Abstract
Objective
Firefighters are chronically exposed to smoke and products of incomplete combustion, which frequently contain PAHs. This study examined the possibility of an association between PAH-induced epigenetic alterations and occupational firefighting exposure.
Methods
Promoter methylation was analyzed in four genes in blood DNA from 18 firefighters (FF) and 20 non-firefighting controls (Non-FF). Jurkat and NPrEC cells were treated with benzo[a]pyrene to ascertain the epigenetic effects of this type of agent.
Results
FF had a higher prevalence of DUSP22 promoter hypomethylation in blood DNA (p=0.03) and the extent of hypomethylation correlated with duration of firefighting service (p=0.04), but not with age. Benzo[a]pyrene reduced promoter methylation and increased gene expression of the same gene in Jurkat and NPrEC cells.
Conclusions
Cumulative occupational exposure to combustion-derived PAHs during firefighting can cause epigenetic changes in promoters of specific genes.
Keywords: Environmental exposure, biomarker, polycyclic aromatic hydrocarbons, DNA methylation, prostate cancer
Introduction
Evidence is rapidly emerging that environmental exposure can perturb the epigenome, resulting in potential biomarkers of exposure or “discriminators” of individual susceptibility1, 2. To our knowledge, epigenetic alteration resulting from exposure from incomplete combustion products, including polycyclic aromatic hydrocarbons (PAHs) has yet to be evaluated. Firefighters work in a special environment and are potentially exposed to a wide range of toxic substances both at the fire scene and fire station, including toxic gases, ultrafine particulates and organic compounds, including PAHs, which may be present in the vapor and/or particle-bound states3-6. These substances may be absorbed via inhalation and ingestion. In addition, firefighters' skin at those sites which have relative highest susceptibility to transdermal absorption is often covered with smoke-derived deposits, suggesting this route is especially significant. Self-contained breathing apparatus (SCBA) may be removed prematurely after initial fire suppression, resulting in unintended inhalation of toxicants5. Firefighters are continuously exposed to PAHs from additional sources, including vehicle diesel exhaust, and smoke-derived deposits on protective gear, clothes and fire-suppression equipment in the fire house while on call4.
In comparison with non-firefighting populations, firefighters are at risk for multiple types of disease, including coronary heart disease, neurodegenerative disease, pulmonary disorders, and several types of cancer4, 7, 8. It has been proposed that increased risk of these diseases are related to occupational PAH exposure3-5.
The chemical class of PAHs includes many carcinogens, atherosclerotic agents and neurotoxins9-12. Benzo(a)pyrene [B(a)P] is the most studied compound in this class, and together with other PAHs, is formed by the incomplete combustion of organic materials. Firefighters are routinely exposed to elevated levels of PAHs during the course of fire suppression. 1-hydroxypyrene, a PAH metabolite, could be detected in the urine of firefighters within 6-7 hours and over four days following a fire suppression event despite the use of appropriate protective equipment3.
PAHs and their metabolites are genotoxic9, as a result of the formation of DNA adducts and oxidative DNA damage. Traditionally, PAH genotoxicity is believed to initiate carcinogenesis, artherogenesis, neurodegeneration, and the induction of inflammation10, 11. Emerging evidence, although sparse as yet, suggests that PAHs can also directly or indirectly induce epigenetic changes relevant to disease development. The best examples recently reported are global or gene promoter-specific DNA methylation changes. Such alterations were reported in blood cell DNA from coke-oven workers13 or in umbilical cord blood cell DNA from children born to mothers living in traffic-laden city environments14. These epigenetic alterations may therefore reflect a history of exposure to PAHs.
DNA methylation is a fundamental mechanism for epigenetic control of gene expression and the maintenance of genomic integrity through preservation of higher order chromatin assembly15. DNA methylation patterns are established by a tightly regulated intrinsic program beginning at pre-implantation and lasting through pre- and perinatal life16. Some cell-/tissue-specific patterns remain flexible and are reprogrammable during susceptible windows of development, as well as throughout later life in response to environmental perturbations1, 16, 17 or disease development1, 18-20. Because of the stable, yet modifiable, nature of DNA methylation, both global and gene-specific changes can serve as biomarkers of exposure to toxicants and/or pre- and established disease states14.
The main objective of this study was to investigate an important but unchartered research area of high relevance to the occupational health of firefighters and others (e.g. emergency responders to oil fires and wildfires, and certain military personnel) exposed to incomplete combustion products. We here examined promoter methylation status of four genes (glutathione S-transferase pi-1 (GSTP1), interferon-γ (IFN-ү), RAD21 homolog (S. pombe) (RAD21), and DUSP22) in whole blood DNA from active professional firefighters (FF) and control individuals not involved in firefighting (Non-FF). The promoter methylation status of these genes was previously reported to be correlated with environmental exposures to traffic-related PAHs, diesel exhaust particles, and smoking1, 14, 21, 22. Whether the prototype PAH and ubiquitous smoke constituent B(a)P could induce analogous epigenetic changes has also been investigated in vitro. The effect of B(a)P on gene expression, the immediate consequence of altered gene promoter methylation status, was also examined. An increased risk for prostate cancer in firefighters has been reported23. To demonstrate whether the study cohort was free of pre-existing or existing occupational-related prostate abnormalities, prostate specific antigen (PSA) was used as a surrogate marker of prostate health. In order to verify that the expression of DUSP22 is regulated by the status of promoter methylation of this gene, cells were treated for assessment of their demethylation susceptibility with 5-aza-deoxycytosine (5-aza-dC), a ubiquitous inhibitor of cytosine methylation on DNA.
