Abstract
The available literature is conflicting on the potential protection of plants against ozone (O3) injury by exogenous jasmonates, including methyl jasmonate (MeJA). Protective antagonistic interactions of O3 and MeJA have been observed in some systems and purely additive effects in others. Here it is shown that chronic exposure to low to moderate O3 concentrations (4–114 ppb; 12 h mean) and to MeJA induced additive reductions in carbon assimilation (A n) and root respiration (R r), and in calculated whole plant carbon balance. Neither this chronic O3 regime nor MeJA induced emission of ethylene (ET) from the youngest fully expanded leaves. ET emission was induced by acute 3 h pulse exposure to much higher O3 concentrations (685 ppb). ET emission was further enhanced in plants treated with MeJA. Responses of growth, allocation, photosynthesis, and respiration to moderate O3 concentrations and to MeJA appear to be independent and additive, and not associated with emission of ET. These results suggest that responses of Pima cotton to environmentally relevant O3 are not mediated by signalling pathways associated with ET and MeJA, though these pathways are inducible in this species and exhibit a synergistic O3×MeJA interaction at very high O3 concentrations.
Keywords: Air pollution, cotton, ethylene, jasmonate, O3, ozone, plant hormones, signalling
Introduction
Ambient ozone (O3) is the most damaging air pollutant to vegetation (Collins et al., 2000; Fuhrer and Booker, 2003), reducing global crop yield in 2000 by up to 121×106 ton (Avnery et al., 2011) through a variety of physiological mechanisms (Wilkinson et al., 2012). O3 generates reactive oxygen species (ROS) in the apoplast, thereby sharing signalling pathways with ROS-mediated responses to biotic challenges (Langebartels et al., 2002; Moeder et al., 2002). These pathways are non-specific (Moons et al., 1997; Baier et al., 2005; Kangasjarvi et al., 2005) and are induced by multiple abiotic stresses including wounding, drought, and salinity, as well as by O3 (Conconi et al., 1996; Wang et al., 2001; Glazebrook, 2005; Browse, 2009).
Methyl jasmonate (MeJA) is a naturally occurring, volatile signal metabolite that functions within and between plants (Farmer and Ryan, 1990; Sembdner and Parthier, 1993; Seo et al., 2001). Of the numerous jasmonate (JA) derivatives found in plants, the isoleucine conjugate jasmonoyl-isoleucine (JA-Ile) is the active form to which others are converted (Browse, 2009), although some jasmonate precursors appear to have distinct biological activity (Dave and Graham, 2012). MeJA has been investigated as a crop growth regulator, reducing fruit retention force in grape and citrus (Hartmond et al., 2000; Burns et al., 2003; Fidelibus et al., 2007), and protecting salinity-treated barley against oxidant stress (Tsonev et al., 1998; Walia et al., 2007). JA inhibits O3-induced lesion proliferation, visible leaf injury, and programmed cell death (PCD) in O3-sensitive Arabidopsis thaliana by suppression of ethylene (ET)-associated signalling pathways (Overmyer et al., 2000, 2003; Rao et al., 2000a , b , 2002; Shoji et al., 2000; Kanna et al., 2003; Tuominen et al., 2004). Sensitivity to O3 increased in hybrid Populus when JA signalling was compromised (Koch et al., 1998, 2000) and decreased in tobacco and Arabidopsis when MeJA was applied exogenously (Orvar et al., 1997; Overmyer et al., 2000; Rao et al., 2000b ; Kanna et al., 2003). These studies suggest that JA and ET are involved in plant response to acute O3. At moderate O3, growth and biomass allocation in Pima cotton were inhibited by both O3 and foliar MeJA, but effects were additive with no protective, anatagonistic interaction (Grantz et al., 2010b).
The role of ET in plant response to O3 is complex. The magnitude of ET emissions is well correlated with the severity of O3-induced injury (Tingey et al., 1976; Tuomainen et al., 1997; Overmyer et al., 2000; Nakajima et al., 2002; Rao et al., 2002; Tamaoki et al., 2003). ET activates defence pathways (Lamb and Dixon, 1997; Leubner-Metzger et al., 1998), yet overexpression of ET enhanced O3 sensitivity in Arabidopsis (Overmyer et al., 2000). This may be mediated by ET suppression of JA-induced genes (Shoji et al., 2000). Wounding or exposure to O3 in tomato induces the classic biphasic emission of ET by activating two sets of 1-aminocyclopropane-1-carboxylate synthase (ACS) genes (Tatsuki and Mori, 1999; Moeder et al., 2002). This leads to tissue generation of H2O2 and ultimately PCD (Moeder et al., 2002; Browse, 2009) through the hypersensitive response (HR), in response to both pathogens (Pennel and Lamb, 1997; Ciardi et al., 2001) and O3 (Schraudner et al., 1998; Overmyer et al., 2000; Rao et al., 2000b ).
Jasmonates play a prominent role in the O3–ET signalling pathway as downstream mediators (Tamaoki et al., 2003). MeJA and ET exhibit both antagonistic and synergistic interactions in different systems (Shoji et al., 2000; Schmelz et al., 2003). A fundamental interaction of JA and ET is mediated by JAZ proteins which repress transcription of JA-responsive genes and interact with downstream transcription factors that mediate responses to ET (Guo and Ecker, 2004; Zhu et al., 2011; Wager and Browse, 2012). High levels of JA (specifically JA-Ile) target JAZ proteins for degradation, thereby providing positive feedback for JA activity. Many defensive and pathogenesis-related transcription factors respond to either ET or JA (Xu et al., 1994; Penninckx et al., 1998; Lorenzo et al., 2003), while necrotrophic pathogen-associated transcription factors respond only to the combination of ET plus JA (Xu et al., 1994; Penninckx et al., 1998; Alonso et al., 1999; Glazebrook et al., 2003; Glazebrook, 2005; Broekaert et al., 2006; Browse, 2009). JA is an essential component in induction of phytoalexin synthesis in Cupressus cultures by yeast elicitor (Zhao et al., 2004).
There is substantial cross-talk among the many components of intraplant signalling networks (Conconi et al., 1996; Baldwin, 1998; Rojo et al., 1999; Kunkel and Brooks, 2002; Glazebrook, 2005; Fujita et al., 2006; Chassot et al., 2008; Spoel and Dong, 2008). For example, responses to JA and abscisic acid are mediated by components of the ET pathway, even in the absence of ET itself (Alonso et al., 1999; Ghassemian et al., 2000). In many species, MeJA induces synthesis of ET. In contrast, induction of jasmonates by ET has been observed only in a few cases (ODonnell et al., 1996; Watanabe et al., 2001).
The role of JA and ET in plant response to environmentally relevant O3 exposures remains unclear. Here the impacts and potential interactions of O3 or MeJA on root and shoot metabolism in Pima cotton, and the role of ET synthesis in mediating these impacts at moderate and very high levels of O3 exposure are evaluated.
Materials and methods
Hypotheses
The impacts of O3 and MeJA on gas exchange of shoots and roots of Pima cotton, and the role of ET synthesis in these impacts, are evaluated. Three null hypotheses are tested using analysis of variance (ANOVA): (i) H1—root and shoot carbon metabolism do not respond to O3 or to MeJA; (ii) H2—ET emission from leaves does not respond to O3 or to MeJA; (iii) H3—there is no antagonistic O3×MeJA interaction.
