Abstract
An adult dog that lived in central British Columbia was examined because of a history of lethargy and vomiting. Histology, immunohistochemistry, and polymerase chain reaction (PCR) examination of a hepatic mass confirmed the presence of an alveolar hydatid cyst, the first description of Echinococcus multilocularis in British Columbia. We provide recommendations for case management and remind practitioners in endemic areas of western Canada that dogs can serve as definitive and, rarely, intermediate hosts for E. multilocularis.
Résumé
Hydatidose alvéolaire (Echinococcus multilocularis) dans le foie d’un chien canadien en Colombie-Britannique, une région nouvellement endémique. Un chien adulte habitant dans le centre de la Colombie-Britannique a été examiné en raison d’une anamnèse d’abattement et de vomissements. L’histologie, l’immunohistochimie et l’amplification en chaîne par la polymérase d’une masse hépatique ont tous confirmé la présence d’un kyste hydatique, la première description d’Echinococcus multilocularis en Colombie-Britannique. Nous présentons des recommandations pour la gestion des cas et rappelons aux praticiens dans les régions endémiques de l’Ouest canadien que les chiens peuvent servir d’hôtes définitifs, et rarement, d’hôtes intermédiaires, pour E. multilocularis.
(Traduit par Isabelle Vallières)
On July 14, 2009 (day 0), a 3.5-year-old, 7.2-kg neutered male shi tzu/bichon frisé cross dog was presented to a small animal practice in Powell River, British Columbia (BC) with a 10-day history of intermittent vomiting and regurgitation. The dog was also noted to have been lethargic and reluctant to jump during this period.
Case description
Palpation of the dog revealed a large firm mass in the cranial aspect of the abdomen; palpation of the mass elicited significant pain. Radiography revealed a 12 to 13 cm diameter soft-tissue mass in the right cranioventral aspect of the abdomen. In association with the mass there was pronounced caudodorsal and moderate leftward displacement of the stomach. The remaining abdominal viscera were also caudally displaced; however, no abnormalities were noted in any of these organs. The mass appeared to be hepatic in origin, but due to the non-specific radiographic findings it was not possible to differentiate focal from generalized liver disease. Abdominal ultrasound was therefore recommended for further differentiation and potential guidance for a fine-needle aspirate. Thoracic radiography revealed no abnormalities.
On day 1, a complete blood (cell) count (CBC) (Scil Vet abc hematology analyzer; Scil Animal Care Company, Gurnee, Illinois, USA) revealed no abnormalities. However, total protein and globulin levels (Abaxis VetScan VS2; Abaxis North America, Union City, California, USA) were both elevated [total protein = 100 g/L; reference interval (RI): 54 to 82 g/L: globulin = 73 g/L; RI: 23 to 52 g/L], most likely indicative of a chronic inflammatory process.
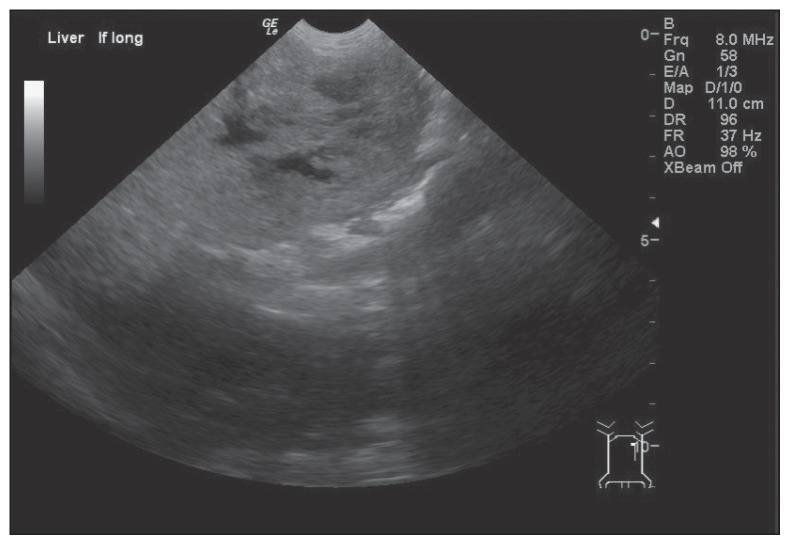
After sedation with medetomidine hydrochloride (Domitor; Pfizer Animal Health, Kirkland, Quebec), 20.9 μg/kg body weight (BW), SC and butorphanol tartrate (Torbugesic; Wyeth Animal Health, Guelph, Ontario), 0.21 mg/kg BW, SC, ultrasound (GE LOGIQ e Portable Ultrasound machine; General Electric, Milwaukee, Wisconsin, USA) revealed a large hepatic mass with mixed echogenicity, cavitated lesions within, and an irregular surface (Figure 1). No abnormalities were found in the spleen, kidneys, bladder, or small intestine. On the basis of these findings, the hepatic mass was considered most likely to be either a tumor or an abscess.