Materials and Methods
Subjects and blood sample collection
We recruited new and experienced firefighters from the City of Cincinnati Fire Service and Radiation Safety officers from the University of Cincinnati. All potential study participants were informed about the study procedures and signed a University of Cincinnati Institutional Review Board consent form. Participants completed a questionnaire containing occupational and medical history and were asked to provide a 5 mL sample of blood. The sample was collected at the beginning of the study and venous blood was collected into BD Vacutainer EDTA tubes (BD corp., Franklin Lakes, NJ) by a member of the attending paramedic team when firefighters returned to the firehouse after a fire event. Blood was transported at 4°C within 1 hour of collection to the laboratory, aliquot, and stored at -80°C.
Cell lines and treatment
Immortalized human Jurkat T-lymphocytes were purchased from ATCC and maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA). The immortalized normal human prostate epithelial cells, NPrEC, were established in our laboratory and maintained in Dulbecco's Modified Eagle Medium (Invitrogen, Carlsbad, CA) and Defined Keratinocyte-SFM (Invitrogen, Carlsbad, CA) with growth supplement (Invitrogen, Carlsbad, CA) as previously described24. For B(a)P treatment experiments, cells were incubated with 0.1, 1, or 10 nM of B(a)P, or with DMSO (Sigma-Aldrich, St. Louis, MO) as vehicle control, for up to two weeks. The culture medium was replenished with fresh B(a)P or DMSO every two days. For 5-aza-dC treatment, cells were exposed to 0.5 or 1 μM 5-aza-dC (Sigma-Aldrich, St. Louis, MO), or DMSO for 5-6 days. Cells were dissociated with trypsin and collected for both DNA and RNA extraction at the end points indicated.
Methylation specific-PCR (MS-PCR) and bisulfite sequencing
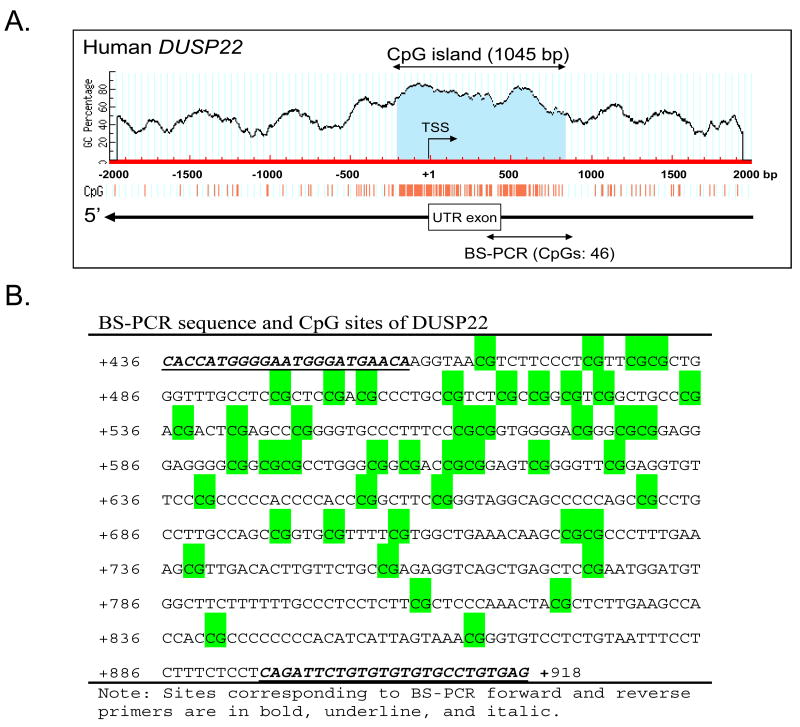
DNA from whole blood or cultured cells was extracted with DNeasy Blood & Tissue kit (Qiagen, Valencia, CA). Two hundred ng of genomic DNA from each blood sample or cell culture was chemically modified with sodium bisulfite using the EZ Methylation Mollification Kit (Zymo Research, CA). Primers for MS-PCR and bisulfite-PCR (BS-PCR) are listed in Supplementary Table 1 (http://links.lww.com/JOM/A91). MS-PCR and bisulfite-PCR were carried out as previous described14, 25. The PCR primers were designed based on the genomic sequences from GenBank databases at the National Center for Biotechnology Information using the online software MethPrimer (www.urogene.org/methprimer/index1.html). Primers for bisulfite sequencing were designed to amplify a 484 bp (436 to 919) fragment downstream of the transcriptional start site (TSS) encompassing the predicted CpG island of dual specificity phosphatase 22 (DUSP22) [BS-PCR- region is indicated in Figure 1A]. BS-PCR amplicons were gel-purified and cloned into the pGEM-T vector (Promega Inc., Madison, WI). Six clones were picked from each sample for sequencing (Macrogen Inc., Rockville, MD). DNA methylation data from sequencing were analyzed using the BiQ Analyzer26. Percentage promoter methylation was calculated by an average of the methylation percentage of 45 CpGs of the six clones in an individual sample.
Figure 1.