Three experiments were performed, each with replication in time and space. Two were chronic, long-term exposures to a range of low to moderate O3 concentrations. The first of these provided measurements of leaf area and root and shoot gas exchange (CHRONIC/GASEX). The second used the same O3 exposure protocol to provide measurements of ET emission from leaves (CHRONIC/ET). The third applied a single acute, pulse exposure to a wide range of low to very high O3 concentrations, also to provide measurements of ET emission from leaves (ACUTE/ET).
Plant growth
Initially, two cultivars of Pima cotton (Gossypium barbadense L.) were used. Seed of cv. Phytogen 800 (P8; Phytogen Seed Company, Indianopolis, IN, USA) was obtained from a commercial source. Seed of cv. S-6 (S6; J.G. Boswell Company, Corcoran, CA, USA) was obtained from foundation seed stock.
Seeds were planted in moist commercial potting mix (Earthgro Potting Soil; Scotts Company, Marysville, OH, USA) in plastic pots (3.8 cm depth×21 cm height) in a research greenhouse (Kearney Research and Extension Center; 103 msl; 36.598'N 119.503'W). Automated drip emitters irrigated all pots to run-through daily and provided a complete fertilizer solution (1.3 g k−1 Miracle Gro, Scotts Miracle-Gro Products Inc., Port Washington, NY, USA) to run-through twice weekly (Grantz et al., 2003, 2008, 2010b). Plants were grown on an open greenhouse bench until the onset of O3 exposure.
Methyl jasmonate application
MeJA (Sigma-Aldrich catalogue no. 392707; 95% purity) was brought to 4.36×10−3 M by dilution of 1 ml to 1000 ml in deionized water. Plants were treated with either 160 μl (160 μg of MeJA) of the MeJA solution (+MeJA) or with 160 μl of H2O (–MeJA). The solution of MeJA was vigorously shaken prior to application and appeared as a single phase. The smallest achievable droplets were applied, using a 0.5–250 μl plastic micropipette tip (Finntip 250; Thermo Electron Corp., Vantaa, Finland). Droplets were distributed uniformly over the adaxial surface of the two youngest fully expanded leaves, twice-weekly, near solar noon.
For CHRONIC/GASEX, application of MeJA began at 24 days after planting (DAP), and for CHRONIC/ET at 21 DAP, in both cases in the O3 exposure chambers. For ACUTE/ET, application of MeJA began at 22 DAP, on the greenhouse bench.
Variations of this method of application have been used previously without inducing local effects at the site of application (Arnold and Schultz, 2002; Henkes et al., 2008; Grantz et al., 2010b). There were no local effects of volatilized MeJA on non-target (–MeJA) control plants located in the same chambers.
Ozone exposure
Exposures to ozone (O3) were performed in cylindrical O3 exposure chambers (continuously stirred tank reactors, CSTRs; Heck et al., 1978) situated in a separate bay of the same greenhouse. Nine CSTRs were arrayed in three blocks, parallel to windows and cooling fans.
O3 was generated by corona discharge (Model SGC-11, Pacific Ozone Technology, Brentwood, CA, USA) from purified oxygen (Series ATF-15, Model 1242, SeQual Technologies Inc., San Diego, CA, USA). Air containing the desired O3 concentration was introduced into each CSTR at one complete air exchange per minute into the orbit of a 120 rpm circulating fan for uniform distribution. O3 was regulated in a single CSTR by a dedicated O3 monitor (Model 49C, Thermo Environmental Instruments, Franklin, MA, USA), with computerized feedback control (Grantz et al., 2003). O3 concentration in the chambers was a linear function of control voltage (r 2=0.997). O3 concentration was maintained proportional to the concentration in a single regulated CSTR, and determined in each CSTR four times per hour with a separate O3 monitor (Model 49C), through continuously purged Teflon dust filters and tubing using a multiport solenoid valve.
CHRONIC/GASEX and CHRONIC/ET exposures were dispensed as daily half-sine waves, with the same nominal exposure each day (7 d week−1). Daily 12 h mean daylight O3 exposures (07:00–19:00 h) were 4, 59, and 114 ppb, with daily maxima near solar noon of 4, 89, and 163 ppb. These are low to moderate O3 concentrations.
ACUTE/ET exposures were dispensed as a single square wave pulse (3.0 h duration). Concentrations of 16±2, 270±4, and 685±13 ppb were imposed in a single block of CSTRs. The O3 control and monitoring system was the same as used for the chronic exposures with the O3 generator operating at a higher voltage. These are low to very high O3 concentrations.
For CHRONIC/GASEX, eight plants were transferred to each CSTR (four per cultivar, two +MeJA, two –MeJA). For CHRONIC/ET, two plants were transferred to each CSTR (one +MeJA, one –MeJA; S6 only). Plants were transferred when cotyledons were fully expanded and the first true leaf was emerging.
For ACUTE/ET, uniform plants of each MeJA treatment (S6 only) were transferred to the CSTRs at ∼09:00 h, at 55 DAP. The O3 pulse was imposed between 10:45–13:45 h Pacific daylight time and the plants returned to the greenhouse bench on the same day.
Leaf and root gas exchange
Leaf area per plant (LA) was determined at 51 DAP with a leaf area meter (LI-3000, LI-COR, Lincoln, NE, USA), following excision between the petiole and stem. Root biomass was obtained from Grantz et al. (2010b).
Gas exchange measurements of net carbon assimilation (A n) and stomatal conductance (g s) were obtained near solar noon at 49 DAP, at steady state on the youngest fully expanded leaves (YFLs). Measurements were obtained with a commercially available gas exchange system (LI-6400; LI-COR Inc.), in situ in the growth CSTR. Photosynthetic photon flux density (PPFD) was controlled at 1000 μmol photons m−2 s−1, provided by 80% red and 20% blue light-emitting diodes (LI-6400-02B). Ambient (reference) CO2 concentration in the cuvette was controlled at 400 μmol mol−1 using complete scrubbing of CO2 in ambient air and an integrated CO2 mixing system (LI-6400-01). Leaf temperature and leaf to air vapour pressure deficit were not controlled and were generally 25–30 °C and 2–3 kPa. A n and g s were expressed relative to projected leaf area.
Root respiration (R r) was measured on freshly sampled fine root tips. Electrodes were calibrated using air-saturated H2O, and oxygen-free H2O obtained by adding a small amount of sodium dithioinite to each chamber. A 2 ml aliquot of H2O was placed in each chamber after several rinsings. When output had become stable (∼10 min), the terminal 3–4 cm of fine root was excised and immediately transferred to a respirometer chamber.
Measurements were conducted in liquid phase, with a Clark-type oxygen electrode (Delieu and Walker, 1972). Four respirometer chambers (Oxygraph Oxygen Electrode System; PP Systems, Haverhill, MA, USA) were run in parallel, interfaced with a computer for data acquisition and analysis (Grantz et al., 2003). A magnetic stir bar was placed in each chamber, separated from the root material by a laboratory-designed porous metal screen. Temperature control (25 °C) was maintained by circulation of water through a precision water bath (Model 9100, Isotemp, Pittsburgh, PA, USA) and through the plastic housing of each respirometer chamber. Specific root respiration (R r) was expressed relative to the oven-dry mass of root material in each respirometer chamber, obtained following the measurement.