Figure 1.
Longitudinal ultrasound image of the left liver. Note the liver’s irregular surface, mixed echogenicity and cavitated lesions within.
On day 2, the dog was prepared for surgery by sedation with acepromazine (Acevet; Vétoquinol Canada, Lavaltrie, Quebec), 0.1 mg/kg BW, SC, atropine (Atro-SA; Rafter 8 Products, Calgary, Alberta), 0.04 mg/kg BW, SC, and butorphanol tartrate (Torbugesic; Wyeth Animal Health), 0.21 mg/kg BW, SC. Intravenous access was established with a catheter in the right cephalic vein. Anesthesia was induced with propofol (Propoflo; Abbott Animal Health, Illinois, USA), 2.8 mg/kg BW, IV, and Lactated Ringer’s solution (Baxter Corporation, Mississauga, Ontario) was administered at 70 to 140 mL/h. Anesthesia was maintained with isoflurane (Isoflo; Abbott Animal Health, Illinois, USA) in oxygen and systolic blood pressure was monitored non-invasively with a Doppler. Perioperative antibiotics consisted of enrofloxacin (Baytril injectable solution; Bayer, Toronto, Ontario), 3.5 mg/kg BW, IM at the time of induction.
An exploratory laparotomy was performed through a ventral midline incision; a large multi-lobulated firm mass was observed originating from the left medial liver lobe with adhesions to the left lateral liver lobe, the greater curvature of the stomach, the spleen, and omentum. Grossly, the mass had an irregular surface. After separating the omentum from the mass, the affected liver lobes were exposed and a TA 30 stapler with a 30-V3 titanium cartridge was used to resect the mass. However, not all abnormal hepatic tissue could be removed. Furthermore, since the splenic blood supply was within the mass, a splenectomy was also performed using a standard double ligation technique and polyglactin 910 suture. Excision of adherent tissue from the greater curvature of the stomach was accomplished by partial gastrectomy, using polydioxanone suture to close the gastrectomy incision in a 2-layer inverting pattern. Examination of all other abdominal organs, including the other liver lobes, revealed no gross abnormalities. The abdomen was then lavaged with warm saline, and the laparotomy incision closed in a routine manner. Post-operatively, the dog was administered morphine (Morphine sulfate injection; Sandoz Canada, Boucherville, Quebec), 0.47 mg/kg BW, SC, and Lactated Ringer’s solution IV was continued at 30 mL/h. Recovery from anesthesia and surgery was uneventful. Subcutaneous administration of morphine (0.47 mg/kg BW) was repeated at 6 and 11 h after the initial dose, and 1 additional dose of enrofloxacin (3.9 mg/kg BW, SC) was given.
