Abstract
Objective
To evaluate the significance of the JAK-STAT pathway in insulin-induced cardioprotection from reperfusion injury.
Methods
In isolated perfused rat hearts subjected to insulin therapy (0.3 mU/ml) ± AG490 (5 μM, JAK-STAT inhibitor), the phosphorylation state of STAT3 and Akt was determined after 15 min of reperfusion. Infarct size was measured after 120 min of reperfusion. Isolated cardiac myocytes from wild type (WT) and cardiac specific STAT3 deficient mice were treated with insulin at reoxygenation following simulated ischemia (SI, 26 h). Cell viability was measured after 120 min of reoxygenation following SI, whereas phosphorylation state of Akt was measured after 15 min of reoxygenation following SI.
Results
Insulin given at reperfusion led to phosphorylation of STAT3 and Akt both of which were inhibited by AG490. AG490 also blocked the insulin-dependent decrease in infarct size, supporting a role for JAK-STAT in cardioprotection. In addition, insulin protection from SI was blocked in myocytes from the STAT3 deficient mice, or in WT mice treated with AG490. Furthermore, insulin failed to phosphorylate Akt in the STAT3 deficient cardiomyocytes.
Conclusion
Insulin-induced cardioprotection at reperfusion occurs through activation of STAT3. Inhibiting STAT3 by AG490, or STAT3 depletion in cardiac myocytes affects activation of Akt, suggesting close interaction between STAT3 and Akt in the cardioprotective signalling pathway activated by insulin treatment at reperfusion.
Keywords: insulin, myocardial infarction, ischemia, signal transduction, STAT3
Introduction
The Janus activated kinase (JAK) and Signal Transducer and Activator of Transcription 3 (STAT3) signalling pathway is responsive to a large number of cytokines, growth factors and hormones [6, 7, 18], and is known to play a vital role in cardioprotection related to ischemia-reperfusion (I/R) injury [2, 13, 16, 24, 26, 28, 29, 39, 46]. This pathway is activated by a diversity of receptors, including receptor tyrosine kinases [38, 45, 51] and G-protein-coupled receptors [11, 33]. Upon activation of JAK, STAT proteins dimerize and translocate to the nucleus, where they mediate gene transcription (reviewed by Sandberg et al. [36]). In myocardial tissue it can be difficult to distinguish which receptor system is responsible for activation of JAK/STAT signalling [12]. Previous reports have shown that administrating insulin at reperfusion following ischemia/reperfusion induces cardioprotection via activation of a cascade of prosurvival protein kinases, including phosphatidylinositol 3-kinase (PI3K), Akt and p70s6 kinase [19, 20].
We hypothesize that activation of STAT3 is an alternative protective pathway for insulin-induced cardioprotection because insulin activates JAK2 [15, 35], which phosphorylates and thereby activates STAT3 and STAT5 [48]. Conversely, cardiac insulin resistance is associated with STAT3 deficiency [32]. We will also propose that an interaction exists between the STAT3 and PI3K/Akt pathways. Experiments were performed in isolated rat hearts subjected to pharmacological STAT3 blockade and isolated cardiomyocytes from STAT3 deficient and wild type mice, examining infarct size and cell death after simulated ischemia in these two models.
Materials and methods
The rat experiments conform to the Guidelines on accommodation and care of laboratory animals (by the European Convention for the protection of vertebrate animals), and the Norwegian Committee on Ethics in Animal Experimentation approved all procedures.
The mouse experiments conform to the Guide for the Care and Use of Laboratory Animals published by the U.S National Institutes of Health (NIH Publication No. 85, revised 1996), and all procedures were approved by the Faculty of Health Sciences Animal Ethics Committee, University of Cape Town.
Isolated rat heart: Langendorff perfusion
Male Wistar rats (350 ± 5 g, n = 101; Scanbur BK, Sollentuna, Sweden) fed a standard diet were heparinized (200 IU intraperitoneally (i.p)) and anesthetized (pentabarbitone sodium, 50 mg/kg i.p). The hearts were excised, immersed in ice-cold Krebs-Henseleit buffer (NaCl 118.5, KCl 4.7, MgSO4 1.2, NaHCO3 25.0, CaCl2 2.4, KH2PO4 1.2, and glucose 11.1 (all mM)) and rapidly mounted on a Langendorff perfusion system by the aortic root. The hearts were perfused with Krebs-Henseleit buffer (pH 7.35–7.45, oxygenated with 95% O2/5% CO2 at 37°C) in a nonrecirculating fashion. A water-filled latex-balloon, connected to a pressure transducer, was inserted into the left ventricle via the left auricle and inflated to set a left ventricular end-diastolic pressure between 0–10 mmHg as baseline. Myocardial temperature was maintained at 37°C throughout the experiment. A 3/0 silk suture was placed around the main branch of the left coronary artery. The ends of the suture were threaded through the tip of a pipette to form a snare. After a stabilization period of 20 min, regional ischemia was induced by tightening the suture around the coronary artery by clamping the tip of the pipette against the epicardial surface and locking it into this position with a second pipette. The hearts were subjected to regional ischemia for 30 min, followed by 120 min of reperfusion.
Isolated rat heart: experimental protocol
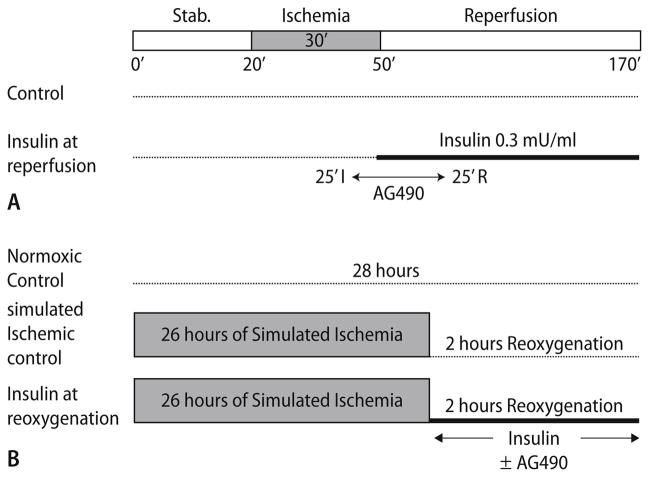
The animals were divided into four groups and the experimental protocols are depicted in Fig. 1a. Group 1 consisted of control hearts (n = 6), subjected to 20 min of stabilization, regional ischemia for 30 min followed by 120 min of reperfusion. In group 2, hearts (n = 4) were subjected to the JAK-STAT inhibitor Tyrphostin AG 490 (AH Diagnostics, Oslo, Norway, 5 μM) for 30 min, administration starting from 25 min of regional ischemia to 25 min of reperfusion. In group 3 (n = 6), insulin (0.3 mU/ml) was administrated from 29 min of regional ischemia throughout the duration of reperfusion (InsR). Group 4 consisted of InsR+AG hearts (n = 7); AG was added from 25 min of regional ischemia to 25 min of reperfusion. The AG 490 compound was dissolved in dimethyl sulfoxide (DMSO), and diluted in Krebs buffer on the day of the experiment (final DMSO concentration < 0.05%).
