Abstract
Four dogs with T2N0M0 transitional cell carcinoma of the lower urinary tract underwent multimodal treatment consisting of neoadjuvant chemotherapy, external-beam radiotherapy, and adjuvant chemotherapy. No significant toxicity was documented. All dogs showed clinical improvement and reduction of tumor volume based on computed tomography (CT).
Résumé
Chimiothérapie et radiothérapie comme traitement pour carcinomes à cellules transitionnelles urothéliaux avec infiltration du muscle dans 4 chiens. Quatre chiens avec des carcinomes à cellules transitionnelles du bas tractus urinaire (T2N0M0) ont été traités avec une approche multimodale consistent en chimiothérapie néodjuvante, radiothérapie externe et chimiothérapie adjuvante. Nous n’avons pas observé une toxicité signifiante. Tous les chiens ont répondu à ce traitement multimodale, défini comme amélioration des symptômes cliniques et réduction des dimensions de la tumeur, indiqué au scanner.
(Traduit par Julia Buchholz)
High-grade, muscle-invasive transitional cell carcinoma (TCC) of the urinary tract is an increasing cause of mortality in dogs, and is characterized by a local aggressive behavior leading to urinary tract obstruction and a high metastatic potential (1). Dogs with invasive TCC have limited treatment options. Regardless of clinical stage, systemic chemotherapy, having shown efficacy in bladder cancer, remains the standard approach for most of these dogs (2–8). Unfortunately, as a single therapy modality, chemotherapy yields survival times that usually do not exceed 1 y (2–8). Due to the typical trigone location, surgery is often not an option, resulting in considerable morbidity and decreased quality of life post-surgically (9–11). External beam radiation therapy for the treatment of urinary TCC has not been used extensively in veterinary medicine and only 1 study has been published (12). A combination of once-weekly coarsely fractioned radiation therapy, mitoxantrone, and piroxicam improved symptoms in 90% of the dogs with TCC; however, survival was not increased compared with dogs given medical treatment only (12).
The following report describes the use of a multimodal treatment strategy consisting of neoadjuvant chemotherapy, external-beam radiation therapy, and adjuvant chemotherapy in 4 dogs with TCC of the lower urinary tract.
Case descriptions
Case 1
A 14.5 kg 9-year-old spayed female beagle dog was evaluated because of stranguria, pollakiuria, and hematuria of 1-month duration that was nonresponsive to piroxicam. The results of physical examination were unremarkable. An abdominal ultrasound revealed a urethral mass extending to the trigone, and a biopsy sample was collected by means of ultrasound-guided traumatic catheterization. The regional lymph nodes and the other abdominal organs were sonographically normal. Thoracic radiography showed no evidence of metastatic disease. Based on imaging and biopsy findings, the tumor was diagnosed as a T2N0M0 TCC of the urethra and bladder. Gemcitabine (Gemcitabin; Ebewe Pharma, Cham, Schweiz) was administered at the dose of 800 mg/m2 IV q7d for 21 cycles in association with piroxicam (Piroxicam-Mepha, Mepha Pharma AG, Aesch/BL), 0.3 mg/kg, PO, SID, with occurrence of 1 episode of VCOG grade 1 bone marrow toxicity (mild) and 2 episodes of grade 2 gastrointestinal toxicity (moderate) [Veterinary Co-operative Oncology Group — Common Terminology Criteria for Adverse Events (VCOG-CTCAE)] (13).