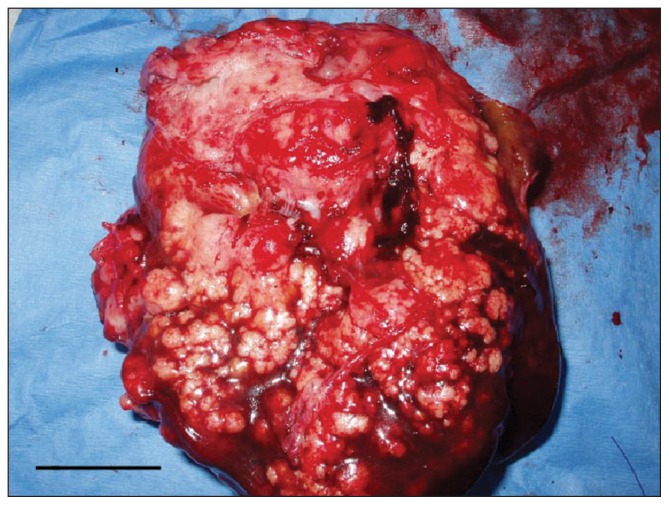
On removal, the mass weighed 570 g and contained multiple coalescing white nodules that were poorly demarcated and appeared to infiltrate adjacent hepatic tissue (Figure 2). One of the cavitated lesions was opened and a swab submitted for bacterial culture and sensitivity. Samples of spleen, liver, and stomach wall were also sent for histopathology.
Figure 2.
Photograph of the resected hepatic mass. Notice the multiple generalized coalescing white nodules that appear to infiltrate hepatic tissue. Bar = 3 cm.
On day 3, the dog was clinically normal, except that bowel movements were absent. The animal was administered enrofloxacin (3.9 mg/kg BW, SC), meloxicam [Metacam 0.5% injection; Boehringer Ingelheim (Canada), Burlington, Ontario], 0.2 mg/kg BW, SC, and Lactated Ringer’s solution IV was continued at 20 mL/h. Approximately 10 h later, the dog received enrofloxacin (3.9 mg/kg BW, SC), butorphanol tartrate, (0.23 mg/kg BW, SC), Lactated Ringer’s solution continued as before, and the animal consumed a small amount of Gastro (Medi-Cal; Royal Canin Canada, Guelph, Ontario) mixed with water.
On day 4, the dog continued to clinically improve and was treated with enrofloxacin (4.0 mg/kg BW, SC, q12h), meloxicam (0.1 mg/kg BW, SC, q24h), and Lactated Ringer’s solution IV was continued at 20 mL/h. Twice during the day the dog consumed Gastro mixed with water. Culture of the swab from the liver mass revealed no growth after 72 h incubation.
On day 5, the IV catheter was removed and the dog was treated with enrofloxacin (4.0 mg/kg BW, SC). Approximately 9 h later, enrofloxacin was administered at 8.1 mg/kg BW. On day 6, the dog was still doing well, was treated with enrofloxacin (8.1 mg/kg BW, PO), and discharged with instructions that it should receive enrofloxacin (8.1 mg/kg BW, PO q12h) for 1 wk and Gastro for its diet.
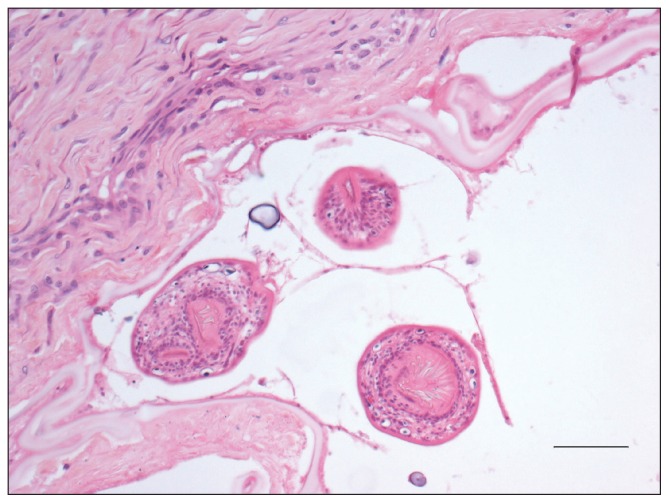
Histopathology of the mass was received on day 6. Within several of the liver sections, hepatic architecture was effaced and replaced by multilocular coalescing cystic structures surrounded by fibrosis. Lining individual cysts was a hyaline capsule wall with an inner lining of basophilic matrix containing occasional calcareous corpuscles. Multiple intraluminal protoscolices with bifringent hooks were present, which were occasionally surrounded by a thin membrane (Figure 3). Hepatic parenchyma immediately adjacent to the multilocular cystic structures was replaced by fibrosis infiltrated by low numbers of mixed inflammatory cells, large areas of necrosis, and multifocal mineralization. The remaining liver parenchyma was characterized by hepatocellular atrophy with biliary hyperplasia and extensive sinusoidal congestion.