Fig. 1.
Treatment protocols for (a) isolated perfused rat hearts and (b) isolated cardiac myocytes. AG490-JAK/STAT inhibitor
After 120 min of reperfusion, the coronary artery was re-occluded, and Evans Blue dye was infused through the aorta to demarcate the myocardial risk zone. The hearts were weighed, frozen overnight and cut into 2-mm thick slices. The slices were incubated for 15 min with 1% triphenyl-tetrazolium chloride (TTC; Sigma-Aldrich, Schnelldorf, Germany) in phosphate buffer (pH 7.4, 37°C) and immersed in 10% formaldehyde to enhance the contrast between viable (stained) and necrotic (unstained) tissue. The slices were then placed between two glass plates 2 mm apart. The area of the left ventricle, the area of the risk zone, and the infarcted area were traced on an acetate transparency, and infarct size was assessed by using computerized planimetry. Infarct size was expressed as percentage of risk zone.
Cardiomyocyte isolation
Cardiac specific STAT3 deficient mice were created by crossing homozygous floxed STAT3 mice [42] with heterozygous MLC2V-driven Cre-recombinase mice [4, 5]. Extraction of genomic DNA, and the presence of floxed STAT3 and MLC2V Cre-recombinase was confirmed by PCR analysis, as previously described [39]. Cardiomyocytes were isolated using the modified method of Zhou et al. [50]. In brief, male wildtype and STAT3 depleted mice (n = 10 per group), aged between 14–16 weeks, were anesthetized (pentobarbitone sodium, 60 mg/kg i.p) and heparinized (25 IU i.p). Hearts were rapidly removed and perfused at 37°C through an aortic cannula with a calcium-free HEPES-buffer (composition: NaCl 116.0, HEPES 17.4, NaH2PO4 0.9, glucose 5.6, KCl 5.4, MgSO4 0.4 (all mM), pH 7.4). Cannulated hearts were perfused with HEPES-buffer containing 0.025 mM EGTA and 0.1% BSA for 10 min to remove all blood. Hearts were then perfused with HEPES-buffer containing collagenase type II (0.14 mg/ml) (Worthington Biochemicals) and CaCl2 (25 μM), in a recirculating manner for 15–25 min to digest the hearts. The collagenase was washed out from well digested hearts with HEPES-buffer containing 25 μM CaCl2 for 10 min, before being removed from the cannula and minced finely with scissors in 25 ml restoration buffer (HEPES-buffer with Na pyruvate 10.0, EGTA 10.0, carnitine 1.6, taurine 5.0, creatine 5.0, CaCl2 0.1 (all mM) and 1% BSA, pH 7.4). This minced tissue suspension was shaken vigorously at 37°C for 15 min, the resulting suspension was filtered through a sterile nylon mesh (40 μm mesh size, Spectrum Laboratories Inc.) and the cells were pelleted at 1,500 rpm for 10 min (Sanyo Harrier 18/80). The supernatant was decanted and the cells resuspended in 25 ml restoration buffer. 100 mM calcium was added in an incremental manner to achieve a final concentration of 1.25 mM over a period of 12 min. Cells were spun down at 1,500 rpm for 10 min and the resulting pellet of cardiomyocytes was resuspended in modified, insulin free, KSLMS (Kawamoto, Sato, Le, McClure, Sato) [23] media. Isolated cardiomyocytes were quantified and a threshold of 60% viable rod shaped myocytes was considered to be a good quality preparation of isolated cardiomyocytes. Isolated myocytes were cultured in standard tissue culture flasks in KSLMS media for 16–24 h at 37°C in a humidified 5% CO2 atmosphere prior to experimentation.
Isolated cardiomyocytes: simulated ischemia
Isolated cardiomyocytes were exposed to simulated ischemia (SI) in a modified buffer [10] containing : NaCl 118.0, KCl 3.6, MgCl2 0.5, CaCl2 0.9, HEPES 4.0 and 2-deoxy-D-glucose (2-DG) 20.0 (all mM) at pH 6.2 for 26 h, at 37°C, in a humidified environment containing 1% O2, 5% CO2 and the balance N2. This was followed by 2 h of reoxygenation in insulin free KLSMS media under normoxic conditions (Fig. 1b). Normoxic myocytes were maintained in insulin free KSLMS media, pH 7.4, in a humidified environment containing 20% O2, 5% CO2 and balance N2 for 28 h. Insulin therapy at reoxygenation was performed by adding insulin (0.3 mU/ml) to KSLMS with or without the JAK-STAT inhibitor AG490 (100 nM) for the entire reoxygenation period (Fig. 1b). In each experiment 350,000–500,000 myocytes were used.
Isolated cardiomyocytes: measurement of cell viability
Cardiomyocyte viability was quantified at the end of the 2 h reoxygenation period by exclusion of trypan blue and rod-shaped morphology [25, 50] by a researcher blinded to the groups, and a total of 100 cells or more was counted per sample. Myocytes were viable if they were light blue in colour and rod shaped. Dead myocytes were dark blue and rounded. Results are expressed as percentage (%) of viable myocytes.
