responsiveness to ozone.
The reduction of lignin pathway activities was consistent with the decreased cambial activity (Table 3) of poplars subjected to ozone. Wood formation was slowed down so lignin biosynthesis activities were reduced. The reductions in radial growth (Fig. 1) and stem biomass (Table 1) were a consequence of decreased cambial activity. It is also shown that photosynthesis was reduced in leaves (Table 2) which suggested that less carbon was supplied to the stems. The allocation of new assimilates to wood or lignin was assessed for the first time, to our knowledge, by performing a 13C pulse–chase experiment. The labelling experiment which showed that wood RSA C was reduced under ozone confirmed that less new carbon was allocated to wood under ozone exposure (Fig. 5A, B). These results are consistent with a decreased phloem loading induced by ozone (Andersen, 2003). Interestingly, the labelling experiment also showed a reduced use of assimilates for lignin biosynthesis since lignin RSA C was decreased by ozone (Fig. 5C, D). The results obtained by 13C labelling, together with the response of enzymes and transcripts involved in lignification, reinforce the hypothesis that ozone reduces effective lignin biosynthesis. It can also be hypothesized that the lignin pathway (enzyme activities and RNA levels) is controlled by carbon supply in poplars subjected to ozone. A control by sugars has also been suggested in normal growth conditions (Rogers et al., 2005).
Lignin biosynthesis was repressed under ozone whereas the lignin content of the cell wall was increased (Fig. 4). Since lignin, cellulose, and hemicellulose are the main components of the cell wall, a modification in the abundance of one component would affect the relative contents of the others. Our results strongly support the notion that lignin biosynthesis was reduced under ozone and that lignin content was increased, probably because one or both of the other components was also affected. Indeed, both cellulose content and cellulose biosynthesis activities were shown to decrease in poplars subjected to ozone treatments (Richet et al., 2011) which is consistent with the thinner cell walls observed in this experiment (Table 3). It can therefore be hypothesized that the synthesis of the cell wall components was reduced and that lignin was less reduced than the other components leading to more lignified cell walls.
Effect of elevated CO2 and ozone on lignification
In our experiment, elevated CO2 and ozone applied separately had opposite effects on many parameters: growth, biomass, photosynthesis, cambial activity, and wood cell wall thickness. Poplar grown under combined elevated CO2 and ozone displayed intermediary effects for all these parameters. The negative effects of ozone were generally limited by elevated CO2. This compensatory effect has been observed in many cases (Karnosky et al., 2003).
Nevertheless, the labelling experiments showed more subtle changes in plants exposed to a combination of elevated CO2 and ozone. Indeed, lower and middle stems displayed different responses. Incorporation of new assimilates into the wood and lignin of the lower stems was moderately reduced compared with the control suggesting that elevated CO2 had a compensatory effect. By contrast, incorporation of new assimilates into the wood and lignin of the middle stems was similar or higher than that observed under elevated CO2. The lower stems responded like the ozone-treated stems and the behaviour of the middle stems resembled that of stems under elevated CO2.
In contrast to other parameters, the lignin content was similarly affected by elevated CO2 and ozone applied separately in poplar wood. The compensatory effect of elevated CO2 was not recorded here under the combined treatment since the lignin content in the wood of poplars grown under elevated CO2 and ozone was higher than in the wood of poplars grown under each treatment (Fig. 4). Thus the effects on lignin content were additive when elevated CO2 was combined with ozone. It is shown that different mechanisms leading to increased lignin content occurred under elevated CO2 and under ozone treatments. Both mechanisms may be involved in the response to combined treatment resulting in an additive effect.
In conclusion, the first detailed analysis of the lignification process in wood of young poplars subjected to elevated CO2 and/or ozone is reported here. Both constraints induced increased lignification of the cell wall but the underlying mechanisms were different. Under elevated CO2, modifications in lignin content were under the control of carbon supply to the stem. Under ozone treatment, changes in lignin content were controlled by the balance between lignin and other cell wall components. Elucidation of these steps should help in identifying key genes involved in hte wood response to elevated CO2 and/or ozone. These candidate genes should be validated on mature trees growing in natural conditions before they could be used in tree improvement for a better adaptation to future climate.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Primers used for quantitative real-time PCR.