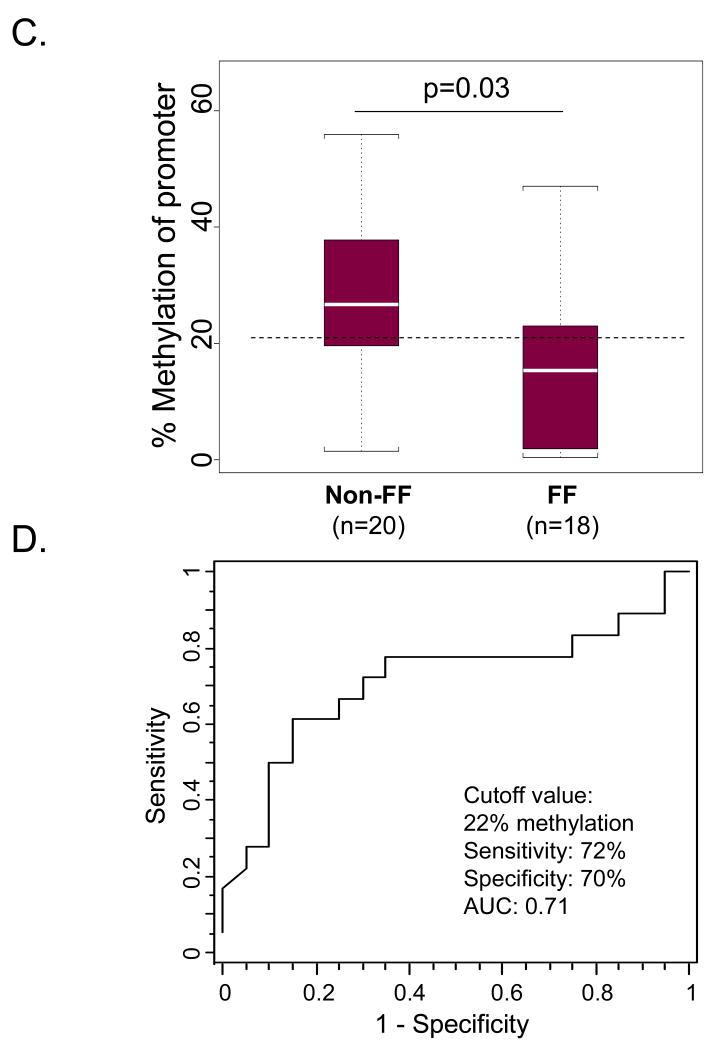
Hypomethylation of the DUSP22 promoter in professional residential firefighters. (A) Schematic diagram of the CG content in the 5′ flanking region of the DUSP22 gene. A 1045 bp CpG island is shaded in blue. The transcription start site (TSS) is marked with a bent arrow. The untranslated exon is marked with a box. The BS-PCR-amplified region is indicated by a left-right arrow. (B) The sequence corresponding to the BS-PCR amplified region. CpG sites are shaded in green. (C) DNA from whole blood of active professional firefighters (FF) and non-firefighting controls (Non-FF) were quantitatively analyzed for % methylation of DUSP22 promoter region by bisulfite sequencing. Six clones from each sample were sequenced. Horizontal dashed line indicates the 22% methylation value. (D) An ROC curve was drawn to graphically assess the discriminating power of the gene promoter methylation for differentiation of samples with or without occupational firefighting exposure.
RNA isolation and quantitative RT-PCR
Total RNA isolation was performed as previously described27. RNA was reverse transcribed to cDNA using SuperScript III First-Strand Synthesis System (Invitrogen). PCR Primers specific for DUSP22 and the house keeping gene hypoxanthine phosphoribosyl transferase-encoding gene (HPRT) have been described previously14. Quantitative PCR was conducted with 2X SYBR Green Universal PCR Master Mix in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The 2-ΔΔCt method was used to calculate the relative expression levels of a transcript by normalization to the level of HPRT mRNA. All reactions were run in triplicate and three independent assays were performed on each sample. Values from vehicle-treated cells were assigned arbitrarily an abundance value of 1.0 which served as a comparison standard for values from other groups with different doses of agents under the same treatment conditions.
Measurement of prostate specific antigen (PSA)
The concentration of blood PSA was measured by sandwich enzyme-linked immunosorbent assay (ELISA) according manufacturer's protocol (Bioquant, San Diego, CA). Plasma was separated from subjects' whole blood and 50ul of plasma, control or PSA standard added to each well of ELISA plate. Each reaction was carried out in duplicate. Samples were independently measured twice. Concentrations of plasma PSA were calculated from a PSA standard curve.
Statistical analysis
For each worker, summary measures of methylation were estimated from the analysis of indicator variables specifying the presence (=1) or absence (=0) of methylation at 45 sites and six clones per site. Subject-specific clones (site averages) were analyzed as outcomes using mixed effects regression modeling. Non-parametric Kruskal-Wallis and Mann-Whitney U tests were performed to compare central tendencies among worker categories, and FF compared with Non-FF. Worker category means and standard errors were obtained from a model assuming that workers were a representative sample of workers from similar occupations (a random effect). From this model, the variability of between-subject means within each worker category was estimated. Worker means and within-subject clone variability were estimated from a model in which worker id was modeled as a fixed effect. Clone variation was estimated from the repeated measurements of clone values within each subject. Sensitivities and specificities corresponding to the continuum of methylation percentages were calculated, and a receiver operating characteristic (ROC) graph was drawn. The optimum cutoff point of percent methylation for discriminating between the two groups was obtained by minimization of the distance from the (1, 1) co-ordinate of the x-y axes to the ROC graph. The analyses were performed using SAS for Windows, Version 9.2, SAS Institute, Cary, NC.