Protein isolation and western blot analysis
Hearts excised from male Wistar rats were Langendorff perfused with Krebs-Henseleit buffer, and subjected to 30 min of regional ischemia followed by 15 min of reperfusion. Area at risk was then separated from area not at risk and snap-frozen in liquid nitrogen for subsequent determination of changes in the levels of phosphorylated STAT3 (Tyr705) in the cytosolic fraction of tissue homogenate by Western blot analysis.
To ensure homogeneity, samples collected from the area at risk were pulverized under liquid nitrogen prior to extraction of protein. 25–35 mg of powder was weighed out and homogenised on ice in 600 μl of suspension buffer containing: EDTA 2.5, EGTA 0.5, β-glycerophosphate 40, sodium pyrophosphate 10, sodium orthovanadate 4, NaF 30, β-mercaptoetoh 10 (all mM), PBS 1 ml, igepal 1%, sodium dodecylsulphate (SDS) 0.1%, sodium deoxycholate 0.5% and complete tablet 1 ml. The homogenised samples were then centrifuged at 14,000 × g for 10 min at 4°C. The pellet was discarded and protein quantification of the supernatant from each protein sample was performed using the Bradford method (Sigma, St.Louis, USA). The protein samples were then diluted in 2 X sample buffer containing: Tris 62.5 mM (pH 6.8), glycerol 10%, SDS 2%, bromophenol blue 0.2% and β-mercaptoetoh 0.5 ml, and boiled for 4 min. Samples (30 μg/lane) were then subjected to electrophoresis on a 10% SDS-polyacrylamid gel and transferred onto nitro-cellulose membranes. The membranes were blocked for 1 h in phosphate buffer saline (PBS, pH 7.6), containing Tween-20 0.1% and non-fat dry milk 5%, and thereafter incubated overnight at 4°C with antibodies for phospho-Akt (Ser473), total Akt (1:1000 dilution), phospho STAT3 (Tyr705) and total STAT3 (1:500 dilution) (all antibodies from CST, USA). The membranes were washed and treated for 1 h with anti-rabbit Ig, Horseradish-peroxidase linked whole antibody. The proteins were detected with ECL reagents (Chemicon, CA, USA) and immunopositive bands were visualized using a Kodak Image Station 1000 (PerkinElmer, USA). Ponceau S staining (Sigma, St. Louis, USA) confirmed equal loading.
Isolated cardiac myocytes from WT and KO mice (n = 4) were subjected to SI for 26 h followed by reoxygenation for 15 min with or without insulin (0.3 mU/ml). Proteins were extracted from the isolated cardiac myocytes and quantitated as previously described [50], resolved by sodium dodecyl sulphatepolyacrylamide gel electrophoresis and transferred to PVDF membranes by electrophoretic transfer. Immunoblotting analysis was performed with Akt Ser473 antibody (CST, USA). The membranes were washed and treated with donkey anti-rabbit Horseradish-peroxidase conjugated antibody (Santa Cruz, CA, USA) and visualised with chemiluminescent reagents (Amersham). Relative peptide levels were measured using densitometric analysis with UVI band (UVI Tech, Cambridge UK) software on a personal computer.
Statistical analysis
All data are presented as mean values ± standard error of the mean (S.E.M). One way analysis of variance (ANOVA or Kruska Wallis one way analysis of variance on ranks) combined with Student-Newman-Keuls post hoc tests was used to test for differences between groups. Non-parametric tests were used for Western blot data. The regression analysis was performed using the GraphPad instat programme. P < 0.05 was considered statistically significant.
Results
Infarct size in Langendorff perfused rat hearts
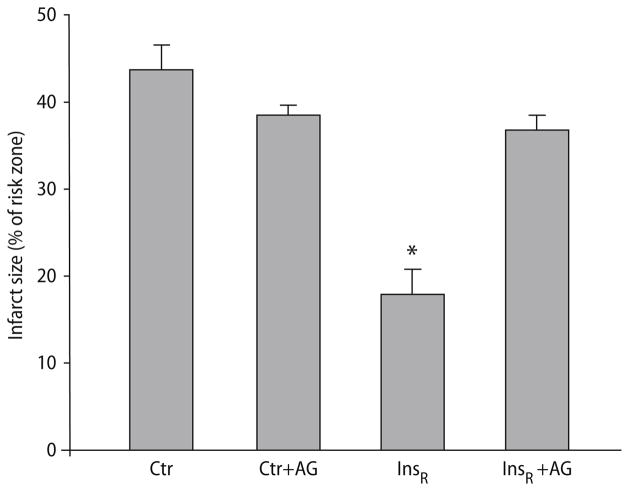
The cardioprotective effect of insulin at reperfusion (InsR) was examined. Infarct size was significantly reduced with InsR (17.9 ± 2.9% vs controls 43.6 ± 3.0, P < 0.001; Fig. 2). To elucidate whether insulin exerts its cardioprotective effect through a JAK/STAT-dependent pathway, we treated the hearts with Tyrphostin AG490, a JAK/STAT inhibitor. The protective effect of insulin was completely abolished by co-administration of AG490 (36.8 ± 1.7 vs InsR 17.9 ± 2.9, P < 0.001; Fig. 2). Administration of the inhibitor alone had no effect on infarct size compared to control (38.4 ± 1.2 vs control 43.6 ± 3.0, P = NS; Fig. 2).
Fig. 2.