Supplementary Table S2. Statistical analyses of transcript levels of different genes involved in lignin biosynthesis.
Supplementary Table S3. Extractives and lignin content on a dry mass basis in opposite wood and tension wood of hybrid poplar grown under different conditions.
Supplementary Fig. S1. Time-course of the activities of SHDH, PAL, and CAD enzymes in opposite wood and tension wood of middle stem from hybrid poplar subjected to different treatments.
Acknowledgments
This work was supported by the French Environment and Energy Management Agency (ADEME) and Région Lorraine. We sincerely thank Frédéric Legée (UMR 1318 AgroParisTech-INRA) and Laurent Cézard (UMR 1318 AgroParisTech-INRA) for the lignin analyses.
Glossary
Abbreviations
- OW
opposite wood
- TW
tension wood
References
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytologist. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell and Environment. 2007;30:258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Andersen CP. Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytologist. 2003;157:213–228. doi: 10.1046/j.1469-8137.2003.00674.x. [DOI] [PubMed] [Google Scholar]
- Atwell BJ, Henery ML, Whitehead D. Sapwood development in Pinus radiata trees grown for three years at ambient and elevated carbon dioxide partial pressures. Tree Physiology. 2003;23:13–21. doi: 10.1093/treephys/23.1.13. [DOI] [PubMed] [Google Scholar]
- Bagard M, Le Thiec D, Delacote E, Hasenfratz-Sauder MP, Banvoy J, Gerard J, Dizengremel P, Jolivet Y. Ozone-induced changes in photosynthesis and photorespiration of hybrid poplar in relation to the developmental stage of the leaves. Physiologia Plantarum. 2008;134:559–574. doi: 10.1111/j.1399-3054.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Baucher M, Halpin C, Petit-Conil M, Boerjan W. Lignin: genetic engineering and impact on pulping. Critical Reviews in Biochemistry and Molecular Biology. 2003;38:305–350. doi: 10.1080/10409230391036757. [DOI] [PubMed] [Google Scholar]
- Blaschke L, Forstreuter M, Sheppard LJ, Leith IK, Murray MB, Polle A. Lignification in beech (Fagus sylvatica) grown at elevated CO2 concentrations: interaction with nutrient availability and leaf maturation. Tree Physiology. 2002;22:469–477. doi: 10.1093/treephys/22.7.469. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annual Review of Plant Biology. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biology. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabané M, Pireaux JC, Leger E, Weber E, Dizengremel P, Pollet B, Lapierre C. Condensed lignins are synthesized in poplar leaves exposed to ozone. Plant Physiology. 2004;134:586–594. doi: 10.1104/pp.103.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MM, Sederoff RR. Variation in lignin content and composition. Mechanisms of control and implications for the genetic improvement of plants. Plant Physiology. 1996;110:3–13. doi: 10.1104/pp.110.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A, Somerville C. Cellulosic biofuels. Annual Review of Plant Biology. 2009;60:165–182. doi: 10.1146/annurev.arplant.043008.092125. [DOI] [PubMed] [Google Scholar]
- Curtis PS, Wang XZ. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- Dence C. The determination of lignin. In: Lin S, Dence C, editors. Methods in lignin chemistry. Berlin, Germany: Springer-Verlag; 1992. pp. 33–62. [Google Scholar]
- Dyckmans J, Flessa H, Polle A, Beese F. The effect of elevated [CO2] on uptake and allocation of 13C and 15N in beech (Fagus sylvatica L.) during leafing. Plant Biology. 2000;2:113–120. [Google Scholar]
- Gillespie KM, Rogers A, Ainsworth EA. Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max) Journal of Experimental Botany. 2011;62:2667–2678. doi: 10.1093/jxb/erq435. [DOI] [PubMed] [Google Scholar]