Ethylene emission
CHRONIC/ET was assayed for ET emission at 54 DAP. ACUTE/ET was assayed at 55 DAP. Incubation for ET emission began immediately following removal from the CSTR in early afternoon. The YFLs were sampled by excising leaf disks with a cork borer (2.3 cm diamter; 4.15 cm2), avoiding the midrib and large veins. Cut edges of the leaf disks were sealed with petroleum jelly held at the melting point. One leaf disk [∼20 mg dry weight (dwt)] was suspended on edge in each glass vial (64.7 ml). Vials were sealed with screw-top, gas-tight serum caps and incubated in darkness for 24 h at 23–24 °C.
At the conclusion of the incubation period, the head space of each vessel was sampled through an air-tight septum with a 22 gauge (0.7 mm) hypodermic needle and a gas-tight syringe. An aliquot of 12 ml was extracted from each vial.
The 12 ml aliquot was injected into a sample collection column of a gas chromatograph (Carle AGC-400, EG and G, Chandler Engineering, Tulsa, OK, USA). A 2.0 ml sample was automatically introduced from the sample collection column into a 30 ml min−1 helium carrier gas and passed through an 8% NaCl/alumina column (F-1, 80/100 mesh), held at 70 °C, followed by a flame ionization detector.
The concentration of ET in the sample gas was determined against an authentic standard also injected as 12 ml into the collection column. Peaks were identified by elution time and quantified relative to local baseline. Emission of ET was expressed as ng g dwt−1 h−1, using an ET concentration derived from peak height, volume of the incubation vessel, biomass of the leaf sample, and incubation time in the vial. The detection threshold was ∼3 ppb, equivalent to < 1 ng g dwt−1 h−1 in the final units.
Experimental design
CHRONIC/GASEX was performed on four plants per CSTR of each of the two related cultivars (two +MeJA and two –MeJA), and repeated once (n=6). The cultivars did not differ in their responses to O3 or to MeJA and were pooled as subsamples. The two plants of each cultivar in each O3×MeJA treatment were also pooled as subsamples. Runs did not differ and were pooled prior to analysis by ANOVA, as a split-plot, randomized complete block design (SAS v. 9.2; PROC GLM), with a non-default error term [O3×block].
CHRONIC/ET was performed on two plants per CSTR (one +MeJA and one –MeJA) of the single cultivar, Pima S-6. The experiment was repeated twice (n=9). Runs were pooled and analysed as a split-plot, randomized complete block design with no subsamples.
ACUTE/ET was performed on six plants per CSTR (three +MeJA and three –MeJA) of the single cultivar, Pima S-6, and was repeated once (n=6). One sample was discarded as an outlier (n=5 for the highest O3). Runs were pooled and analysed by ANOVA as a completely randomized design.
ET emission data were log transformed to approximate more closely a normal distribution prior to ANOVA. In all cases, mean separation (P < 0.05) was performed with Duncan’s multiple range test.
Results
Leaf responses
The CHRONIC/GASEX protocol led to a systemic response to MeJA, observed as darkly pigmented circular areas (∼500 μm diameter) on the adaxial surface of leaves (Fig. 1). This pigmentation was apparent on all leaves of all +MeJA plants, independent of O3 exposure level, including those leaves younger and older than the two leaves that received direct application (e.g. four leaf insertion levels are shown in Fig. 1). This response was not observed on the cotyledons nor on leaves of control (–MeJA) plants in the same CSTR. MeJA did not induce any localized injury at the sites of foliar microapplication nor additional symptoms on the leaves receiving direct application.
Fig. 1.

Response of leaf surface appearance of a low O3-treated plant to application of MeJA (+MeJA) to young leaves of Pima cotton, cv. S-6 in the CHRONIC/GASEX experiment. The discrete dark-pigmented, areas were observed on all leaves of +MeJA plants, and independently of O3 exposure.
O3 accelerated leaf senescence, so that O3-induced visible symptoms of bronzing and purple discoloration were observed on older leaves (not shown). No O3-induced pigmentation was observed on the two YFLs.
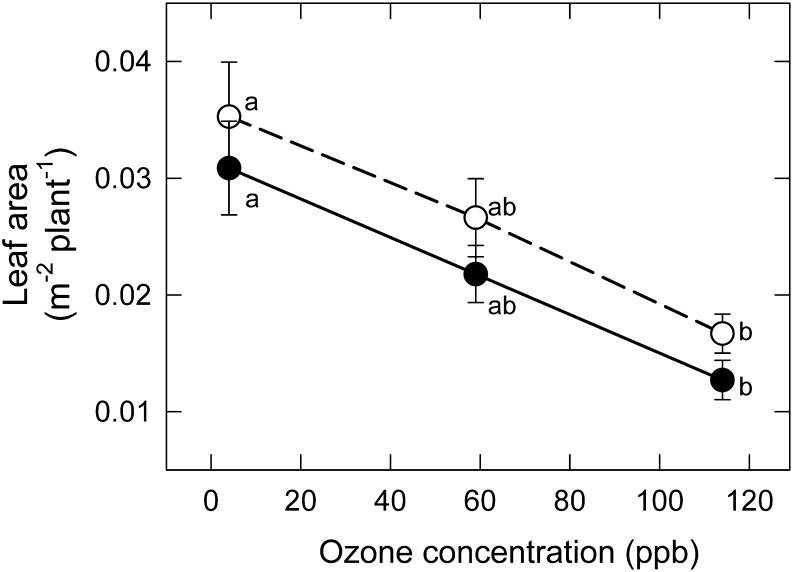
LA was reduced by ∼15% by MeJA (Fig. 2; compare the dotted and solid lines) and by ∼55% by moderate O3 in –MeJA plants (Fig. 2; open symbols). Responses of LA to increasing O3 were parallel in +MeJA and –MeJA plants, so that the highly significant impacts of both factors were strictly additive (i.e. with no significant O3×MeJA interaction) (Table 1).
Fig. 2.
Response of whole plant leaf area, LA, of Pima cotton to chronic exposure to a range of low to moderate O3 in the CHRONIC/GASEX experiment, in leaves treated with MeJA (+MeJA; 160 μg plant−1; filled symbols, solid lines) or untreated (–MeJA; open symbols, dashed lines). Points associated with the same letter within a line do not differ (P < 0.05). The effects of O3 and MeJA were highly significant (P < 0.01; Table 1).
Table 1.