The dog experienced clinical improvement based on subsided signs of stranguria, pollakiuria, and hematuria within 1 cycle of treatment. An abdominal ultrasound was performed by the same operator 4 wk after the start of chemotherapy. For measurement standardization purposes, the owner was instructed to offer water ad libitum and not to allow the dog to urinate for at least 1 h before the ultrasound examinations. To obtain consistent bidimensional measurements over multiple examinations, the dog was always positioned in lateral recumbence and the bladder had to be distended, as evidenced by a bladder wall of < 2 mm (14,15). Based on the measurements of the primary tumor, compared with those obtained during the previous ultrasonographic examination, the dog experienced partial remission. The symptoms reappeared 150 d after the initiation of chemotherapy. Pre- and post-contrast CT studies (GE Bright Speed 8-Slice Helical CT unit; GE Healthcare, Milan, Italy) were performed for diagnostic purposes as well as for radiation therapy planning with a slice thickness of 1.25 mm (Figure 1).
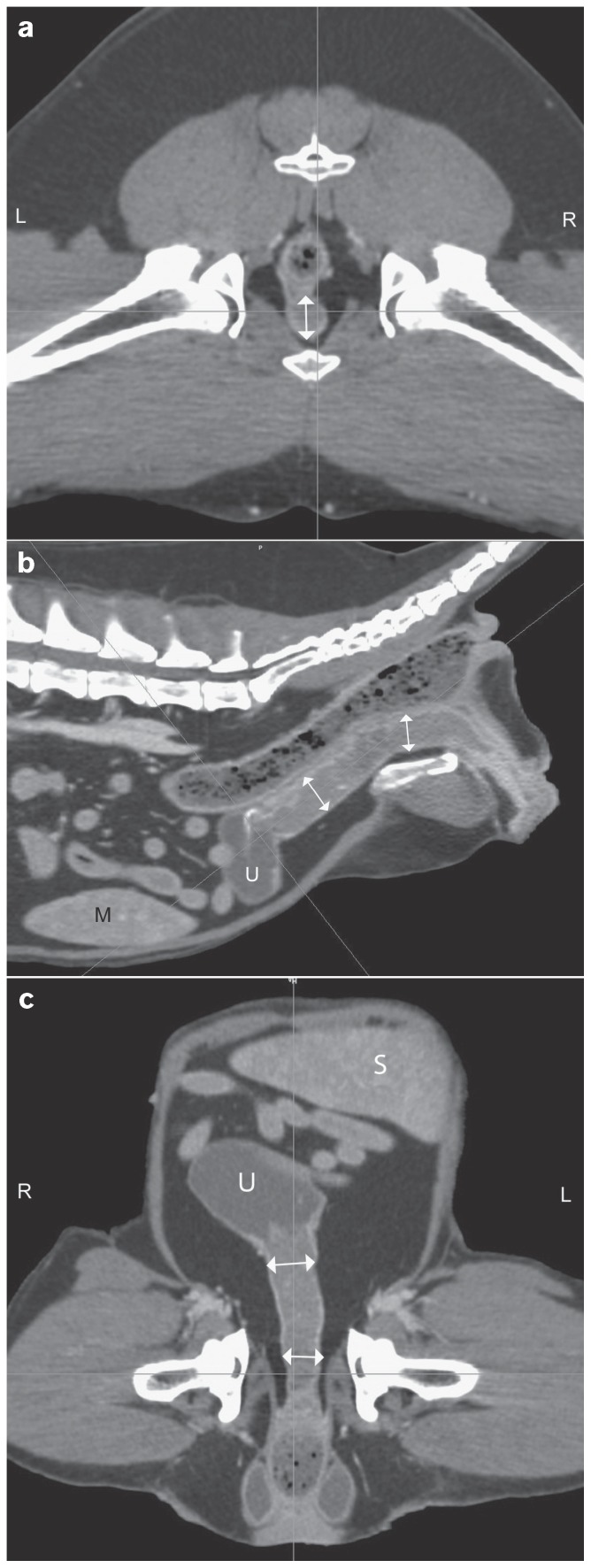
Figure 1.
Computed tomographic images of the caudal abdomen of a 10-year-old, female spayed beagle dog with transitional cell carcinoma of the urethra (↕). Axial image on the level of the hips (a), sagittal (b) and dorsal (c) multiplanar reconstructions. (S — spleen, U — urinary bladder).