Infarct size expressed as percentage of the area at risk in isolated rat hearts treated with insulin (0.3 mU/ml) at reperfusion after ischemia, with or without the JAK/STAT inhibitor, AG490 (5 μM). Values represent means ± S.E.M. *P < 0.001, significant difference compared to controls
Insulin-induced phosphorylation of STAT3 and Akt in isolated rat hearts was abrogated by AG490
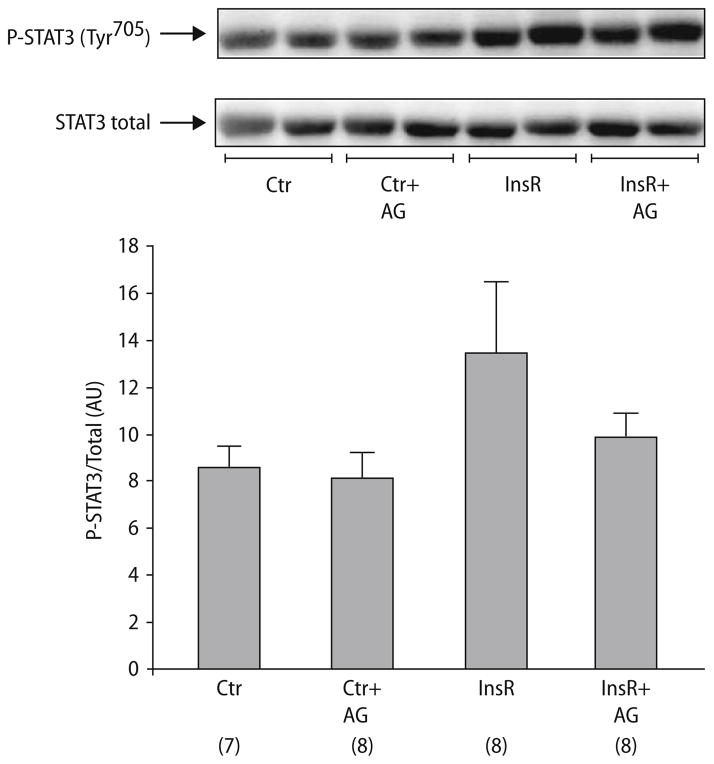
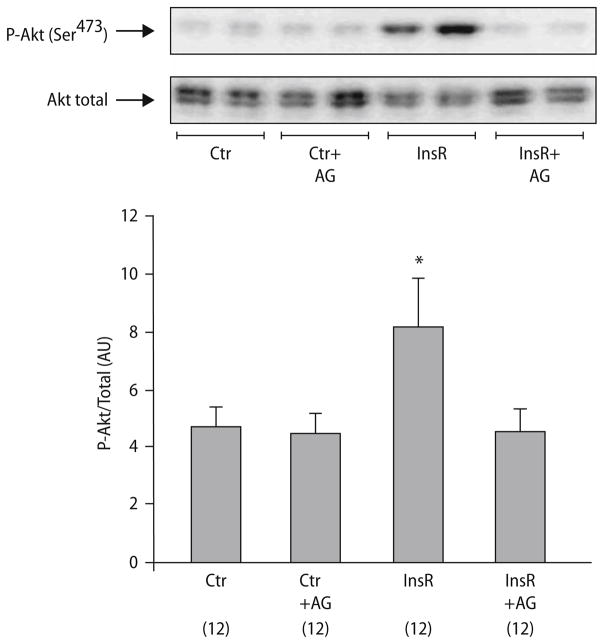
After confirming the ability of insulin to increase the level of phosphorylated Akt and STAT3 under standard conditions in untreated non-ischemic hearts (data not shown), we tested insulin’s ability to phosphorylate STAT3 in isolated rat hearts subjected to insulin (0.3 mU/ml) for 15 min at reperfusion after 30 min of regional ischemia. Although there was no significant difference between the groups, there was a tendency towards insulin-induced phosphorylation of STAT3 at Tyr705 compared to controls (13.46 ± 2.96 vs 8.56 ± 0.86 AU, P = 0.139; Fig. 3). Co-administration of AG 490 attenuated phosphorylation of STAT3 (10.00 ± 0.89 AU, Fig. 3), whereas AG490 had no effect on the phosphorylation of STAT3 on its own (8.06 ± 1.11AU, Fig. 3). Furthermore, to explore whether JAK/STAT could also be an upstream target in the Akt-pathway, the effect of insulin-induced activation on Akt in the presence of JAK/STAT inhibitor was examined. After stimulation with insulin, phosphorylation of Akt at Ser473 was observed in the isolated rat heart (8.16 ± 1.65 for InsR vs 4.68 ± 0.68 in controls (all AU), P < 0.05; Fig. 4). Co-administration of AG 490 attenuated the insulin induced phosphorylation of Akt (4.54 ± 0.75 AU, P = NS vs control; Fig. 4), whereas administration of AG alone had no effect on the phosphorylation level of Akt (4.42 ± 0.72 AU, P = NS vs control, Fig. 4). Additionally, no differences were seen when comparing the control to a group treated with DMSO (data not shown).
Fig. 3.
Representative western blot analysis and relative densitometry (arbitrary units) of phospho-STAT3 Tyr705/Total STAT3 for insulin-treated tissue samples from isolated rat hearts with or without AG490. Ctr control, InsR insulin at reperfusion (0.3 mU/ml), AG AG490 (5 μM). (n = samples/group, shown in parentheses). Values represent means ± S.E.M
Fig. 4.
Representative western blot analysis and relative densitometry (arbitrary units) of phospho-Akt Ser473 in insulin at reperfusion hearts, with or without AG490. (n = samples/group, shown in parentheses). Values represent means ± S.E.M. *P < 0.05, significant difference compared to control
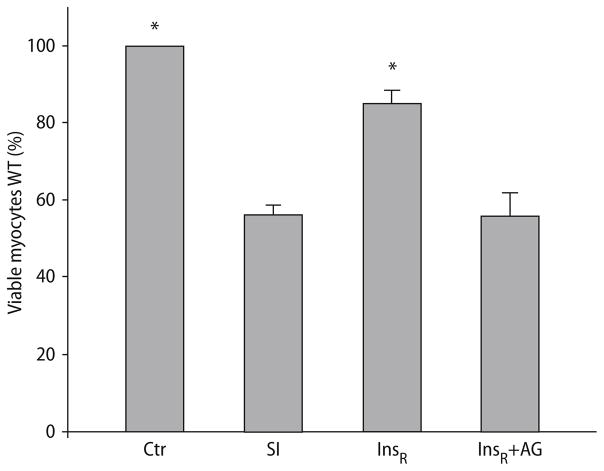
Increase in cell viability induced by administrating insulin at reoxygenation after SI in WT cardiomyocytes was abrogated by inhibition of STAT3
Simulated ischemia in WT cardiomyocytes reduced cell viability (56.3 ± 2.08 vs normoxic controls, P < 0.001; Fig. 5). Administrating insulin at reoxygenation after SI afforded protection to the WT myocytes (viability increased to 85.0 ± 3.4% vs WT, P < 0.001; Fig. 5), this protection was abrogated by co-administration of AG490 (viability of 55.9 ± 5.9% vs SI, P = NS; Fig. 5).