Analysis of variance (P-values) of midday photosynthetic gas exchange, root respiration, and calculated whole plant carbon balance, following long-term exposure to moderate O3 in Pima cotton (CHRONIC/GASEX experiment)
| Pima cotton | |||
| O3 | MeJA | O3×MeJA | |
| Leaf area (m2 plant−1) | 0.008 | 0.000 | 0.945 |
| Assimilation (μmol m−2 s−1) | 0.001 | 0.000 | 0.671 |
| Stomatal conductance (μmol m−2 s−1) | 0.000 | 0.007 | 0.742 |
| Root respiration (μmol g dwt−1 s−1) | 0.941 | 0.001 | 0.623 |
| Carbon balance (μmol plant−1) | 0.001 | 0.000 | 0.469 |
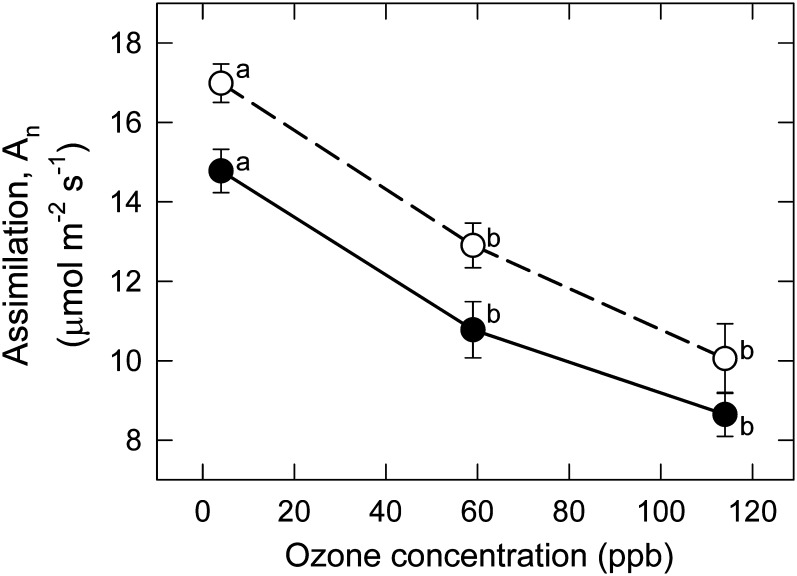
Photosynthetic activity of the YFLs, observed as midday net carbon assimilation (A n), was reduced by 13% by MeJA at low O3 (Fig. 3; compare open and filled symbols). A n was also reduced by O3 (Fig. 3; open symbols; Table 1). These impacts were both highly significant but these responses to O3 in the +MeJA and –MeJA plants were also parallel, and effects were strictly additive with no significant O3×MeJA interaction (Table 1). The effect on A n of O3 was much larger than that of MeJA (Fig. 3).
Fig. 3.
Response of net carbon assimilation, A n, of Pima cotton to chronic exposure to a range of low to moderate O3 in the CHRONIC/GASEX experiment, in leaves treated with MeJA (+MeJA; 160 μg plant−1; filled symbols, solid lines) or untreated (–MeJA; open symbols, dashed lines). Points associated with the same letter within a line do not differ (P < 0.05). The effects of O3 and MeJA were highly significant (P < 0.01; Table 1).
Root responses
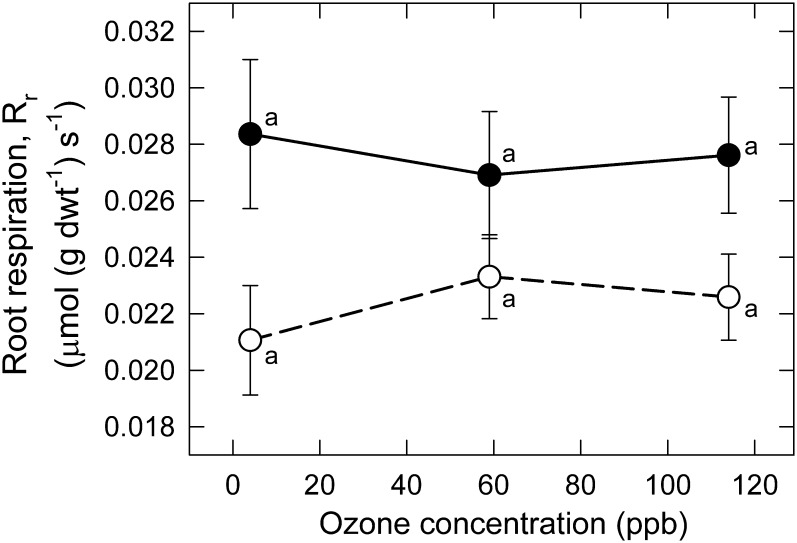
In contrast to effects on A n, the CHRONIC/GASEX protocol stimulated root respiration (R r) substantially (Fig. 4). The response of R r to MeJA was greatest (35%) at low O3, though enhancement was significant at all O3, and there was no significant O3×MeJA interaction (Table 1). In contrast to MeJA, O3 had little effect on R r, inducing an upward trend of 7–10% (non-significant) in –MeJA plants (Fig. 4; open symbols). O3 had little effect and MeJA a large effect on R r, in contrast to effects on A n (Fig. 4).
Fig. 4.
Response of root respiration, R r, of Pima cotton to chronic exposure to a range of low to moderate O3 in the CHRONIC/GASEX experiment, in leaves treated with MeJA (+MeJA; 160 μg plant−1; filled symbols, solid lines) or untreated (–MeJA; open symbols, dashed lines). Points associated with the same letter within a line do not differ (P < .05). The effect of MeJA was highly significant (P < 0.01; Table 1).
Shoot productivity and whole root system respiration declined proportionally, for example by ∼70% each with increasing O3 in – MeJA plants. Loss of shoot productivity was driven by similar inhibition of LA and A n, while declines in root system respiration were driven by O3 impacts on allocation of biomass to roots. The effects of O3 and MeJA on A n and R r both negatively impacted plant carbon balance (CB), suggesting that impacts on productivity might be more closely associated with CB than with the individual gas exchange components. CB was estimated as the difference between A n scaled for leaf area, photoperiod, and contribution to canopy carbon acquisition (shoot productivity), and R r scaled for total root biomass (Grantz et al., 2010b) (root system respiration). CB declined by approximately a third in response to MeJA at all O3 and by ∼75% at the highest chronic O3 in both +MeJA and –MeJA (not shown). Despite substantial additive impacts of MeJA and O3 on CB, there was no O3×MeJA interaction (not shown).
The responses of LA, A n, R r, and CB allow rejection of hypothesis H1, that growth and metabolism of root and shoot do not respond to O3 or to MeJA. In contrast, hypothesis H3 cannot be rejected under these conditions of low to moderate chronic O3 exposure. There was no evidence of O3×MeJA interaction that could provide the basis for protection by MeJA against ambient O3.
Ethylene is not induced at moderate O3 concentrations
The unexpected absence of an antagonistic and potentially protective O3×MeJA interaction suggested that the required signalling components may by absent in this system. Tests were carried out to determine whether CHRONIC/ET exposure to the range of low to moderate O3 concentrations affected the JA–ET signalling pathways.
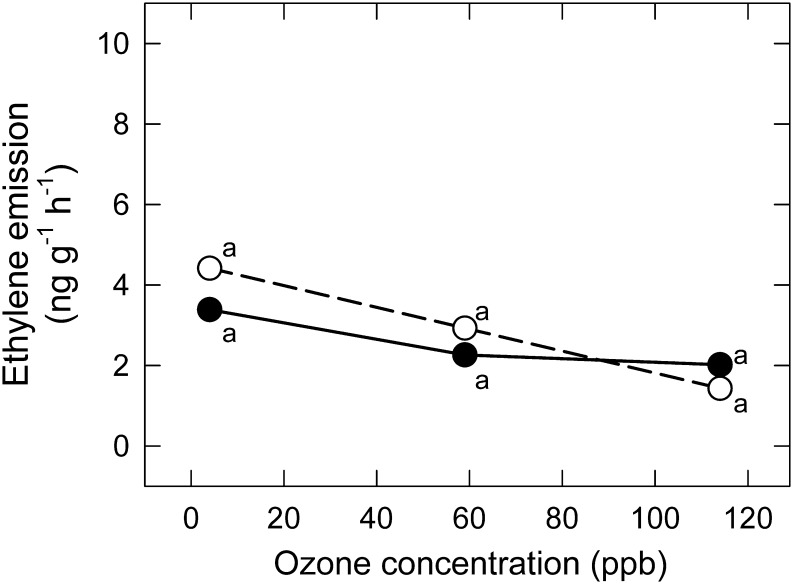
Very low emissions of ET from the YFL (<5 ng g dwt−1 h−1) were observed under all combinations of O3 and MeJA exposure conditions (Fig. 5). There was a slight downward trend in ET emission (non-significant; Table 2) with increasing O3. Exogenous MeJA also had no significant effect on emission of ET (Fig. 5; Table 2), and the MeJA×O3 interaction was non-significant (Table 2).