Results
Study Participants
We recruited 38 participants into the study including 18 active professional firefighters (FF) and 20 controls with no previous professional firefighting experience (Non-FF). FF had a fire repression service ranging from 9 to 25 years. Non-FF had never participated in fire suppression. Subject characteristics are listed in Table 1.
Table 1. Subject characteristics and occupation exposure.
| Sample | FF N=18 | Non-FF N=20 |
|---|---|---|
| Gender | ||
| Male | 17 | 18 |
| Female | 1 | 2 |
| Age | ||
| Age years | 30-53 | 23-53 |
| Mean | 41 | 34 |
| Race | ||
| White | 14 | 20 |
| Black | 4 | 0 |
| Firefighting service years | 9-36 | 0 |
| Mean | 18 | 0 |
| Cigarette smoking | ||
| Current smoker | 2 | 3 |
| Never smoker | 16 | 17 |
FF: Active professional firefighter
Non-FF: non-firefighting control
DUSP22 promoter is hypomethylated in active professional firefighters (FF)
To investigate the relationship between the extent of promoter methylation and length of occupational firefighting experience, we assessed the promoter methylation status of four human genes: GSTP1, IFN-ү, RAD21, and DUSP22 in whole blood DNA. The promoter regions of GSTP1, IFN-ү and RAD21 showed no significant difference in their methylation statuses between FF and Non-FF (Supplementary Table 2, http://links.lww.com/JOM/A92). Only DUSP22 (see Figure 1A and B for gene organization and location of primers) was found to be significantly hypomethylated in its promoter region in active firefighters (FF) compared with the control group (Non-FF) (Figure 1C). The percentage promoter methylation was calculated as the average percentage methylation of the 45 CpGs across the CpG-rich island (CGI) of DUSP22 (see Materials and Methods and Figure 1). The percentage promoter methylation of DUSP22 in blood was statistically different between FF (median=15%) and non-FF (median=27%) with p=0.03 (Figure 1C). There was extensive hypomethylation (<10% methylation of CpGs in the promoter region), in 7/18 (39%) FF, and 2/20 (10%) Non-FF. An ROC graph drawn (Figure 1D) to graphically assess the power of gene promoter methylation to discriminate between samples from FF and non-FF showed an optimal discrimination at 22% methylation, corresponding to 72% sensitivity (95% CI= 47-88%) and 70% specificity (95% CI= 48-88%). We have included a table in Supplemental 3 (http://links.lww.com/JOM/A93) to describe the magnitude of two sources of variability of the data, including estimated standard deviations of methylation percentage among workers in each category, and estimated standard deviations of clone methylation percentage in each category. The results indicated that firefighting activity is associated with hypomethylation of the DUSP22 promoter.
Hypomethylation of the DUSP22 promoter region is not age-related but is correlated with years of occupational experience
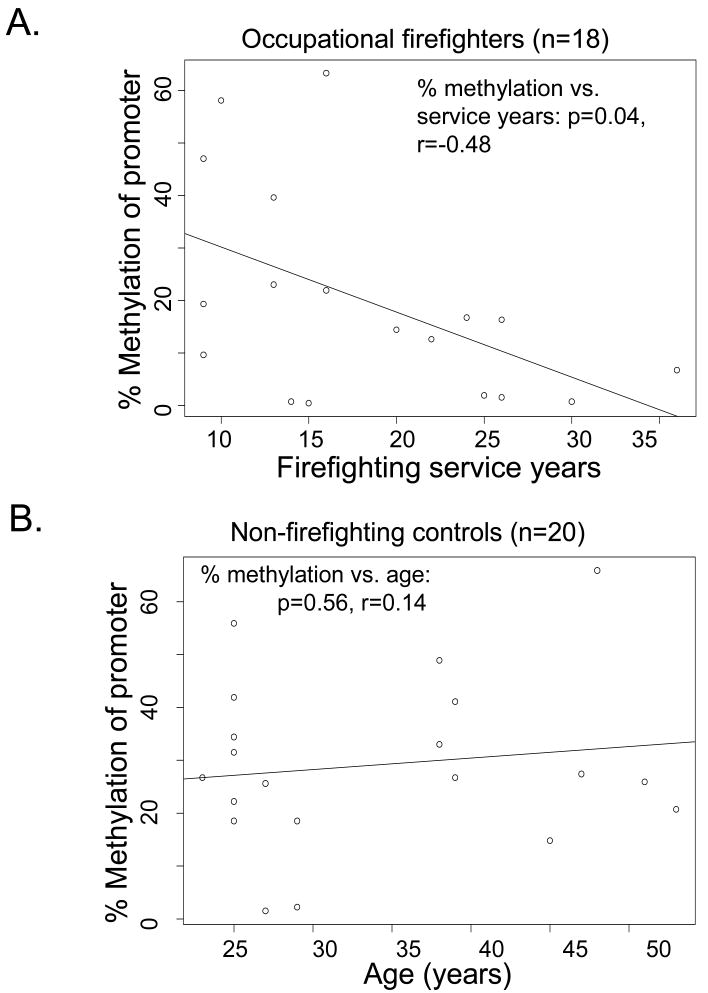
Firefighters are chronically exposed to a wide range of toxic agents. Accordingly, the effects of some of these agents might be expected to accumulate with years of active service. We found that the methylation status of the DUSP22 promoter region was significantly correlated with firefighting service years (r=-0.48, p=0.04) in FF (Figure 2A) but not with age (r=0.14, p=0.56) in non-FF (Figure 2B).