Fig. 5.
Primary cardiomyocyte viability from WT mice under control conditions (ctr), following 26-h of simulated ischemia (SI) and insulin at reoxygenation, with or without AG490 (InsR and InsR + AG, respectively). Bars represent mean ± S.E.M. *P < 0.001, significant difference compared to ischemic controls
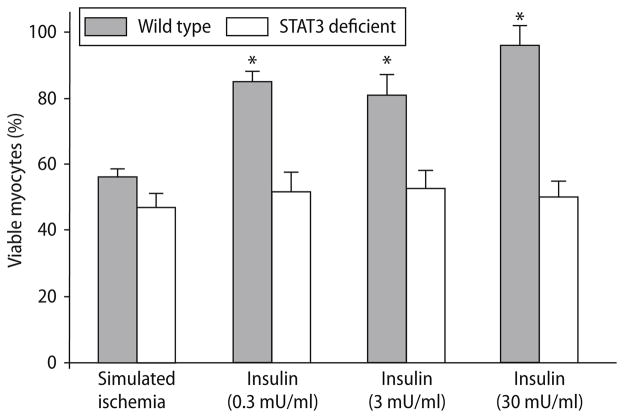
Insulin therapy at reoxygenation after SI in STAT3 deficient cardiomyocytes
To examine the necessity of STAT3 in the cardioprotective signalling pathway activated by insulin therapy, we administrated insulin at reperfusion in cardiomyocytes from STAT3 deficient mice. Simulated ischemia reduced cell viability in the STAT3 deficient cardiomyocytes, to the same degree as WT (47.1 ± 4.17 vs WT 56.3 ± 2.08, P = NS; Fig. 6). Different doses of insulin (0.3, 3 and 30 mU/ml) improved cell viability in WT myocytes (85.2 ± 3.4%, 81.2 ± 6.2% and 96.2 ± 5.7%; P < 0.001 vs ischemic control; Fig. 6), but not in myocytes isolated from STAT3 deficient hearts (51.8 ± 5.6%, 52.8 ± 5.3% and 50.2 ± 4.7%; P = NS vs ischemic control; Fig. 6).
Fig. 6.
Primary cardiomyocyte viability from WT and STAT3 deficient mice following 26-h of simulated ischemia, and insulin at reoxygenation (0.3, 3 and 30 mU/ml). Bars represent mean ± S.E.M. *P < 0.001, significant difference compared to ischemic control
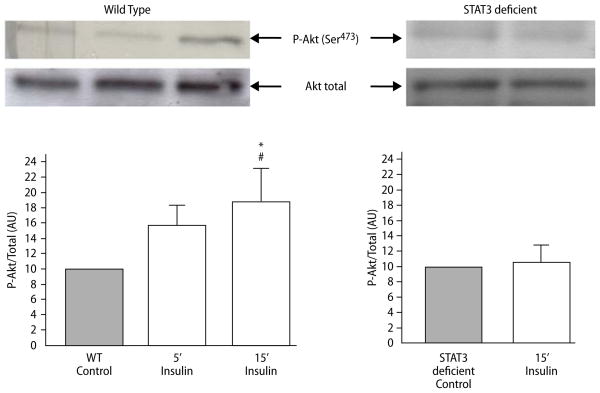
Genetic depletion of STAT3 in the mouse heart abrogated insulin-induced phosphorylation of Akt
We examined whether genetic depletion of STAT3 had any effect on insulin-induced activation of Akt. Western blot analysis after 5 and 15 min of insulin administration at reoxygenation showed increased phosphorylation of Akt in WT cardiomyocytes (15.6 ± 2.2 and 18.7 ± 3.8 respectively vs 10.0 ± 0.0 in controls; Fig. 7, Wild type). Phosphorylation of Akt was increased by 15 min of insulin-administration (18.7 ± 3.8 vs 10.0 ± 0.0 in controls; P = 0.05; Fig. 7, Wild type). Regression analysis was performed to compare 5 and 15 min of insulin administration (r2 = 0.60 and P = 0.04; Fig. 7, Wild type). However in STAT3 deficient cardiomyocytes, insulin failed to induce phosphorylation of Akt (Fig. 7, STAT3 deficient).
Fig. 7.
Representative Western blots and relative densitometry showing the effect of insulin therapy on phospho-Akt Ser473 in wild type (WT) and in STAT3 deficient mice. Bars represent means ± S.E.M. *P = 0.05 difference vs controls and #P = 0.04 by regression analysis. Comparison of all the data points at each time gives significance (P = 0.04; r2 = 0.60) on the regression analysis
Discussion
The present study investigated the involvement of the JAK-STAT pathway in the cardioprotective signalling activated by insulin therapy at reperfusion in isolated rat hearts and cardiac mouse myocytes. Our results delineated STAT3 as an important novel mediator in the cardioprotective pathway activated by insulin therapy. Blocking of STAT3 activity by a JAK-STAT inhibitor, AG490, led to loss of cardioprotection offered by insulin administration at reperfusion in both isolated rat hearts and isolated WT cardiac myocytes. Administration of insulin did not activate Akt in STAT3 deficient cardiac myocytes, and pharmacological inhibition of JAK-STAT in the perfused rat heart completely abolished the insulin induced increase in phosphorylation of Akt at reperfusion, therefore strongly suggesting that an interaction exists between the STAT3 and PI3K/Akt pathways [40]. Furthermore, in cardiac myocytes from STAT3 deficient mice, administration of insulin at reoxygenation failed to offer protection. Additional support for the role of STAT3 activation in insulin-mediated cardioprotective signalling was provided by the strong trend towards abolition of insulin-induced phosphorylation of STAT3 by AG490 in isolated rat hearts.