Fig. 5.
Response of emission rate of ET (dry weight basis) of Pima cotton to (a) chronic exposure to a range of low to moderate O3 in the CHRONIC/ET experiment, in leaves treated with MeJA (+MeJA; 160 μg plant−1; filled symbols, solid lines) or untreated (–MeJA; open symbols, dashed lines). Points associated with the same letter within a line do not differ (P < 0.05). Neither the effect of O3 nor the effect of MeJA, nor the O3×MeJA interaction, was significant (Table 2).
Table 2.
Analysis of variance (P-values) of ethylene emission following long-term exposure to moderate O3 or pulse exposure to high O3 (CHRONIC/ET and ACUTE/ET experiments)
| O3 | MeJA | O3×MeJA | ||
| Long-term moderate O3 (n=9) | Log ethylene emission [log (ng g−1 h−1)] | 0.304 | 0.581 | 0.402 |
| Pulse high O3 (n=6) | Log ethylene emission [log (ng g−1 h−1)] | 0.000 | 0.017 | 0.094 |
ET was not induced despite the substantial O3 concentrations imposed (up to 163 ppb, midday hourly average) and clear impacts of both O3 and MeJA on multiple endpoints. Hypotheses H2 and H3, that ET is not induced by MeJA or moderate O3, and that there is no antagonistic O3×MeJA interaction under these conditions, cannot be rejected. This result provides a potential rationale for the lack of protective O3×MeJA interaction under the CHRONIC/GASEX conditions.
Ethylene is induced at very high O3 concentrations
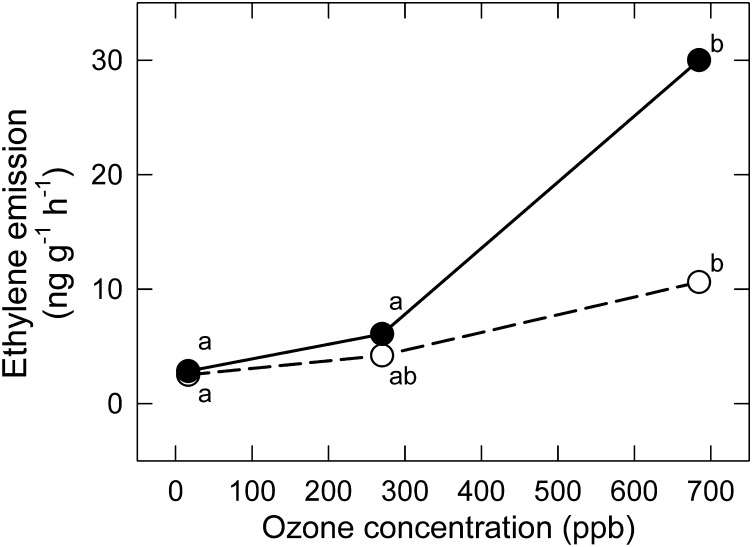
The absence of ET emission in CHRONIC/ET raised the question of whether the leaves of Pima cotton are competent in O3-induced ET emission and the HR and PCD signalling pathways that MeJA could influence. This was tested in plants exposed to a 3 h square wave pulse to a broad range of low to very high O3 concentrations (16±2, 270±4, and 685±13 ppb).
At low O3 (14 ppb and 16 ppb), the results of CHRONIC/ET and ACUTE/ET were consistent, with similar ET emissions (2.74 ng g dwt−1 h−1 and 2.66 ng g dwt−1 h−1; compare Figs 5 and 6). The intermediate O3 concentration in the pulse protocol (270 ppb) was greater than twice the concentration at the high end of the moderate exposure regime. These exposures did not induce a significant increase in ET emission, in either –MeJA or +MeJA leaves, though both exposures are well above typical ambient levels.
Fig. 6.
Response of emission rate of ET (dry weight basis) of Pima cotton to an acute pulse exposure to a range of low to very high O3 in the ACUTE/ET experiment, in leaves treated with MeJA (+MeJA; 160 μg plant−1; filled symbols, solid lines) or untreated (–MeJA; open symbols, dashed lines). Points associated with the same letter within a line do not differ (P < 0.05). Both the effect of O3 and the effect of MeJA were significant (P < 0.01; Table 2). The O3×MeJA interaction was significant at P < 0.10 (P = 0.094; Table 2).
At the highest acute O3 exposure (685 ppb) in the ACUTE/ET protocol, ET emission was induced by O3, increasing significantly by 324% (Fig. 6; open symbols) in –MeJA leaves, and by 1604% in +MeJA leaves (Fig. 6; filled symbols). The O3 effect on ET emission was highly significant (Table 2), regardless of whether ET emissions were expressed relative to leaf mass (Fig. 6) or leaf area (not shown).
The MeJA stimulation of ET emission was also significant (Table 2). MeJA enhanced ET emissions by 13% at low O3, but this increased to 45% and 354% in +MeJA leaves (relative to –MeJA leaves) at successively higher O3 exposures (Fig. 6). This O3×MeJA interaction (P = 0.094; Table 2) was not observed under more moderate O3 exposure regimes.
At very high O3, both hypotheses H2 and H3 may be rejected. Pima cotton exhibited significant induction of ET signaling pathways under appropriate conditions of O3 exposure.
Discussion
Acute and chronic exposures to O3 have been shown to reduce productivity, photosynthetic gas exchange, and allocation of biomass below-ground (Cooley and Manning, 1987; Reiling and Davison, 1992; Grantz et al., 2006; Booker et al., 2009; Chen et al., 2009). While the spatial heterogeneity of foliar symptoms may be more pronounced under shorter regimes of higher O3 concentration than under chronic exposure protocols (Chen et al., 2009), differences in the induction of signalling pathways are not well defined. This may lead to potential confusion in development of protective strategies based on emerging knowledge of signalling metabolites.
Growth and gas exchange
A range of MeJA application rates produced concentration-specific effects on growth and allocation in Pima cotton (Grantz et al., 2010b). These paralleled those induced by moderate O3. These ranges of O3 and MeJA did not induce necrotic lesions indicative of HR but were sufficient to induce systemic impacts. Similar responses to O3 and to MeJA of leaf and root tip gas exchange were observed in the present study. For all endpoints examined to date, including growth, gas exchange, and ET emission, there was no O3×MeJA interaction at moderate O3.
Leaf tissues in the YFLs have direct access to both O3 and MeJA through stomatal uptake and cuticular diffusion. Above-ground processes are more accessible and thus typically better characterized than their below-ground counterparts. In the present study, O3 reduced A n of the YFLs and LA of the whole plant substantially, consistent with previous observations of gas exchange, leaf chlorophyll, and shoot growth, both in this species (Grantz and Yang, 1996; Grantz and Shrestha, 2006; Grantz et al., 2006, 2010b) and in many others (Cooley and Manning, 1987; Reiling and Davison, 1992; Morgan et al., 2003; Ashmore, 2005; Booker et al., 2009). O3 impacts on A n are associated with reduced transcript, protein, and activity of Rubisco (Dann and Pell, 1989).