Figure 3.
Photomicrograph of a section of cystic tissue from the mass shown in Figure 2. Note the 3 intraluminal protoscolices surrounded by a thin membrane. The cyst is lined by a hyaline membrane (“laminated layer”). Hematoxylin and eosin, Bar = 50 μm.
Adherent to the serosal surface of the gastric wall was a thick layer of fibrous stroma dissected by numerous multilocular cystic structures that were occasionally lined by a hyaline membrane. However, intraluminal protoscolices were not observed. Infiltrating the stroma were moderate numbers of lymphocytes, plasma cells and macrophages. Multifocal areas of necrosis were present throughout this tissue. The overlying muscularis, submucosal, and mucosal layers were not affected. Moderate congestion of the splenic red pulp was present. However, there was no evidence of extension of the fibrotic or inflammatory lesion, and protoscolices were not observed.
Collectively these findings were consistent with the presence of an Echinococcus species. However, in order to obtain definitive identification of the species involved, analysis using molecular techniques was required.
In light of the Echinococcus sp. identification, a fecal sample was collected on day 9, submitted for analysis (Idexx Laboratories) and examined using a sodium acetate-acetic acid-formalin sedimentation method (1); no parasite eggs were identified.
On day 10, the owner reported that the dog vomited bile or clear fluid approximately 4 times and was lethargic. It was therefore administered only clear liquids, 12.5 mg dimenhydri-nate (Gravol; Church & Dwight Canada Corp., Mississauga, Ontario), q12h to q8h and 2.5 mL bismuth subsalicylate (Pepto-Bismol; Procter & Gamble, Toronto, Ontario), q12h to q6h. On day 14, the owner reported that the dog had vomited clear fluid and food on multiple occasions. As a result, the dog was administered metoclopramide (Reglan; Alaven, Pharmaceutical LLC, Marietta, Georgia, USA), 0.4 mg/kg BW, PO q12h for 10 d. Thereafter, metoclopramide was administered at 0.4 mg/kg BW, PO q24h as required. On day 16, the vomiting was reported to have ceased. On day 17, the collective opinion of several parasitologists was that histologically and grossly the lesion appeared most likely to be the metacestode (intermediate) stage of Echinococcus multilocularis. Since dogs are typically a definitive host for this parasite the dog was treated on 1 occasion between days 18 and 25 with praziquantel (5 mg/kg)/pyrantel pamoate (5 mg/kg)/febantel (25 mg/kg BW) (Drontal Plus; Bayer, Toronto, Ontario) PO. On day 42, the owner reported that the dog was doing well. However, whenever treatment with metoclopramide was stopped the vomiting started. As a result, metoclopramide (0.4 mg/kg BW) was thereafter administered PO q24h to q12h, as needed. On day 50, the dog was reported to have been dull and lethargic for a few days, and to have vomited Gastro; the dog was being treated with metoclopramide, 0.4 mg/kg BW, PO q24h at night. Feeding was changed to 4 small meals of Gastro per day, and the dose of metoclopramide was increased to 0.4 mg/kg BW, PO q12h.
On day 55, frozen tissue from the excised liver mass was sent for analysis to the Institut für Parasitologie, Universität Bern, Switzerland. Firstly, polymerase chain reaction (PCR) was carried out to amplify a section of the mitochondrial 12S rRNA gene. The resultant PCR fragment was sequenced and shown to be 100% identical to the sequence of reference E. multilocularis (2). Secondly, restriction fragment length polymorphism analysis was performed on PCR-generated fragments of the mitochondrial NADH dehydrogenase 1 and 12S rRNA genes (3); the obtained PCR fragments matched those of reference E. multilocularis. Finally, direct immunofluorescence of frozen tissue sections indicated that the tissue was positive for E. multilocularis antigen (4). A definitive diagnosis of E. multilocularis was therefore made, and conveyed to the attending veterinarian on day 85.