Figure 2.
Hypomethylation of DUSP22 promoter is not age-related but correlated with duration of occupation. Spearman correlations were calculated to assess associations in each category. (A) 18 firefighter veterans were analyzed for correlation of duration of firefighting service with percentage of methylation. (B) 20 controls without firefighting exposure were analyzed for association of age with methylation percentage.
All subjects have normal serum PSA levels
Firefighters have been reported to have higher prevalence of prostate cancer4. The serum PSA test is a first line assay for early detection of prostatitis, hyperplasia and cancer in the prostate. To determine if firefighters in our cohort had abnormal prostate functions as a result of chronic exposure to smoke-related toxicants and to further analyze the association of epigenetic changes with prostate disease as well as rule out pre-exist prostate diseases in controls we measured blood PSA concentrations of study subjects. They were all within the normal range (data not shown).
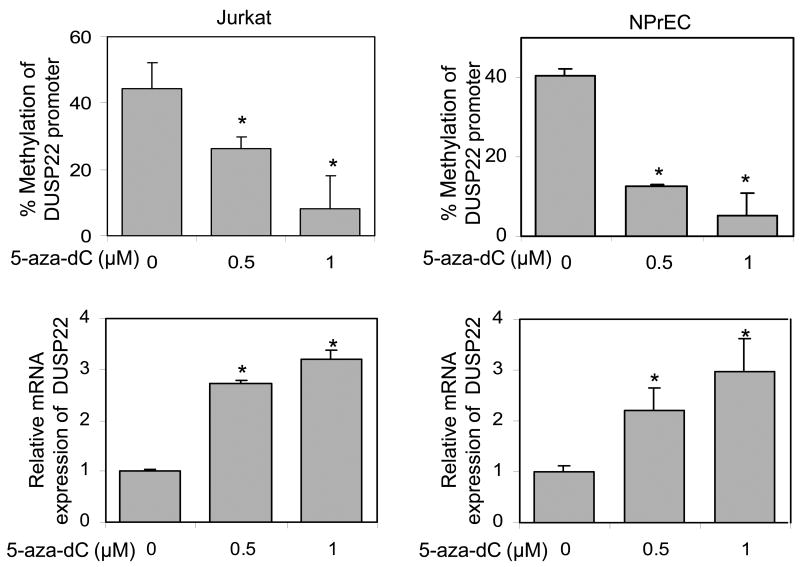
5-aza-dC induces promoter hypomethylation and increases transcript expression of DUSP22
Treatment of Jurkat cells and NPrEC with 5-aza-dC for 5-6 days resulted in a dose-dependent hypomethylation of the DUSP22 promoter compared with vehicle treatment (Figure 3, upper panel). A significant increase in mRNA expression of DUSP22 in 5-aza-dC treated cells was concurrently detected (Figure 3, lower panel), indicating that the expression of DUSP22 was regulated by the status of promoter methylation of this gene.
Figure 3.
5-aza-dC induced hypomethylation of DUSP22 promoter and transcript expression. Jurkat and NPrEC cells were treated with 5-aza-dC for six days. DNA and RNA were extracted for determination of the induction of promoter hypomethylation (upper panel) and mRNA expression (lower panel). Six clones from each sample were sequenced. *: Statistically significance compared with mock treatment (0 nM).
B(a)P induces promoter hypomethylation and increases transcript expression of DUSP22 in vitro
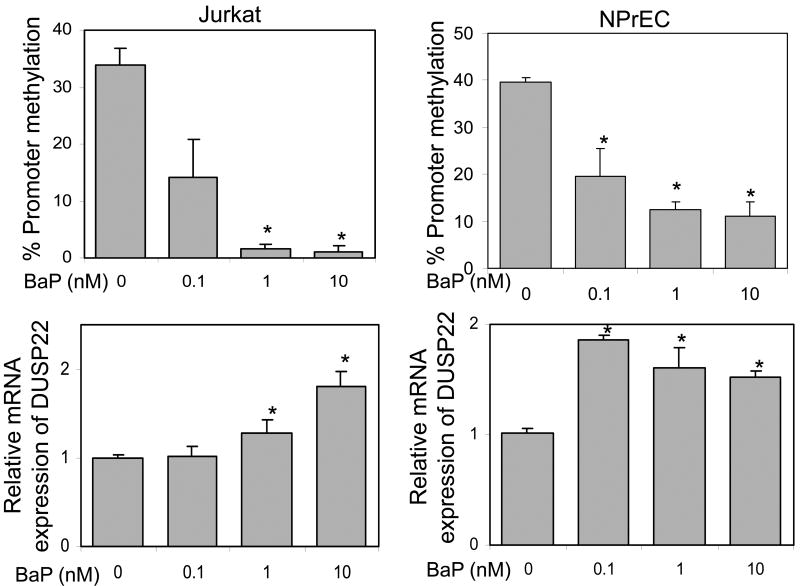
We first carried out a pilot experiment to examine the toxicity of B(a)P on cell growth and optimized the concentration required for induction of DNA methylation in NPrEC cells. We found that B(a)P at concentrations ≤10 nM did not significantly affect cell growth, but ≥100 nM inhibited cell growth and caused cell death. We further found that hypomethylation of the gene promoter could be induced at B(a)P concentrations ≥1 nM after one week and ≥0.1 nM after two weeks of treatment. Therefore, two weeks of treatment withB(a)P at concentrations of 0.1-10 nM was chosen for our study.