Signalling pathways involved
We have previously reported that insulin alone; via PI3K/Akt and p70s6 kinase signalling confers cardioprotection by anti-apoptotic pathways [20]. Insulin can activate JAK2 [15, 35], an upstream activator of STAT3, and the present study indicates STAT3 as a novel cytoprotective cell signalling pathway in insulin-induced cardioprotection at reperfusion. Co-administration of the JAK-STAT inhibitor AG490 with insulin abolished the cardioprotective effect of insulin in isolated Langendorff perfused rat hearts, and reduced the amount of viable cardiomyocytes from WT mice. In addition, cardiac specific STAT3 deficient mice were not protected by insulin, therefore implicating a role for the JAK-STAT pathway in insulin-induced cardioprotection. In isolated rat hearts, we could not observe a significant increase in STAT3 phosphorylation following insulin stimulation, possibly due to the time point when the samples were collected (15 min of reperfusion), because insulin induced tyrosine phosphorylation of STAT3 in wild type mice exposed to 2 h of hyperinsulinemic-euglycemic clamp experiments [32], and in C2C12 cells exposed to insulin for 72 h [43]. These results could also be explained by the fact that our Western blots were performed only on cytosolic extracts only while most of phopho-STAT3 had probably translocated to the nucleus.
The use of STAT3 deficient mice has demonstrated the importance of STAT3 phosphorylation in the cardioprotective pathway activated by ischemic [28] and pharmacological [24, 27] preconditioning. In tumor necrosis factor alpha (TNFα)-mediated preconditioning, this phosphorylation confers cardioprotection independently of the phosphorylation of Akt, therefore suggesting a parallel and independent pathway [40]. The inhibitor AG490 attenuated insulin-induced phosphorylation of both STAT3 and Akt. This could imply but does not prove that Akt is placed downstream of STAT3 in the cardioprotective signalling pathway activated by insulin therapy. Alternately there could be cross-talk between these two signals. The first possibility was supported by experiments in STAT3 deficient mice, where insulin failed to phosphorylate Akt. Ueda et al. [44] recently reported that granulocyte colony stimulating factor (G-CSF) activated JAK-STAT, PI3K and Akt. Use of the JAK2 inhibitor AG490 abrogated G-CSF induced phosphorylation of JAK2, STAT3, Akt; and the PI3K inhibitor LY294002 suppressed G-CSF induced phosphorylation of Akt, but not JAK2 or STAT3. The authors therefore suggested that JAK-STAT is placed upstream of Akt.
Previous studies have implicated molecular cross-talk between JAK/STAT and PI3K pathways in nonmyocardial cells activated by luteinizing hormone or carbon monoxide [3, 49] and in myocardial cells activated by opioids [13]. Furthermore, coupling of PI3K to the interferon surface receptor-1 through STAT3 has been reported [34]. This is in concordance with the assumption of STAT3 as an upstream regulator of Akt activity. However, PI3K may act via Akt-independent pathways as shown by O’Neill et al. [30]. It is also appropriate to stipulate that it is not necessarily the activity of Akt, but rather the location of Akt which determines the phenotypic effect resulting from Akt activation [41].
Recently mammalian target of rapamycin complex 2 (mTORC2) has been reported as an activator of Akt at Ser473 [37], whereas phosphoinositide-dependent kinase 1 (PDK1) has been identified as the kinase responsible for phosphorylating Akt at Thr308 [1]. This raises the possibility of two parallel pathways leading to activation of Akt, so that the JAK/STAT pathway could be connected to mTORC2 and phosphorylation of Akt at the Ser residue. On the other hand, Jonassen et al. identified mTOR-p70s6 kinase as downstream from Akt [20]. Suleman et al. [40] have suggested that in ischemic preconditioning, both the JAK/STAT and the PI3K-Akt pathways are activated with a close interaction in that activation of both pathways was required, and inhibition of the one blocked functioning of the other. A proposal of interest is that STAT3, besides having a nuclear site of action, could serve as an adaptor protein linking together two proteins outside the nucleus [13].
The present data and other studies raise the issues of close interactions between STAT3, Akt and PI3K (Table 1). We speculate that STAT-3 may have direct links to Akt as an upstream target, or that Akt phosphorylation results from insulin-stimulated JAK2 activation and association with IRS and thereby involvement in the PI3K-Akt-pathway.
Table 1.
Comparison of studies exploring both Akt and STAT3 in Ischemia-reperfusion
| Author | Present | Suleman (2008) [40] | Gross, (2006) [13] | Lecour (2005) [24] |
|---|---|---|---|---|
| Preparation | Perfused rat heart, myocytes | Perfused rat heart, myocytes | Rat heart in situ | Perfused rat heart |
| Stimulus | Insulin | Ischemic PC | Opioid-PC | TNF-PC |
| Phase of protection | Protection at reperfusion | Trigger | Trigger and reperfusion | Reperfusion |
| STAT3 KO | No protection | No protection | No data | No protection |
| STAT3-P | AG490 ↓↓ | Wortmannin ↓↓ AG490↓↓ | Wortmannin↓↓ AG490↓↓ | AG490↓↓ |
| PI3K-Akt-P | No data | Wortmannin↓↓ | Wortmannin↓↓ | Wortmannin no effect |
| Akt-P | Not in STAT3 KO | Not in STAT3 KO AG490↓↓ | AG490↓↓ | Not involved |
| Interacting signals | Akt, STAT3 | PI3K, Akt, STAT3 | PI3K, Akt, STAT3 | STAT3, no interaction with PI3K, Akt |
PC preconditioning, TNF tumor necrosis factor, STAT3 KO signal transducer and activator of transcription 3 knock out, AG490 tyrphostin AG490, PI3K phosphatidylinositol 3-kinase, P phosphorylation
Possible clinical application
Insulin alone, or, as part of the metabolic cocktail glucose-insulin-potassium (GIK) [22], as well as opioids [14] and atrial natriuretic peptide [47] all reduce infarct size when administered during the reperfusion phase. To confer its cardioprotection, insulin needs to be given at least during the first 15 min of reperfusion in an isolated rat heart model where it activates PI3K signalling pathways [19–21]. Insulin may also reduce mortality as suggested by a retrospective analysis of patients with acute myocardial infarction undergoing reperfusion therapy [9]. Using insulin infusions in the same dosage range as in the isolated heart and cardiomycocyte experiments to protect the ischemic myocardium upon reperfusion would constitute a safe and cheap technique. Two such trials are currently underway [31]. The effects of insulin as a hormone are well characterized, and since insulin naturally occurs in the body, treatment with insulin is unlikely to cause tumorigenesis such as other anti-apoptotic treatments may do.