A n was also substantially reduced by MeJA, consistent with previous observations in other species (Wiedhase et al., 1987; Staswick et al., 1992; Creelman and Mullet, 1995; Tung et al., 1996; Arnold and Schultz, 2002; Henkes et al., 2008).
Below-ground structures have no direct access to O3 or O3 breakdown products (Turner et al., 1973), nor to MeJA. Root impacts are also more difficult to characterize than those in the shoot. In the present study, O3 had only a modest stimulatory effect on R r, but a large inhibitory impact on root biomass (Grantz et al., 2010b), while MeJA had a large stimulatory impact on R r and a modest inhibition of root biomass. In previous studies, R r increased significantly with increasing O3 exposure in Pima cotton and muskmelon (Cucumis melo L.; Grantz et al., 2003), but little impact on R r was observed in yellow nutsedge (Cyperus esculentus; Grantz et al., 2010a ). The responses to O3 by the root system were similar to earlier observations (Cooley and Manning, 1987; Reiling and Davison, 1992; Grantz et al., 2006). Variability in physiological processes below-ground is typically large (Bryla et al., 1997; Lambers et al., 2002), and often contributes the largest errors in determination of whole plant CB (Ryan, 1991).
Jasmonates down-regulate core metabolism and photosynthesis, reduce allocation below-ground, and accelerate senescence, while up-regulating production of feeding deterrents and toxins (Herms and Mattson, 1992; Feys et al., 1994; Berger et al., 1996; Henkes et al., 2008; Browse, 2009). The below-ground responses to MeJA observed in the present study were consistent with earlier observations (Staswick et al., 1992; Creelman and Mullet, 1995; Tung et al., 1996; Arnold and Schultz, 2002; Henkes et al., 2008; Grantz et al., 2010b). In Arabidopsis, these responses are regulated by both ET and MeJA (Schmidt et al., 2010).
Reductions in the estimates of whole plant CB induced by both O3 and MeJA were substantial. Nevertheless, at moderate O3 there was no O3×MeJA interaction. Root system respiration was highest and shoot productivity was lowest in the +MeJA plants subjected to the highest chronic O3 exposure. These whole plant impacts were driven nearly equally by effects on total shoot productivity and total root system respiration. However, the responses of these components were driven differently by changes in carbon allocation (dominant for roots) and in physiological activity (co-dominant with allocation in shoots). In C3 and C4 grasses, a similar estimate of CB declined in response to simulated herbivory (Thorne and Frank, 2009). In yellow nutsedge, CB was positively correlated with reproductive output (Grantz et al., 2010a). Further evaluation of the CB parameter, with appropriate measurements at whole shoot and root system scales, may provide considerable insight into the impacts of O3 on vegetation.
Ethylene
ET emission is highly correlated with O3 injury (Tingey et al., 1976; Tamaoki et al., 2003), and clearly linked to induction of HR and PCD (Overmyer et al., 2000; Rao et al., 2000b ). Lesion proliferation is limited by JA (Kanna et al., 2003; Overmyer et al., 2003; Tuominen et al., 2004), apparently serving to deny palatable necrotic tissue to necrotrophs.
A potential explanation for the consistent lack of protection by MeJA against moderate O3 exposure in Pima cotton is that the JA–ET signalling network is not induced. This is supported by the present data. The exposures to moderate O3 or MeJA did not induce ET signalling. The alternative explanation, excessive cuticular or stomatal resistance that prevented penetration of either material, can be dismissed based on the systemic responses to both O3 and MeJA that were observed. Another alternative explanation can be dismissed based on the ACUTE/ET protocol. ET was assayed after 4 weeks of exposure in the chronic experiment, and after 3 h of exposure in the pulse protocol, suggesting that a burst of ET at the onset of moderate O3 exposure could have been overlooked. However, the intermediate pulse exposure (270 ppb) did not elicit emission of ET, though the O3 concentration was substantially higher than the highest O3 in the chronic exposure.
Despite the lack of ET signalling at moderate O3, the presence of these pathways and the sensivity of ET emission in Pima cotton to O3 were confirmed under very high O3 exposure conditions, using the acute 3 h square wave pulse exposure. In both the CHRONIC/ET and ACUTE/ET experiments, the low and intermediate O3 exposures were consistent in not inducing significant emission of ET. Only at much higher O3 (685 ppb) was ET induced and a synergistic O3×MeJA interaction observed. This interaction does not imply protection by MeJA, since ET emission is associated with O3 injury (Tingey et al., 1976; Tuomainen et al., 1997; Overmyer et al., 2000; Nakajima et al., 2002; Rao et al., 2002; Tamaoki et al., 2003).
In Pima cotton, exposure to O3 at ambient levels or slightly above induces substantial developmental and physiological responses without inducing emission of ET. These responses were consistently additive and independent of ET induction. At higher O3, ET is induced and an O3×MeJA interaction is observed. Superambient O3 may be required to elicit the ET and JA coordination of responses to O3 that are well known in responses to other biotic and abiotic stresses, mediated through PCD and HR (Kangasjarvi et al., 1994, 2005; Schlagnhaufer et al., 1995; Overmyer et al., 2005). The present data appear to rule out application of MeJA as an anti-ozonant in Pima cotton under current global conditions.
Acknowledgments
The authors thank Dr R. Hutmacher for providing seed of cv. Phytogen 800, Dr R. Percy for providing foundation seed of cv. S-6, Dr Mary Lu Arpaia for providing access to the gas chromatography facility, Mr James Seiber for considerable advice and assistance in determinations of ethylene, and Mr Caesar Aguilar for assistance with destructive harvests.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis . Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Arnold T, Schultz JU. Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus . Oecologia. 2002;130:585–593. doi: 10.1007/s00442-001-0839-7. [DOI] [PubMed] [Google Scholar]
- Ashmore MR. Assessing the future global impacts of ozone on vegetation. Plant, Cell and Environment. 2005;28:949–964. [Google Scholar]
- Avnery S, Mauzerall DL, Liu J, Horowitz LW. Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmospheric Environment. 2011;45:2284–229. [Google Scholar]
- Baier M, Kandlbinder A, Golldack D, Dietz K-J. Oxidative stress and ozone: perception, signaling and response. Plant, Cell and Environment. 2005;29:854–868. [Google Scholar]
- Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proceedings of the National Academy of Sciences, USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Bell E, Mullet JE. Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiology. 1996;111:525–531. doi: 10.1104/pp.111.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker F, Muntifering R, McGrath M, Burkey K, Decoteau D, Fiscus E, Manning W, Krupa S, Chappelka A, Grantz D. The ozone component of global change: potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. Journal of Integrative Plant Biology. 2009;51:337–351. doi: 10.1111/j.1744-7909.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Delaure SL, De Bolle MF, Cammue BP. The role of ethylene in host–pathogen interactions. Annual Review of Phytopathology. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- Bryla DR, Bouma TJ, Eissenstat DM. Root respiration in citrus acclimates to temperature and slows during drought. Plant, Cell and Environment. 1997;20:1411–1420. [Google Scholar]
- Burns JK, Pozo LV, Arias CR, Hockema B, Rangaswamy V, Bender CL. Coronatine and abscission in citrus. Journal of the American Society forHorticultural Science. 2003;128:309–315. [Google Scholar]