Treatment of Jurkat cells and NPrEC with B(a)P at low concentrations (0.1-10 nM) for two weeks resulted in a significant dose-dependent hypomethylation of the DUSP22 promoter region (Figure 4, upper panel), and concordant increases in DUSP22 transcript expression, when compared with vehicle treatment in both cell lines (Figure 4, lower panel). Treatment of Jurkat and NPrEC cells with B(a)P over the same concentration range (0.1-10 nM) did not affect cell growth as measured by cell counting and the MTT assay (data not shown). Taken together, these data indicated that B(a)P is not cytotoxic to at concentrations that can elicit promoter hypomethylation and increases gene expression in DUSP22 in Jurkat cells and NPrEC.
Figure 4.
BaP- induced hypomethylation of DUSP22 promoter and degree of transcription. Jurkat and NPrEC cells were treated with various concentrations of BaP for two weeks. DNA and RNA were extracted for determination of the promoter methylation status (upper panel) and mRNA expression (lower panel). Six clones from each sample were sequenced. *: Statistically significance compared with mock treatment (0 nM).
Discussion
Although there is evidence that certain PAHs cause cancer and other diseases via genotoxicity10, 11, we hypothesize that PAHs may contribute to development of these diseases through epigenetic reprogramming1 in firefighters as a result of long term occupational exposure. Here we provide the first evidence for this hypothesis. A higher prevalence (39% versus 10%) and degree (median: 15% versus 27%) of DUSP22 promoter hypomethylation was detected in active professional firefighters when compared with a control group (non-FF). Promoter hypomethylation of the DUSP22 gene was found not to be age-related but correlated with length of occupational service. Of the seven cases with extensive promoter hypomethylation, six had at least 14 years of firefighting experience, and one had 9 service years and was also a current smoker. Of the two control individuals with significant DUSP22 promoter hypomethylation, one was reported to be constantly exposed to second-hand smoking and the other not to have an apparent contributing factor. At this point it is not possible to conclude whether smoking contributes to epigenetic alteration of the DUSP22 promoter, due to the low number of smokers in our cohort (2 FF and 3 Non-FF, Table 1).
Observational studies have consistently indicated an increased prostate cancer incidence in firefighters4. Here we showed that exposure of NPrEC to low concentrations of the smoke-derived carcinogen B(a)P induced DUSP22 promoter hypomethylation and increased gene expression. Since some of the subjects in our cohort (FF = 30-53 year old; Non-FF = 23-53 year old) are older than 45, the possibility existed that some of them might have harbored prostate cancer. It was therefore logical to question if DUSP22 promoter hypomethylation is a reflection of prostate disease in this cohort. We therefore measured serum PSA levels, a mainstay test for prostate diseases (benign and malignant growth and prostatitis, and blood DNA GSTP1 promoter hypermethylation, a widely confirmed biomarker of prostate cancer22,28 in our subjects. Collectively, our data suggested that the observed hypomethylation of DUSP22 promoter was not a consequence of prostate disease but likely related to exposure to smoke or products of incomplete combustion.
One limitation of this study is that we did not examine biomarkers of other diseases associated with firefighting. In this regard, it will be of significant interest to measure biomarkers associated with atherosclerosis-related cardiovascular disease and oxidative stress-related disorders. These may include homocysteine, C-reactive protein, and other cardiovascular/atherosclerosis-biomarkers of clinical values in our future studies. Applying the same reasoning, our impending studies will include blood markers of gonadal function (serum levels of testosterone, luteinizing hormone, anti-Mullerian hormone, and inhibin-B) and other malignancies such as ovarian (CA 12529); and testicular (α-fetoprotein30) cancer. Such an approach is expected to generate new insights into the health of firefighters because PAHs and other smoke-derived toxicants are known to have adverse impacts on fertility and reproductive function31, which could be major health concerns for younger firefighters.
In a previous study we identified RAD21 and DUSP22 as genes whose promoter methylation statuses were linked to transplacental PAH exposure using umbilical cord blood DNA from a cohort of children born to mothers living in a traffic-laden city neighborhood14. Asthma incidence (∼30%) in this cohort is amongst the highest in the nation. Recently, we reported that IFN-γ also exhibits a similar relationship with maternal PAH exposure in the same cohort of children (Tang YW, unpublished data). Interestingly, amongst these three genes, we only found the promoter of DUSP22, but not RAD21 or IFN-γ, to be modified by firefighting activity. This result was in contrast to findings in our children's study and raised the intriguing question of why firefighting triggered promoter hypomethylation in DUPS22 but not in the other two genes. Our in vitro experiments clearly demonstrated that low concentrations of B(a)P (0.1-10 nM) induced DUSP22 promoter hypomethylation and increased gene expression… The IFN-γ promoter has also been shown to be sensitive to low concentrations of B(a)P and undergo promoter hypermethylation and gene silencing in vitro in our laboratory (Tang YW, unpublished data) and in BALB/c mice inhaling diesel exhaust particles in other laboratory21. An overall rational interpretation of these findings is therefore that epigenetic alteration of gene-specific promoters is highly dependent on the context of the exposure. PAH exposure in the children's study was traffic-related and transplacental whereas in the current firefighters' study exposure was in the form of a mixture of smoke-derived toxicants, likely absorbed via inhalation or ingestion or by transdermal absorption.