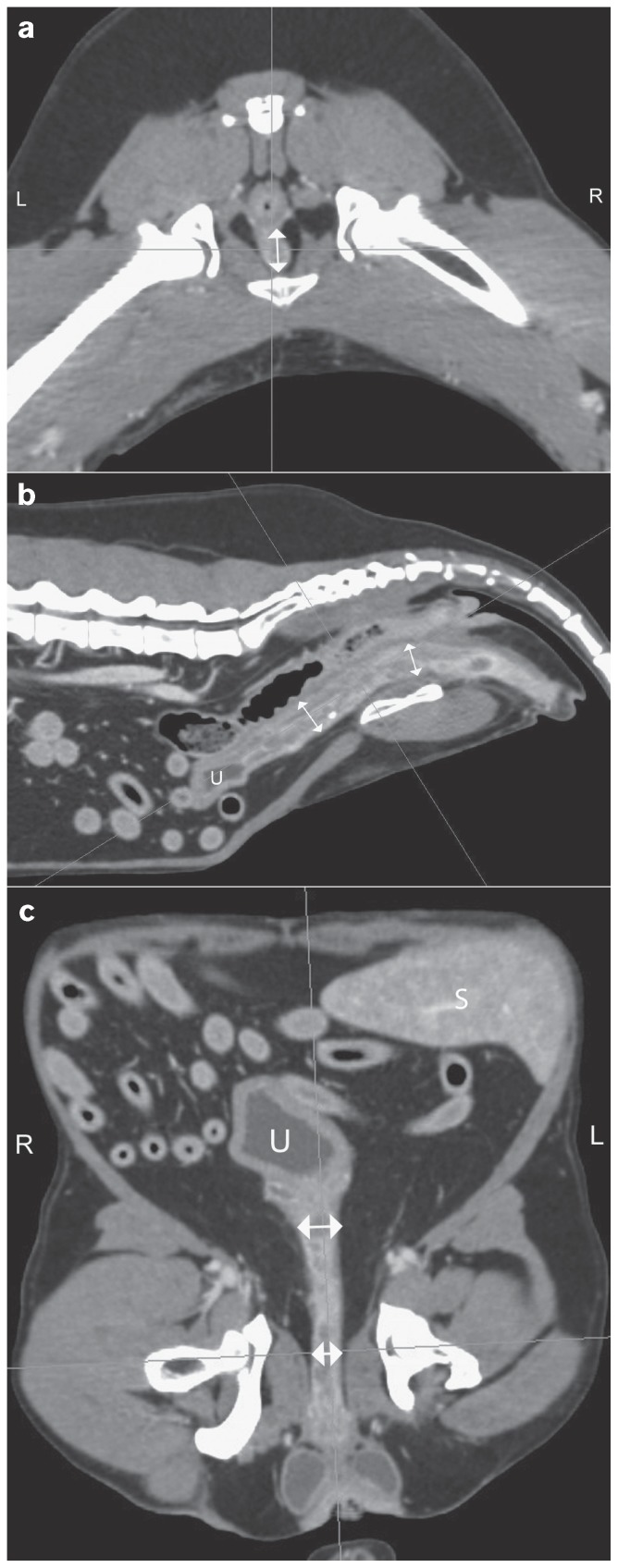
Pre- and post-contrast scans were imported into the radiation treatment planning system (Eclipse 8.1; Varian Medical Systems, Palo Alto, California, USA); body, colon, rectum, spinal cord, and bladder were contoured. Gross tumor volume (GTV), clinical tumor volume (CTV), and planning target volume (PTV) were delineated. The CTV and PTV included the entire urethra plus 50.3% and 69.4% of the bladder volume, respectively. Three to 4 field plans were utilized; multi-leaf collimators and dynamic wedges were used for plan optimization concerning sparing of sensitive structures/organs and dose homogeneity, respectively. Radiation was performed with a 6 MV linear accelerator (Clinac; Varian Medical Systems). The dog received 12 fractions of 3.3 Gy for a total dose of 39.6 Gy over a 3-week period. No adverse effects of radiation treatment were observed [Veterinary Radiation Therapy Oncology Group (VRTOG) grade 0] (16) and after 6 fractions the dog started urinating normally. Two weeks after the end of radiation therapy, the dog received 4 cycles of carboplatin (Carboplatin, Ebewe Pharma, Cham, Schweiz), 240 mg/m2, IV, q3wk and piroxicam was discontinued to avoid enhanced nephrotoxicity. The treatment was well-tolerated with no occurrence of any side effects. A follow-up total-body CT scan was repeated 3 mo after radiation therapy, showing a 25% reduction of the tumor size (Figure 2) and no evidence of regional or distant metastasis. Afterwards, thoracic radiography and abdominal ultrasound were repeated at 2-month intervals, showing no progression of the disease. Eight months after the end of the multimodal treatment, the dog developed acute pancreatitis and died at home 576 d after the initial diagnosis. Necropsy was not permitted.