Since some of the hepatic mass was not removed at surgery, treatment of the dog with albendazole (Summit Compounding Pharmacy, Toronto, Ontario),10 mg/kg BW, PO q24h (5) began on approximately day 123; a similar treatment protocol is used in humans with hepatic alveolar echinococcosis (6). Because this dose can cause bone marrow suppression in dogs, it was recommended that a CBC be carried out weekly for the first month then once every 3 to 4 mo.
On day 153, the owner reported that the dog’s appetite was reduced, that it had vomited several times over the previous few nights, and that it had lost approximately 0.3 kg in weight over the previous week. Treatment with albendazole was stopped and the dog was administered metoclopramide, 0.4 mg/kg BW, PO q24h to q12h, as needed. Four days later, the dog was clinically normal. Resolution of the clinical signs suggested they were an adverse reaction to albendazole. However, since there is no proven alternative treatment for the metacestode stage of E. multilocularis in dogs, it was recommended that albendazole treatment be re-started at the same dosage. The owner was advised to stop treatment as soon as any gastrointestinal signs occurred, allow the dog to recover, and then check the CBC 2–3 days later; if normal, treatment could resume. If any abnormalities were present (e.g., neutropenia), it was advised that the CBC should be rechecked every 5 d and that the albendazole treatment should only be re-started when the CBC was normal. Due to concerns about side effects, the owner decided not to proceed further with albendazole treatment.
Discussion
The dog described herein appears to be the first documented instance of a canid infected with the metacestode of E. multilocularis in North America. Echinococcus multilocularis is a zoonotic tapeworm that is found in much of Europe, and in parts of northern Asia and North America (7,8). The adult stage is a small tapeworm that resides in the distal small intestine of its definitive host and reaches a maximum length of 5 mm. Within the intestine, the tapeworm releases eggs that are passed into the environment during defecation (9).
Eggs of E. multilocularis are immediately infective and the life cycle continues when an intermediate host ingests viable eggs. Once consumed, the hexacanth embryo is released from the egg, travels through the intestinal wall, and migrates to the liver via the hepatic portal circulation (6). The metacestode stage of E. multilocularis is an alveolar hydatid cyst, composed of numerous small vesicles lined with a germinal epithelium from which multiple protoscolices develop. This stage is characterized by exogenous budding that frequently results in spread of metacestodes to other locations within the intermediate host (6). The life cycle is completed when an infected intermediate host is consumed by a definitive host; within the digestive tract, the protoscolices attach to the intestinal wall and mature. Although the rate of development of E. multilocularis in definitive hosts can vary, the typical prepatent period is approximately 5 wk (9).
Within its endemic range, E. multilocularis is sustained through a sylvatic lifecycle. In temperate areas, multiple definitive and intermediate hosts are capable of perpetuating the parasite’s lifecycle; the red fox (Vulpes vulpes) is usually considered the most important definitive host, although other species, including coyotes (Canis latrans), raccoon-dogs (Nyctereutes procyonoides) and wolves (Canis lupus), are also suitable hosts (10). The most notable intermediate hosts in temperate areas are species of arvicoline and neotomine rodents including voles, lemmings, and deer mice (6,11).
Currently, E. multilocularis in North America is thought to be endemic in 2 distinct regions. The first, known as the Northern Tundra Zone, begins along the west coast of Alaska and extends northward and eastward to occupy most of the Canadian Arctic (12,13), although actual reports in Canada are limited (14,15). The second region, further south, is the North Central Region and consists of the southern portions of the Canadian provinces Alberta, Saskatchewan, and Manitoba, along with 13 neighboring USA states (North Dakota, South Dakota, Iowa, Minnesota, Montana, Wyoming, Nebraska, Illinois, Wisconsin, Indiana, Ohio, Missouri, Michigan) (13,16–18).