The DUSP22 gene encodes an enzyme that belongs to a family of atypical dual specificity phosphatases32. The long 3.0 kb transcript of DUSP22 has a wide tissue distribution but the shorter 1.3 kb variant has restricted expression in the testis and liver33. The enzyme is an upstream regulator of several mitogen-activated protein kinase pathways but its function is highly dependent on cell type and context. Ectopic expression of DUSP22 in COS7 cells preferentially dephosphorylates p38 and JNK, but not ERK 34, whereas it dephosphorylates ERK in Jurkat cells35. Mouse embryonic stem cell experiments clearly demonstrate the central role of DUSP22 in cytokine-induced JNK activation33. The enzyme is also involved in estrogen action as it can dephosphorylate the estrogen receptor-α and negate estrogen-induced IL6/STAT3 signaling36. A recent study reports a novel chromosome translocation [t(6;7)(p25.3;q32.3)] that leads to suppression of DUSP22 in approximately half of anaplastic large cell lymphomas37, a type of non-Hodgkin lymphoma, a cancer type that has been reported to be elevated in firefighters4. Based on these reports, DUSP22 appears to play a key role in inflammatory and proliferative disorders. Findings from our study have now placed DUSP22 as one of the few genes whose expression is modified epigenetically in response to environmental exposure. In this regard, we showed that DUSP22 expression is upregulated upon B(a)P-induced hypomethylation of its promoter in vitro and that DUSP22 promoter hypomethylation in peripheral blood DNA is associated with firefighting and the duration of active service. It will be of interest for future studies to investigate whether promoter hypomethylation of DUSP22, in addition to being a biomarker of toxic exposure to PAH also has the capability to predict later-life diseases (such as prostate cancer) resulting from long-term exposure to smoke- or other incomplete-combustion-derived toxicants (such as PAHs). Findings from such investigations will undoubtedly deepen our understanding of the role played by epigenetics in exposure biology in settings beyond firefighting, particularly those which also involve exposure to products of incomplete combustion of organic materials, such as in situ-burning of oil spills, both during peacetime and as a result of military operations (e.g. Gulf War I).
This proof-of-concept study indicates that epigenetic changes occur in professional firefighting populations. These modifications may be toxicant- and/or exposure context-specific and result from exposure to complex mixtures of toxic substances emitted from burning and overheated materials. It is therefore essential to identify a panel of epigenetic biomarkers that are linked to the most common toxicant exposures or exposure contexts encountered by firefighters, and that could estimate the risk of later-life diseases in this, as well as in other, exposed populations. Such biomarkers may aid in the development of new strategies/technologies for exposure surveillance and disease prevention in the future.
Supplementary Material
Clinical Significance.
This study is the first to report that epigenetic changes are associated with residential firefighting. Benzo[a]pyrene, a common ubiquitously existing PAH was further shown to induce hypomethylation of DUSP22 promoter in vitro cell studies. Our results suggest that epigenetic biomarkers may be used in monitoring occupational exposure to toxicants.
Acknowledgments
Source of Funding: This work is in part supported by National Institutes of Health grants: CA 112532 (SMH), ES015584 (SMH), ES018758 (SMH), ES018789 (SMH), ES015584 (SMH), ES018789 (SMH), ES015905 (SMH), ES019480 (SMH), ES006096 (SMH; ENH), ES016531 (ENH), ES017362 (ENH).
Abbreviations
- DUSP22
Dual specificity phosphatase 22
- PAHs
polycyclic aromatic hydrocarbons
- B(a)P
Benzo[a]pyrene
- MS-PCR
methylation-specific polymerase chain reaction
- BS-PCR
bisulfite-specific PCR
- 5-aza-dC
5-aza-2′-deoxycytidine
- NPrEC
normal prostate epithelial cells
- PCa
prostate cancer
- Non-FF
non-firefighting controls
- FF
professional firefighters
- ROC
receiver operating characteristic
- GSTP1
glutathione S-transferase π-1
- IFN-ү
interferon-γ
- RAD21
RAD21 homolog (S. pombe)
- ERKs
extracellular-signal-regulated kinases
- SAPK
stress-activated protein kinase
- JNK
Jun amino-terminal kinase
Footnotes
Conflicts of Interest: All authors have no direct or indirect commercial financial incentive associated with publishing the article.