Reservations
An important reservation is that at present there are no specific pharmacological inhibitors available for STAT3. Furthermore, when using pharmacological inhibitors, nonspecific effects may occur, and the inhibitory effect of AG490 on Akt as we observed, could be such a non-specific effect. However, previous studies with STAT3 deficient mice have shown involvement of STAT3 in the cardioprotective signalling pathways activated by ischemic [39] and pharmacological preconditioning [13, 24], including protection of cardiomyocytes from apoptosis [17, 39]. In non-cardiac studies, AG490 was described as a selective inhibitor of JAK2 kinase, having no effect on the kinase activity of other protein tyrosine kinases [8, 27]. Negoro et al. [28] reported that AG490 reduced the phosphorylation of STAT3 through inhibition of JAK2, while AG490 had no direct effect on either the mitogen activated protein kinase (MAPK) family or PI3K signalling. Thus, the inhibitory effect of AG490 on Akt in presence of insulin that was observed in this study could be due to an involvement of JAK2 but not STAT3 in PI3K-Akt signalling. Furthermore, insulin-stimulated activation of JAK2 results in a physical association between JAK2 and the insulin receptor substrate (IRS) [15, 35]. In our study, lack of insulin-induced phosphorylation of Akt in STAT3 deficient cardiomyocytes was observed, suggesting that STAT3 is activated upstream of Akt, or that JAK2 activation leads to parallel stimulation of both Akt and STAT3.
Conclusions
In summary, insulin-induced cardioprotection at reperfusion occurs through activation of STAT3. STAT3 cardiac deficient mice failed to be protected by insulin and pharmacological inhibition of STAT3 by the JAK-STAT inhibitor AG490 abolished cardioprotection offered by insulin therapy at reperfusion. Furthermore, inhibiting STAT3 by AG490, or knocking out STAT3 in cardiac myocytes affected phosphorylation of Akt, therefore suggesting that STAT3 phosphorylation and activation of Akt are closely associated in the cardioprotective signalling pathway activated by insulin treatment at reperfusion. The involvement of STAT3 in prosurvival signalling pathways activated by different pharmacological agents is of current interest [13, 17, 24, 40], and further studies should elucidate the significance of STAT3 activation in the attenuation of reperfusion-induced injury.
Acknowledgments
This work was supported by the Norwegian Research Council, the SOUTH AFRICA-NORWAY programme on research co-operation, and by the Medical Research Council of South Africa. B.N.F was supported by the Norwegian Council on Cardiovascular Diseases, The Norwegian National Health Association. N.S was supported by the South African National Research Foundation. S.L was supported by a Servier Senior Fellowship in Heart Failure. We thank Shizuo Akira for the floxed STAT3 mouse and Kenneth R Chien for the MLC2V cre-recombinase mice, and Professor Kirsti Ytrehus for helpful comments.
Contributor Information
Britt N. Fuglesteg, Email: brittf@fagmed.uit.no, Dept. of Medical Physiology, Faculty of Medicine, University of Tromsøm, 9037 Tromsø, Norway, Tel.: +47/776-44874, Fax: +47/776-45440
Naushaad Suleman, Hatter Institute for Cardiovascular Research, Dept. of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Crina Tiron, Dept. of Biomedicine, Faculty of Medicine, University of Bergen, Bergen, Norway.
Tambuzai Kanhema, Dept. of Biomedicine, Faculty of Medicine, University of Bergen, Bergen, Norway.
Lydia Lacerda, Hatter Institute for Cardiovascular Research, Dept. of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Thomas V. Andreasen, Dept. of Medical Physiology, Faculty of Medicine, University of Tromsøm, 9037 Tromsø, Norway, Tel.: +47/776-44874, Fax: +47/776-45440
Michael N. Sack, 10 NIH/NHLBI, Bethesda (MD), USA
Anne K. Jonassen, Dept. of Biomedicine, Faculty of Medicine, University of Bergen, Bergen, Norway
Ole D. Mjøs, Dept. of Medical Physiology, Faculty of Medicine, University of Tromsøm, 9037 Tromsø, Norway, Tel.: +47/776-44874, Fax: +47/776-45440
Lionel H. Opie, Hatter Institute for Cardiovascular Research, Dept. of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
Sandrine Lecour, Hatter Institute for Cardiovascular Research, Dept. of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
References
- 1.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R, Dawn B, Xuan YT. Emerging role of the JAK-STAT pathway as a mechanism of protection against ischemia/reperfusion injury. J Mol Cell Cardiol. 2001;33:1893–1896. doi: 10.1006/jmcc.2001.1469. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho CRO, Carvalheira JBC, Lima MHM, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJA. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Kubalak SW, Minamisawa S, Price RL, Becker KD, Hickey R, Ross J, Jr, Chien KR. Selective requirement of myosin light chain 2v in embryonic heart function. J Biol Chem. 1998;273:1252–1256. doi: 10.1074/jbc.273.2.1252. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a noncell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125:1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- 6.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 7.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 8.De Vos J, Jourdan M, Tarte K, Jasmin C, Klein B. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells. Br J Haematol. 2000;109:823–828. doi: 10.1046/j.1365-2141.2000.02127.x. [DOI] [PubMed] [Google Scholar]
- 9.Díaz R, Paolasso EA, Piegas LS, Tajer CD, Moreno MG, Corvalán R, Isea JE, Romero G. Metabolic modulation of acute myocardial infarction: The ECLA glucose-insulin-potassium pilot trial. Circulation. 1998;98:2227–2234. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- 10.Esumi K, Nishida M, Shaw D, Smith TW, Marsh JD. NADH measurements in adult rat myocytes during simulated ischemia. Am J Physiol. 1991;260:1743–1752. doi: 10.1152/ajpheart.1991.260.6.H1743. [DOI] [PubMed] [Google Scholar]
- 11.Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 12.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102:279–297. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- 13.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt and GSK-3β. Am J Physiol Heart Circ Physiol. 2006;291:827–834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 14.Gross ER, Hsu AK, Gross GJ. GSK3β inhibition and KATP channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol. 2007;102:341–349. doi: 10.1007/s00395-007-0651-6. [DOI] [PubMed] [Google Scholar]