In North America, there are few documented instances of E. multilocularis infecting the intestinal tract of domestic dogs and cats. Most notably, in 1951, 12% of dogs belonging to the Alaskan Inuit on St. Lawrence Island were positive for E. multilocularis on postmortem examination (19). High numbers of rodents and the high prevalence of infection in dogs are believed to be the reasons that St. Lawrence Island had one of the highest rates of human alveolar echinococcosis in the world (7,20). However, to the best of the authors’ knowledge, cases of dogs with patent E. multilocularis infections have not been described elsewhere in North America. Although cats can be definitive hosts for E. multilocularis, very few cases have been reported in North America [3 in Saskatchewan (21) and 2 in North Dakota (16)]. No other cases were identified in the literature.
Human infections with E. multilocularis are extremely uncommon in the USA and Canada. Other than the aforementioned cases in Alaska, only 2 cases of human alveolar echinococcosis acquired in North America are described; a man from Winnipeg, Manitoba (22), and a woman from Minnesota (23,24).
The 3-year-old dog described herein was primarily a house dog, and from the time of purchase at 8 wk of age had lived approximately 8 km southeast of Quesnel in central BC. The dog was obtained from a breeder who lived on a farm near Quesnel and had never travelled beyond an area 100 km northwest of Quesnel, Powell River, and Victoria, BC. Approximately twice per week, from the time of purchase, the dog routinely visited a farm 8 km northwest of Quesnel. It is noteworthy that foxes and coyotes were known to reside in the area surrounding the home. Furthermore, while on the farm the dog had unsupervised access to fields, and occasionally returned home covered in strong-smelling material that was suspected to be fox feces; foxes were known to live in the area surrounding the farm.
As mentioned, the dog was infected with the metacestode stage of E. multilocularis, characteristic of an intermediate host. Additional work has indicated that the parasite strain was most likely European in origin (25). Although uncommon, similar cases of hepatic metacestode infections in domestic dogs have been described in Belgium (26), Germany (27), and Switzerland, in particular (5,28–30). In some cases, metacestode lesions were present in the omentum, abdominal cavity, or lungs (27–29). Amongst these cases, the most common clinical signs at presentation were progressive abdominal enlargement, intermittent inappetence, and vomiting. It should be noted, however, that in contrast to the case from BC, in which E. multilocularis was previously thought to be absent, all these cases occurred in highly endemic areas.
It generally is thought that a dog can become infected with the metacestode stage of E. multilocularis via 2 routes. The first is through ingestion of large numbers of infective eggs (28). The second is a consequence of infection with adult parasites that release eggs in the dog’s intestine; the eggs hatch in the intestine, invade the intestinal wall and proceed to develop (28). Thus, dogs can be infected simultaneously with both adult and metacestode stages of E. multilocularis (31). It is unclear which route of infection is more likely in the dog described herein. The sedimentation method used to examine the dog’s feces on day 9 was negative, suggesting that the metacestode stage developed as a result of ingestion of infective eggs. This route of infection would be consistent with the dog’s suspected behavior of rolling in fox feces, which could have resulted in ingestion of high numbers of parasite eggs. However, the diagnostic sensitivity of examining 1 fecal sample is not 100% (32). As a result, it is not possible to conclusively rule out intestinal infection with adult tapeworms.
In summary, veterinary practitioners in endemic areas should recognize that dogs may serve as both definitive and intermediate hosts for E. multilocularis, and that the metacestode of this zoonotic parasite is a rare potential differential diagnosis for hepatic masses. While infection of domestic dogs with E. multilocularis probably will remain rare, intestinal infections are likely to be under-diagnosed as the eggs of E. multilocularis are morphologically indistinguishable from those of E. granulosus and Taenia species (33); although they can be distinguished using molecular methods (3,34). Owners of dogs diagnosed with E. multilocularis (either the larval stage or adults) should consult with medical practitioners to ensure that they themselves have not been exposed, either through eggs shed by their dog or through common exposure of both dog and owner to eggs in feces of other carnivores. In endemic areas, dogs and cats that hunt and scavenge rodents should be routinely dewormed with a cestocide. Finally, this report is an excellent reminder of the role of veterinary practitioners in detecting the presence of pathogens with public health significance in animal sentinels in newly endemic regions. Future work includes determining the current distribution and genetic diversity of E. multilocularis within western Canada, important for assessing the risks of infection for wildlife, companion animals, and humans.