- Chassot C, Buchala A, Schoonbeek H-J, Metraux J-P, Lamotte O. Wounding of Arabidopsis leaves causes a powereful but transient protection against Botrytis infection. The Plant Journal. 2008;55:555–567. doi: 10.1111/j.1365-313X.2008.03540.x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Frank TD, Long SP. Is a short, sharp shock equivalent to long-term punishment? Contrasting the spatial pattern of acute and chronic ozone damage to soybean leaves via chlorophyll fluorescence imaging. Plant, Cell and Environment. 2009;32:327–335. doi: 10.1111/j.1365-3040.2008.01923.x. [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Jones JB, Klee HJ. Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. Vesicatoria. Molecular Plant-Microbe Interactions. 2001;14:487–495. doi: 10.1094/MPMI.2001.14.4.487. [DOI] [PubMed] [Google Scholar]
- Collins WJ, Stevenson DS, Johnson CE, Derwent RG. The European regional ozone distribution and its links with the global scale for the years 1992 and 2015. Atmospheric Environment. 2000;34:255–267. [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA. The octadecanoid signaling pathway in plants mediates a response to UV radiation. Nature. 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- Cooley DR, Manning WJ. The impact of ozone on assimilate partitioning in plants: a review. Environmental Pollution. 1987;47:95–113. doi: 10.1016/0269-7491(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Dave A, Graham IA. Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA) Frontiers in Plant Science. 2012;3:42. doi: 10.3389/fpls.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann MS, Pell EJ. Decline of activity and quantity of ribulose bisphosphate carboxylase oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiology. 1989;91:427–432. doi: 10.1104/pp.91.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delieu T, Walker DA. An improved cathode for the measurement of photosynthetic oxygen evolution by isolated chloroplasts. New Phytologist. 1972;71:201–225. [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proceedings of the National Academy of Sciences, USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. The Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidelibus MW, Cathline KA, Burns JK. Potential abscission agents for raisin, table, and wine grapes. HortScience. 2007;42:1626–1630. [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Fuhrer J, Booker F. Ecological issues of ozone: agricultural issues. Environment International. 2003;29:141–154. doi: 10.1016/S0160-4120(02)00157-5. [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. The Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang H-S, Nawrath C, Metraux J-P, Zhu T, Katagiri F. Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. The Plant Journal. 2003;34:217–228. doi: 10.1046/j.1365-313x.2003.01717.x. [DOI] [PubMed] [Google Scholar]
- Grantz DA, Gunn S, Vu H-B. Ozone impacts on plant development: a meta-analysis of root/shoot allocation and growth. Plant, Cell and Environment. 2006;29:1193–1209. doi: 10.1111/j.1365-3040.2006.01521.x. [DOI] [PubMed] [Google Scholar]
- Grantz D, Shrestha A. Tropospheric ozone and interspecific competition between yellow nutsedge and pima cotton. Crop Science. 2006;46:1879–1889. [Google Scholar]
- Grantz DA, Shrestha A, Vu H-B. Early vigor and ozone response in horseweed (Conyza Canadensis) biotypes differing in glyphosate resistance. Weed Science. 2008;56:224–230. [Google Scholar]
- Grantz DA, Shrestha A, Vu H-B. Ozone impacts on assimilation and allocation to reproductive sinks in the vegetatively propagated C4 weed, yellow nutsedge. Crop Science. 2010a;50:246–252. [Google Scholar]
- Grantz DA, Silva V, Toyota M, Ott N. Ozone increases root respiration but decreases leaf CO2 assimilation in cotton and melon. Journal of Experimental Botany. 2003;43:2375–2384. doi: 10.1093/jxb/erg261. [DOI] [PubMed] [Google Scholar]
- Grantz DA, Vu H-B, Aguilar C, Rea MA. No interaction between methyl jasmonate and ozone in Pima cotton: growth and allocation respond independently to both. Plant, Cell and Environment. 2010b;33:717–728. doi: 10.1111/j.1365-3040.2009.02096.x. [DOI] [PubMed] [Google Scholar]
- Grantz D, Yang S. Effect of O3 on hydraulic architecture in pima cotton: biomass allocation and water transport capacity of roots and shoots. Plant Physiology. 1996;112:1649–1657. [Google Scholar]
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Hartmond U, Yuan R, Burns JK, Grant A, Kender WJ. Citrus fruit abscission induced by methyl-jasmonate. Journal of the American Society for Horticultural Science. 2000;125:547–552. [Google Scholar]
- Heck WW, Philbeck RB, Denning JA. A continuous stirred tank reactor (CSTR) system for exposing plants to gaseous air pollutants. 1978 USDA, Publication No. ARS-5-181, Washington DC. [Google Scholar]
- Henkes GJ, Thorpe MR, Minchin PEH, Schurr U, Rose USR. Jasmonic acid treatment to part of the root system is consistent with simulated leaf herbivory, diverting recently assimilated carbon towards untreated roots within an h. Plant, Cell and Environment. 2008;31:1229–1236. doi: 10.1111/j.1365-3040.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Quarterly Review of Biology. 1992;67:283–335. [Google Scholar]
- Kangasjarvi J, Jaspers P, Kollist H. Signalling and cell death in ozone-exposed plants. Plant, Cell and Environment. 2005;28:1021–1036. [Google Scholar]
- Kangasjarvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defense systems induced by ozone. Plant, Cell and Environment. 1994;17:783–794. [Google Scholar]
- Kanna M, Tamaoki M, Kubo A, Nakajima N, Rakwal R, Agrawal GK, Tamogami S, Ioki M, Ogawa D, Saji H, Aono M. Isolation of an ozone-sensitive and jasmonate-semi-insensitive Arabidopsis mutant (oji1) Plant and Cell Physiology. 2003;44:1301–1310. doi: 10.1093/pcp/pcg157. [DOI] [PubMed] [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR. Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiology. 2000;123:487–496. doi: 10.1104/pp.123.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiology. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross-talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology. 2002;5:325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lambers H, Atkin OK, Millenaar FF. Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York: Marcel Dekker, Inc.; 2002. pp. 323–362. [Google Scholar]
- Langebartels C, Schraudner M, Heller W, Ernst D, Sandermann H. Oxidative stress and defense reactions in plants exposed to air pollutants and UV-B radiation. In: Inze D, van Montagu M, editors. Oxidative stress in plants. London: Taylor and Francis; 2002. pp. 105–135. [Google Scholar]
- Leubner-Metzger G, Pertruzzelli L, Waldvogel R, Vogeli-Lange R, Meins FMJ. Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I β-1,3-glucanase during tobacco seed germination. Plant Molecular Biology. 1998;38:785–795. doi: 10.1023/a:1006040425383. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serano JJ, Solano R. ETHYLENE RESPONSE FACTOR 1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeder W, Barry CS, Tauriainen J, Utriainen M, Grierson D, Sandermann H, Langabartles C, Kangasjarvi J. Ethylene synthesis regulated by bi-phasic induction of ACC synthase and ACC oxidase genes is required for H2O2 accumulation and cell death in ozone-exposed tomato. Plant Physiology. 2002;130:1918–1926. doi: 10.1104/pp.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, van Montagu M. Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. The Plant Cell. 1997;9:2243–2259. doi: 10.1105/tpc.9.12.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PB, Ainsworth EA, Long SP. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant, Cell and Environment. 2003;26:1317–1328. [Google Scholar]
- Nakajima N, Itoh T, Takikawa S, Asai N, Tamaoki M, Aono M, Kubo A, Azumi Y, Kamada H, Saji H. Improvement in ozone tolerance of tobacco plants with an antisense DNA for 1-aminocyclopropane-1-carboxylate synthase. Plant, Cell and Environment. 2002;25:727–736. [Google Scholar]
- ODonnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Orvar BL, McPherson J, Ellis BE. Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. The Plant Journal. 1997;11:203–212. doi: 10.1046/j.1365-313x.1997.11020203.x. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Kangasjarvi J. Reactive oxygen species and hormonal control of cell death. Trends in Plant Science. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Pellinen R, Kuittenen T, Tuominen H, Ahlfors R, Keinanen M, Saarma M, Scheel D, Kangasjarvi J. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death 1 mutant. Plant Physiology. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, Kangasjarvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. The Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennel RI, Lamb C. Programmed cell death in plants. The Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis . The Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Koch JR, Davis KR. Ozone: a tool for probing programmed cell death in plants. Plant Molecular Biology. 2000a;44:345–358. doi: 10.1023/a:1026548726807. [DOI] [PubMed] [Google Scholar]
- Rao MV, Lee HI, Creelman RA, Mullet JA, Davis KR. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. The Plant Cell. 2000b;12:1633–1646. doi: 10.1105/tpc.12.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Lee HI, Davis KR. Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. The Plant Journal. 2002;32:447–456. doi: 10.1046/j.1365-313x.2002.01434.x. [DOI] [PubMed] [Google Scholar]
- Reiling K, Davison AW. The response of native, herbaceous species to ozone; growth and fluorescence screening. New Phytologist. 1992;120:29–37. [Google Scholar]
- Rojo E, Leon J, Sanchez-Serrano JJ. Cross-talk between wound signaling pathways determines local versus systemic gene expression in. Arabidopsis thaliana. The Plant Journal. 1999;20:135–142. doi: 10.1046/j.1365-313x.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Ryan MG. Effects of climate change on plant respiration. Ecological Applications. 1991;1:157–167. doi: 10.2307/1941808. [DOI] [PubMed] [Google Scholar]
- Schlagnhaufer CD, Glick RE, Arteca RN, Pell EJ. Molecular cloning of an ozone-induced 1-aminocyclopropane-1-carboxylate synthase cDNA and its relationship with a loss of rbcS in potato (Solanum tuberosum L.) plants. Plant Molecular Biology. 1995;28:93–103. doi: 10.1007/BF00042041. [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH. Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in. Zea mays. Physiologia Plantarum. 2003;117:403–412. doi: 10.1034/j.1399-3054.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Hummel GM, Schottner M, Schurr U, Walter A. Jasmonic acid does not mediate root growth responses to wounding in Arabidopsis thaliana . Plant, Cell and Environment. 2010;33:104–116. doi: 10.1111/j.1365-3040.2009.02062.x. [DOI] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese C, van Camp W, Inze D, Langebartels C, Sandermann H. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. The Plant Journal. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:569–589. [Google Scholar]
- Seo HS, Song JT, Cheong J-J, Lee YH, Lee YW, Hwang I, Lee JS, Chol YD. Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proceedings of the National Academy of Sciences, USA. 2001;98:4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Nakajima K, Hashimoto T. Ethylene suppresses jasmonate-induced gene expression in nicotine biosynthesis. Plant and Cell Physiology. 2000;41:1072–1076. doi: 10.1093/pcp/pcd027. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host and Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proceedings of the National Academy of Sciences, USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Nakajima N, Kubo A, Aono M, Matsuyama T, Saji H. Transcriptome analysis of O3-exposed Arabidopsis reveals that multiple signal pathways act mutually antagonistically to induce gene expression. Plant Molecular Biology. 2003;53:443–456. doi: 10.1023/B:PLAN.0000019064.55734.52. [DOI] [PubMed] [Google Scholar]
- Tatsuki M, Mori H. Rapid and transient expression of 1-aminocyclopropane-1-carboxylate synthase isogenes by touch and wound stimuli in tomato. Plant and Cell Physiology. 1999;40:709–715. doi: 10.1093/oxfordjournals.pcp.a029597. [DOI] [PubMed] [Google Scholar]
- Thorne MA, Frank DA. The effects of clipping and soil moisture on leaf and root morphology and root respiration in two temperate and two tropical grasses. Plant Ecology. 2009;200:205–215. [Google Scholar]
- Tingey DT, Standley C, Field RW. Stress ethylene evolution: a measure of ozone effects on plants. Atmospheric Environment. 1976;10:969–974. doi: 10.1016/0004-6981(76)90204-3. [DOI] [PubMed] [Google Scholar]
- Tsonev TD, Lazova GN, Stoinova ZG, Popova LP. A possible role for jasmonic acid in adaptation of barley seedlings to salinity stress. Journal of Plant Growth Regulation. 1998;17:153–159. [Google Scholar]
- Tung P, Hooker TS, Tampe PA, Reid DM, Thorpe TA. Jasmonic acid: effects on growth and development of isolated tomato roots cultured in vitro . International Journal of Plant Sciences. 1996;157:713–721. [Google Scholar]
- Tuomainen J, Betz C, Kangasjarvi J, Ernst D, Yin SH, Langebartels C, Sandermann H., Jr Ozone induction of ethylene emission in tomato plants: regulation by differential transcript accumulation for the biosynthetic enzymes. The Plant Journal. 1997;12:1151–1162. [Google Scholar]
- Tuominen H, Overmyer K, Keinanen M, Kollist H, Kangasjarvi J. Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis . The Plant Journal. 2004;39:59–69. doi: 10.1111/j.1365-313X.2004.02107.x. [DOI] [PubMed] [Google Scholar]
- Turner NC, Rich S, Waggoner PE. Removal of ozone by soil. Journal of Environmental Quality. 1973;2:259–264. [Google Scholar]
- Wager A, Browse J. Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Frontiers in Plant Science. 2012;3:41. doi: 10.3389/fpls.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Close TJ. Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant, Cell and Environment. 2007;30:410–421. doi: 10.1111/j.1365-3040.2006.01628.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mopper S, Hasenstein KH. Effects of salinity on endogenous ABA, IAA, JA and SA in Iris hexagona. Journal of Chemical Ecology. 2001;27:327–339. doi: 10.1023/a:1005632506230. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Seo S, Sakai S. Wound-induced expression of a gene for 1-aminocyclopropane-1-carboxylate synthase and ethylene production are regulated by both reactive oxygen species and jasmonic acid in Cucurbita maxima . Plant Physiology and Biochemistry. 2001;39:121–127. [Google Scholar]
- Wiedhase RA, Lehmann J, Kramell H, Sembdner G, Parthier B. Degradation of ribulose-1,5-bisphosphate carboxylase and chlorophyll in senescing barley leaf segments triggered by jasmonic acid methyl ester, and counteraction by cytokinin. Physiologia Plantarum. 1987;69:161–166. [Google Scholar]
- Wilkinson S, Mills G, Illidge R, Davies WJ. How is ozone pollution reducing our food supply? Journal of Experimental Botany. 2012;63:527–536. doi: 10.1093/jxb/err317. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chang L, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. The Plant Cell. 1994;6:1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zheng S-H, Fujita K, Sakai K. Jasmonate and ethylene signaling and their interaction are integral parts of the elicitor signaling pathway leading to β-thujaplicin biosynthesis in Cupressus lusitanica cell cultures. Journal of Experimental Botany. 2004;55:1003–1012. doi: 10.1093/jxb/erh127. [DOI] [PubMed] [Google Scholar]
- Zhu Z, An F, Feng Y, et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis . Proceedings of the National Academy of Sciences, USA. 2011;108:12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]