- 15.Gual P, Baron V, Lequoy V, Van Obberghen E. Interaction of Janus kinases JAK-1 and JAK-2 with the insulin receptor and the insulin-like growth factor-1 receptor. Endocrinology. 1998;139:884–893. doi: 10.1210/endo.139.3.5829. [DOI] [PubMed] [Google Scholar]
- 16.Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, Siddiqui MAQ, Das DK. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001;33:1929–1936. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 17.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 18.Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 19.Jonassen AK, Mjøs OD, Sack MN. p70s6 kinase is a functional target of insulin activated Akt cell-survival signaling. Biochem Biophys Res Commun. 2004;315:160–165. doi: 10.1016/j.bbrc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Jonassen AK, Sack MN, Mjøs OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 21.Jonassen AK, Brar BK, Mjos OD, Sack MN, Latchman DS, Yellon DM. Insulin administered at reoxygenation exerts a cardioprotective effect in myocytes by a possible anti-apoptotic mechanism. J Mol Cell Cardiol. 2000;32:757–764. doi: 10.1006/jmcc.2000.1118. [DOI] [PubMed] [Google Scholar]
- 22.Jonassen AK, Aasum E, Riemersma RA, Mjøs OD, Larsen TS. Glucoseinsulin-potassium reduces infarct size when administered during reperfusion. Cardiovasc Drugs Ther. 2000;14:615–623. doi: 10.1023/a:1007802630604. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto T, Sato JD, McClure DB, Sato GH. Serum-free medium for the growth of NS-1 mouse myeloma cells and the isolation of NS-1 hybridomas. Meth Enzymol. 1986;121:266–277. doi: 10.1016/0076-6879(86)21024-1. [DOI] [PubMed] [Google Scholar]
- 24.Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH. Pharmacological preconditioning with tumor necrosis factor-α activates signal tranducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–3918. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 25.Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA, Lochner A. Activation of p38 MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. J Mol Cell Cardiol. 2001;33:769–778. doi: 10.1006/jmcc.2001.1347. [DOI] [PubMed] [Google Scholar]
- 26.Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, Siddiqui MAQ. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–329. doi: 10.1161/01.cir.104.3.325. [DOI] [PubMed] [Google Scholar]
- 27.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a JAK-2-inhibitor. Nature. 1996;379:645–647. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 28.Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- 29.Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, Yamauchi-Takihara K. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47:797–805. doi: 10.1016/s0008-6363(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, Boudina S, Wayment B, Litwin SE, Shioi T, Izumo S, Birnbaum MJ, Abel ED. A conserved role for phospatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opie LH. Metabolic management of AMI comes to the fore and extends beyond control of hyperglycemia. Circulation. 2008;117:1610–1619. doi: 10.1161/CIRCULATIONAHA.108.780999. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Cho YR, Finck BN, Kim HJ, Higashimori T, Hong EG, Lee MK, Danton C, Deshmukh S, Cline GW, Wu JJ, Bennett AM, Rothermel B, Kalinowski A, Russell KS, Kim YB, Kelly DP, Kim JK. Cardiac-specific overexpression of peroxisome proliferators-activated receptor-α causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier S, Duhamel F, Coulombe P, Popoff MR, Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol Cell Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeffer LM, Mullersman JE, Pfeffer SR, Murti A, Shi W, Yang CH. STAT3 as an adapter to couple phosphatidylinositol 3-kinase to the IFNAR1 chain of the type I interferon receptor. Science. 1997;276:1418–1420. doi: 10.1126/science.276.5317.1418. [DOI] [PubMed] [Google Scholar]
- 35.Saad MJ, Carvalho CR, Thirone AC, Velloso LA. Insulin induces tyrosine phosphorylation of JAK2 in insulin-sensitive tissues of the intact rat. J Biol Chem. 1996;271:22100–22104. doi: 10.1074/jbc.271.36.22100. [DOI] [PubMed] [Google Scholar]
- 36.Sandberg EM, Wallace TA, Godeny MD, VonDerLinden D, Sayeski PP. Jak2 tyrosine kinase. A true jak of all trades? Cell Biochem Biophys. 2004;41:207–231. doi: 10.1385/cbb:41:2:207. [DOI] [PubMed] [Google Scholar]
- 37.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 38.Shuai K, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- 39.Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, Sack MN. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–616. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Suleman N, Somers S, Smith R, Opie LH, Lecour S. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn067. in press. [DOI] [PubMed] [Google Scholar]
- 41.Sussman M. “AKT”ing lessons for stemcells: regulation of cardiacmyocytes and progenitor cell proliferation. Trends Cardiovasc Med. 2007;17:235–240. doi: 10.1016/j.tcm.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 43.Taler M, Shpungin S, Salem Y, Malovani H, Pasder O, Nir U. Fer is a downstream effector of insulin and mediates the activaton of signal transducer and activator of transcription 3 in myogenic cells. Mol Endocrinol. 2003;17:1580–1592. doi: 10.1210/me.2002-0328. [DOI] [PubMed] [Google Scholar]
- 44.Ueda K, Takano H, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, Komuro I. Granulocyte colony stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-Endothelial NO synthase pathway. Arterioscler Thromb Vasc Biol. 2006;26:108–113. doi: 10.1161/01.ATV.0000219697.99134.10. [DOI] [PubMed] [Google Scholar]
- 45.Vignais ML, Gilman M. Distinct mechanisms of activation of Stat1 and Stat3 by platelet-derived growth factor receptor in a cell-free system. Mol Cell Biol. 1999;19:3727–3735. doi: 10.1128/mcb.19.5.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAKSTAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang XM, Philipp S, Downey JM, Cohen MV. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol. 2006;101:311–318. doi: 10.1007/s00395-006-0587-2. [DOI] [PubMed] [Google Scholar]
- 48.Zecchin GH, De Souza CT, Prada PO, Carvalheira JBC, Velloso LA, Saad MJA. Effect of obesity on insulin signaling through JAK2 in rat aorta. Vascul Pharmacol. 2005;43:346–352. doi: 10.1016/j.vph.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 50.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:429–436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 51.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulinlike growth factor I receptor. J Biol Chem. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]