Acknowledgments
We thank Drs. Tom Gibson and Stephanie Nykamp for editorial assistance with text concerning surgery and imaging, respectively. CVJ
Footnotes
Reprints will not be available from the authors.
A brief abstract with information contained in this case report has been published in the Proceedings of the Annual Meeting of the American Association of Veterinary Parasitologists, July 31–August 3, 2010, Atlanta, Georgia, USA.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Junod L. Technique coprologique novelle essentiellement destinée a la concentration des trophozoites d’amibes. Bul Soc Pathol Exot. 1972;65:390–398. [PubMed] [Google Scholar]
- 2.Dinkel A, von Nickisch-Rosenegk M, Bilger B, Merli M, Lucius R, Romig T. Detection of Echinococcus multilocularis in the definitive host: Coprodiagnosis by PCR as an alternative to necropsy. J Clin Microbiol. 1998;36:1871–1876. doi: 10.1128/jcm.36.7.1871-1876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- 4.Deplazes P, Gottstein B. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology. 1991;103:41–49. doi: 10.1017/s0031182000059278. [DOI] [PubMed] [Google Scholar]
- 5.Scharf G, Deplazes P, Kaser-Hotz B, et al. Radiographic, ultrasonographic, and computed tomographic appearance of alveolar echinococcosis in dogs. Vet Radiol Ultrasound. 2004;45:411–418. doi: 10.1111/j.1740-8261.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 6.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert J, Schantz PM, Gasser RB, et al. Geographic distribution and prevalence. In: Eckert J, Gemmell MA, Meslin FX, et al., editors. WHO/OIE manual on echinococcosis in humans and animals: A public health problem of global concern. Paris, France: Office international des épizooties; 2001. pp. 119–153. [Google Scholar]
- 8.Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006;55:S187–S191. doi: 10.1016/j.parint.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MA, Coop RL, Wall RL. Veterinary Parasitology. 3rd ed. Oxford, UK: Blackwell Publishing; 2007. pp. 377–379. [Google Scholar]
- 10.Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Polley L. Visceral larva migrans and alveolar hydatid disease, dangers real or imagined. Vet Clin North Am. 1978;8:353–378. doi: 10.1016/s0091-0279(78)50041-5. [DOI] [PubMed] [Google Scholar]
- 12.Rausch RL. Life cycle patterns and geographic distribution of Echinococcus species. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and hydatid disease. Wallingford, UK: CABI; 1995. pp. 88–134. [Google Scholar]
- 13.Eckert J, Conraths FJ, Tackmann K. Echinococcosis: An emerging or re-emerging zoonosis? Int J Parasitol. 2000;30:1283–1294. doi: 10.1016/s0020-7519(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 14.Choquette LPE, Macpherson AH, Cousineau JG. Note on the occurrence of Echinococcus multilocularis Leuckart, 1863 in the Arctic fox in Canada. Can J Zool. 1962;40:1167. [Google Scholar]
- 15.Eaton RD, Secord DC. Some intestinal parasites of Arctic fox, Banks Island, N.W.T. Can J Comp Med. 1979;43:229–230. [PMC free article] [PubMed] [Google Scholar]
- 16.Leiby P, Kritsky DC. Echinococcus multilocularis: A possible domestic life cycle in central North America and its public health implications. J Parasitol. 1972;58:1213–1215. [PubMed] [Google Scholar]
- 17.Storandt ST, Virchow DR, Dryden MW, Hygnstrom SE, Kazacos KR. Distribution and prevalence of Echinococcus multilocularis in wild predators in Nebraska, Kansas, and Wyoming. J Parasitol. 2002;88:420–422. doi: 10.1645/0022-3395(2002)088[0420:DAPOEM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Kazacos KR. Cystic and alveolar hydatid disease caused by Echinococcus species in the contiguous United States. Suppl Compend Contin Educ Pract Vet. 2003;25:16–20. [Google Scholar]