- Hamelinck CN, van Hooijdonk G, Faaij APC. Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass and Bioenergy. 2005;28:384–410. [Google Scholar]
- IPCC. Climate change 2007: synthesis report. Summary for policy makers. 2007. Intergovernmental Panel on Climate Change, Fourth Assessment Report. [Google Scholar]
- Isebrands JG, McDonald EP, Kruger E, Hendrey G, Percy K, Pregitzer K, Sober J, Karnosky DF. Growth responses of Populus tremuloides clones to interacting elevated carbon dioxide and tropospheric ozone. Environmental Pollution. 2001;115:359–371. doi: 10.1016/s0269-7491(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Kaakinen S, Kostiainen K, Ek F, Saranpaa P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E. Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Global Change Biology. 2004;10:1513–1525. [Google Scholar]
- Karnosky DF. Impacts of elevated atmospheric CO2 on forest trees and forest ecosystems: knowledge gaps. Environment International. 2003;29:161–169. doi: 10.1016/S0160-4120(02)00159-9. [DOI] [PubMed] [Google Scholar]
- Karnosky DF, Skelly JM, Percy KE, Chappelka AH. Perspectives regarding 50 years of research on effects of tropospheric ozone air pollution on US forests. Environmental Pollution. 2007;147:489–506. doi: 10.1016/j.envpol.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Karnosky DF, Zak DR, Pregitzer KS, et al. Tropospheric O3 moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project. Functional Ecology. 2003;17:289–304. [Google Scholar]
- Kilpelainen A, Peltola H, Ryyppo A, Sauvala K, Laitinen K, Kellomaki S. Wood properties of Scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiology. 2003;23:889–897. doi: 10.1093/treephys/23.13.889. [DOI] [PubMed] [Google Scholar]
- King JS, Kubiske ME, Pregitzer KS, Hendrey GR, McDonald EP, Giardina CP, Quinn VS, Karnosky DF. Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2 . New Phytologist. 2005;168:623–635. doi: 10.1111/j.1469-8137.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Kostiainen K, Jalkanen H, Kaakinen S, Saranpaa P, Vapaavuori E. Wood properties of two silver birch clones exposed to elevated CO2 and O3 . Global Change Biology. 2006;12:1230–1240. [Google Scholar]
- Kostiainen K, Kaakinen S, Saranpaa P, Sigurdsson BD, Lundqvist SO, Linder S, Vapaavuori E. Stem wood properties of mature Norway spruce after 3 years of continuous exposure to elevated CO2 and temperature. Global Change Biology. 2009;15:368–379. [Google Scholar]
- Kostiainen K, Kaakinen S, Warsta E, Kubiske ME, Nelson ND, Sober J, Karnosky DF, Saranpaa P, Vapaavuori E. Wood properties of trembling aspen and paper birch after 5 years of exposure to elevated concentrations of CO2 and O3 . Tree Physiology. 2008;28:805–813. doi: 10.1093/treephys/28.5.805. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants face the future. Annual Review of Plant Biology. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- Luo ZB, Calfapietra C, Scarascia-Mugnozza G, Liberloo M, Polle A. Carbon-based secondary metabolites and internal nitrogen pools in Populus nigra under Free Air CO2 Enrichment (FACE) and nitrogen fertilisation. Plant and Soil. 2008;304:45–57. [Google Scholar]
- Luo ZB, Polle A. Wood composition and energy content in a poplar short rotation plantation on fertilized agricultural land in a future CO2 atmosphere. Global Change Biology. 2009;15:38–47. [Google Scholar]
- Maillard P, Guehl J-M, Muller J-F, Gross P. Interactive effects of elevated CO2 concentration and nitrogen supply on partitioning of newly fixed 13C and 15N between shoot and roots of pedunculate oak seedlings (Quercus robur) Tree Physiology. 2001;21:163–172. doi: 10.1093/treephys/21.2-3.163. [DOI] [PubMed] [Google Scholar]
- Marino BD, McElroy MB. Isotopic composition of atmospheric CO2 inferred from carbon in C4 plant cellulose. Nature. 1991;349:127–131. [Google Scholar]