References
- 1.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010;126:453–465. doi: 10.1016/j.jaci.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Ho SM. Epigenetics meets endocrinology. J Mol Endocrinol. 2011;46:R11–R32. doi: 10.1677/jme-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caux C, O'Brien C, Viau C. Determination of firefighter exposure to polycyclic aromatic hydrocarbons and benzene during fire fighting using measurement of biological indicators. Appl Occup Environ Hyg. 2002;17:379–386. doi: 10.1080/10473220252864987. [DOI] [PubMed] [Google Scholar]
- 4.Lemasters GK, Genaidy AM, Succop P, et al. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J Occup Environ Med. 2006;48:1189–1202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 5.Edelman P, Osterloh J, Pirkle J, et al. Biomonitoring of chemical exposure among New York City firefighters responding to the World Trade Center fire and collapse. Environ Health Perspect. 2003;111:1906–1911. doi: 10.1289/ehp.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter CS, Ross CS, Fabian T, et al. Ultrafine particle exposure during fire suppression--is it an important contributory factor for coronary heart disease in firefighters? J Occup Environ Med. 2010;52:791–796. doi: 10.1097/JOM.0b013e3181ed2c6e. [DOI] [PubMed] [Google Scholar]
- 7.Byczek L, Walton SM, Conrad KM, Reichelt PA, Samo DG. Cardiovascular risks in firefighters: implications for occupational health nurse practice. AAOHN J. 2004;52:66–76. [PubMed] [Google Scholar]
- 8.Schulte PA, Burnett CA, Boeniger MF, Johnson J. Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991. Am J Public Health. 1996;86:1281–1288. doi: 10.2105/ajph.86.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45:106–114. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- 10.Ramos KS, Moorthy B. Bioactivation of polycyclic aromatic hydrocarbon carcinogens within the vascular wall: implications for human atherogenesis. Drug Metab Rev. 2005;37:595–610. doi: 10.1080/03602530500251253. [DOI] [PubMed] [Google Scholar]
- 11.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Chen A, Dietrich KN, Huo X, Ho SM. Developmental neurotoxicants in e-waste: an emerging health concern. Environ Health Perspect. 2011;119:431–438. doi: 10.1289/ehp.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavanello S, Bollati V, Pesatori AC, et al. Global and gene-specific promoter methylation changes are related to anti-B[a]PDE-DNA adduct levels and influence micronuclei levels in polycyclic aromatic hydrocarbon-exposed individuals. Int J Cancer. 2009;125:1692–1697. doi: 10.1002/ijc.24492. [DOI] [PubMed] [Google Scholar]
- 14.Perera F, Tang WY, Herbstman J, et al. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 16.Szyf M. The implications of DNA methylation for toxicology: toward toxicomethylomics, the toxicology of DNA methylation. Toxicol Sci. 2011;120:235–255. doi: 10.1093/toxsci/kfr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8:173–182. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 19.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe Y, Maekawa M. Methylation of DNA in cancer. Adv Clin Chem. 2010;52:145–167. doi: 10.1016/s0065-2423(10)52006-7. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Ballaney M, Al-alem U, et al. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008;102:76–81. doi: 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YJ, Chen Y, Ahsan H, et al. Silencing of glutathione S-transferase P1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett. 2005;221:135–143. doi: 10.1016/j.canlet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Bates MN. Registry-based case-control study of cancer in California firefighters. Am J Ind Med. 2007;50:339–344. doi: 10.1002/ajim.20446. [DOI] [PubMed] [Google Scholar]
- 24.Mobley JA, Leav I, Zielie P, et al. Branched fatty acids in dairy and beef products markedly enhance alpha-methylacyl-CoA racemase expression in prostate cancer cells in vitro. Cancer Epidemiol Biomarkers Prev. 2003;12:775–783. [PubMed] [Google Scholar]
- 25.Ho SM, Tang WY, Belmonte de FJ, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21:4067–4068. doi: 10.1093/bioinformatics/bti652. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang B, Bracken B, Burke B, Chung E, Liang J, Ho SM. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol. 2009;181:2508–2513. doi: 10.1016/j.juro.2009.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu T, Giovannucci E, Welge J, Mallick P, Tang WY, Ho SM. Measurement of GSTP1 promoter methylation in body fluids may complement PSA screening: a meta-analysis. Br J Cancer. 2011;105:65–73. doi: 10.1038/bjc.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cragun JM. Screening for ovarian cancer. Cancer Control. 2011;18:16–21. doi: 10.1177/107327481101800103. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich Y, Baniel J. Late relapse of testis cancer. Urol Clin North Am. 2007;34:253–258. doi: 10.1016/j.ucl.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Perrin J, Tassistro V, Mandon M, Grillo JM, Botta A, Sari-Minodier I. Tobacco consumption and benzo(a)pyrene-diol-epoxide-DNA adducts in spermatozoa: in smokers, swim-up procedure selects spermatozoa with decreased DNA damage. Fertil Steril. 2011;95:2013–2017. doi: 10.1016/j.fertnstert.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 33.Chen AJ, Zhou G, Juan T, et al. The dual specificity JKAP specifically activates the c-Jun N-terminal kinase pathway. J Biol Chem. 2002;277:36592–36601. doi: 10.1074/jbc.M200453200. [DOI] [PubMed] [Google Scholar]
- 34.Aoyama K, Nagata M, Oshima K, Matsuda T, Aoki N. Molecular cloning and characterization of a novel dual specificity phosphatase, LMW-DSP2, that lacks the cdc25 homology domain. J Biol Chem. 2001;276:27575–27583. doi: 10.1074/jbc.M100408200. [DOI] [PubMed] [Google Scholar]
- 35.Alonso A, Merlo JJ, Na S, et al. Inhibition of T cell antigen receptor signaling by VHR-related MKPX (VHX), a new dual specificity phosphatase related to VH1 related (VHR) J Biol Chem. 2002;277:5524–5528. doi: 10.1074/jbc.M107653200. [DOI] [PubMed] [Google Scholar]
- 36.Sekine Y, Ikeda O, Hayakawa Y, et al. DUSP22/LMW-DSP2 regulates estrogen receptor-alpha-mediated signaling through dephosphorylation of Ser-118. Oncogene. 2007;26:6038–6049. doi: 10.1038/sj.onc.1210426. [DOI] [PubMed] [Google Scholar]
- 37.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117:915–919. doi: 10.1182/blood-2010-08-303305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.