- 19.Schantz PM, Chai J, Craig PS, et al. Epidemiology and control of hydatid disease. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and Hydatid Disease. Wallingford, UK: CABI; 1995. pp. 231–233. [Google Scholar]
- 20.Stehr-Green JK, Stehr-Green PA, Schantz PM, Wilson JF, Lanier A. Risk factors for infection with Echinococcus multilocularis in Alaska. Am J Trop Med Hyg. 1988;38:380–385. doi: 10.4269/ajtmh.1988.38.380. [DOI] [PubMed] [Google Scholar]
- 21.Wobeser G. The occurrence of Echinococcus multilocularis (Leukart, 1863) in cats near Saskatoon, Saskatchewan. Can Vet J. 1971;12:65–68. [PMC free article] [PubMed] [Google Scholar]
- 22.James E, Boyd W. Echinococcus alveolaris (with the report of a case) Can Med Assoc J. 1937;36:354–356. [PMC free article] [PubMed] [Google Scholar]
- 23.Gamble WG, Segal M, Schantz PM, Rausch RL. Alveolar hydatid disease in Minnesota. First human case acquired in the contiguous United States. J Am Med Assoc. 1979;241:904–907. doi: 10.1001/jama.241.9.904. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki H, Nakao M, Nakaya K, Schantz PM, Ito A. Short report: Genetic analysis of Echinococcus multilocularis originating from a patient with alveolar echinococcosis occurring in Minnesota in 1977. Am J Trop Med Hyg. 2008;79:245–247. [PubMed] [Google Scholar]
- 25.Jenkins EJ, Peregrine AS, Hill JE, et al. Detection of European strain of Echinococcus multilocularis in North America. Emerg Infect Dis. 2012;18:1010–1012. doi: 10.3201/eid1806.111420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losson BJ, Coignoul F. Larval Echinococcus multilocularis infection in a dog. Vet Rec. 1997;141:49–50. doi: 10.1136/vr.141.2.49. [DOI] [PubMed] [Google Scholar]
- 27.Geisel O, Barutzki D, Minkus G, Hermanns W, Löscher T. Hunde als Finnenträger (Intermediärwirt) von Echinococcus multilocularis. Kleintierpraxis. 1990;35:275–280. [Google Scholar]
- 28.Haller M, Deplazes P, Guscetti F, Sardinas JC, Reichler I, Eckert J. Surgical and chemotherapeutic treatment of alveolar echinococcosis in a dog. J Am Anim Hosp Assoc. 1998;34:309–314. doi: 10.5326/15473317-34-4-309. [DOI] [PubMed] [Google Scholar]
- 29.Deplazes P, Eckert J. Veterinary aspects of alveolar echinococcosis — A zoonosis of public health significance. Vet Parasitol. 2001;98:65–87. doi: 10.1016/s0304-4017(01)00424-1. [DOI] [PubMed] [Google Scholar]
- 30.Heier A, Geissbühler U, Sennhauser D, Scharf G, Kühn N. A case of alveolar hydatid disease in a dog: Domestic animals as rare incidental intermediate hosts for Echinococcus multilocularis. Schweiz Arch Tierheilk. 2007;149:123–127. doi: 10.1024/0036-7281.149.3.123. [DOI] [PubMed] [Google Scholar]
- 31.Deplazes P, Arnold P, Kaser-Holtz B, et al. Concurrent infections of the liver and intestine with Echinococcus multilocularis in dogs. Arch Int Hidatid. 1997;31:202–203. [Google Scholar]
- 32.Katagiri S, Oliveira-Sequeira TCG. Comparison of three concentration methods for the recovery of canine intestinal parasites from stool samples. Exp Parasitol. 2010;126:214–216. doi: 10.1016/j.exppara.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Zajac AM, Conboy GA. Veterinary Clinical Parasitology. 7th ed. Ames, Iowa: Blackwell Publishing; 2006. p. 60. [Google Scholar]
- 34.Himsworth CG, Jenkins E, Hill JE, et al. Emergence of sylvatic Echinococcus granulosus as a parasitic zoonosis of public health concern in an indigenous community in Canada. Am J Trop Med Hyg. 2010;82:643–645. doi: 10.4269/ajtmh.2010.09-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]