- Mattson WJ, Julkunen-Tiitto R, Herms DA. CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth differentiation balance models? Oikos. 2005;111:337–347. [Google Scholar]
- Matyssek R, Karnosky DF, Wieser G, Percy K, Oksanen E, Grams TEE, Kubiske M, Hanke D, Pretzsch H. Advances in understanding ozone impact on forest trees: messages from novel phytotron and free-air fumigation studies. Environmental Pollution. 2010;158:1990–2006. doi: 10.1016/j.envpol.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Oren R, Ellsworth DS, Johnsen KH, et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- Overdieck D, Fenselau K. Elevated CO2 concentration and temperature effects on the partitioning of chemical components along juvenile Scots pine stems (Pinus sylvestris L.) Trees: Structure and Function. 2009;23:771–786. [Google Scholar]
- Penuelas J, Estiarte M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends in Ecology and Evolution. 1998;13:20–24. doi: 10.1016/s0169-5347(97)01235-4. [DOI] [PubMed] [Google Scholar]
- Perré P. Meshpore: a software able to apply image-based meshing techniques to anisotropic and heterogeneous porous media. Drying Technology. 2005;23:1993–2006. [Google Scholar]
- Richet N, Afif D, Huber F, Pollet B, Banvoy J, El Zein R, Lapierre C, Dizengremel P, Perré P, Cabané M. Cellulose and lignin biosynthesis is altered by ozone in wood of hybrid poplar (Populus tremula×alba) Journal of Experimental Botany. 2011;62:3575–3586. doi: 10.1093/jxb/err047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen J, Lindsberg MM, Holopainen T, Oksanen E, Lappi J, Peltonen P, Vapaavuori E. Silver birch and climate change: variable growth and carbon allocation responses to elevated concentrations of carbon dioxide and ozone. Tree Physiology. 2004;24:1227–1237. doi: 10.1093/treephys/24.11.1227. [DOI] [PubMed] [Google Scholar]
- Rogers LA, Dubos C, Cullis IF, Surman C, Poole M, Willment J, Mansfield SD, Campbell MM. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. Journal of Experimental Botany. 2005;56:1651–1663. doi: 10.1093/jxb/eri162. [DOI] [PubMed] [Google Scholar]
- Runion GB, Entry JA, Prior SA, Mitchell RJ, Rogers HH. Tissue chemistry and carbon allocation in seedlings of Pinus palustris subjected to elevated atmospheric CO2 and water stress. Tree Physiology. 1999;19:329–335. doi: 10.1093/treephys/19.4-5.329. [DOI] [PubMed] [Google Scholar]
- Shi R, Sun Y-H, Li Q, Heber S, Sederoff R, Chiang VL. Towards a systems approach for lignin biosynthesis in Populus trichocarpa: transcript abundance and specificity of the monolignol biosynthetic genes. Plant and Cell Physiology. 2010;51:144–163. doi: 10.1093/pcp/pcp175. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:34.31–34.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingarzan R. A review of surface ozone background levels and trends. Atmospheric Environment. 2004;38:3431–3442. [Google Scholar]
- Weng JK, Li X, Bonawitz ND, Chapple C. Emerging strategies of lignin engineering and degradation for cellulosic biofuel production. Current Opinion in Biotechnology. 2008;19:166–172. doi: 10.1016/j.copbio.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Wittig VE, Ainsworth EA, Long SP. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant, Cell and Environment. 2007;30:1150–1162. doi: 10.1111/j.1365-3040.2007.01717.x. [DOI] [PubMed] [Google Scholar]
- Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biology. 2009;15:396–424. [Google Scholar]
- Wustman BA, Oksanen E, Karnosky DF, Noormets A, Isebrands JG, Pregitzer KS, Hendrey GR, Sober J, Podila GK. Effects of elevated CO2 and O3 on aspen clones varying in O3 sensitivity: can CO2 ameliorate the harmful effects of O3? Environmental Pollution. 2001;115:473–481. doi: 10.1016/s0269-7491(01)00236-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.