Figure 2.
Follow-up CT images 3 months after radiation therapy. The diameter of the urethral transitional carcinoma markedly decreased (↕; approximately 25%). Axial image at the level of the hips (a), sagittal (b) and dorsal (c) multiplanar reconstructions. (S — spleen, U — urinary bladder).
Case 2
A 12-kg 11-year-od spayed female Chihuahua dog was referred with a cytological diagnosis of TCC after having failed 1 cycle of mitoxantrone (Novantron, Meda, Schweiz), administered at 6 mg/m2 IV 1 mo before, and piroxicam at standard dose. At presentation, the dog was not able to urinate because of complete urethral obstruction as demonstrated by cystoscopy. Pathological examination of retrieved specimen revealed TCC. A total-body CT scan showed a heterogeneous mass arising from the urethra with evidence of invasion of the bladder wall; there was no evidence of pelvic or abdominal lymphadenopathy and distant metastases (clinical stage T2N0M0). Because this dog was not able to urinate spontaneously, a percutaneous prepubic cystostomy tube was placed. Radiation therapy was performed as previously described and after 2 fractions the dog started urinating on its own. The CTV and PTV included the entire urethra plus 19.0% and 44.8% of the bladder volume, respectively. The cystostomy tube was not removed until the end of radiation therapy to facilitate reproducibility of bladder volume prior to radiation. Toxicity due to radiation therapy was not observed. Two weeks after the end of radiation therapy, piroxicam was discontinued and the dog completed 4 cycles of carboplatin, administered every 3 wk, which were well-tolerated.
A follow-up total-body CT scan 3 mo after radiation therapy showed a 10% reduction of the tumor size without evidence of regional or distant metastasis. Regular re-staging work-up performed at 2-month intervals showed no disease progression. Eight months after the end of multimodal treatment and 1 y after initial presentation, the dog became lethargic and anorectic and showed severe abdominal pain. Serum biochemical analysis showed increased creatinine [1213 mmol/L, reference range (RR): 0 to 125 mmol/L] and urea (80.2 mmol/L, RR: 3.8 to 9.4 mmol/L). An abdominal ultrasound showed stable disease of the urinary TCC with no evidence of metastatic disease. A diagnosis of acute renal failure was made and the owners elected euthanasia. Necropsy was not permitted.
Case 3
A 33.9-kg 11-year-old spayed female pit bull terrier dog was referred with a complaint of stranguria, pollakiuria, hematuria, and abdominal pain of 2-months duration, not responding to piroxicam. The owner reported no other clinical signs. Cystoscopy revealed a proximal urethral mass with an unremarkable bladder; biopsies confirmed TCC. Initial staging work-up, consisting of total-body CT, showed no evidence of regional or distant metastatic disease (clinical stage T2N0M0). The dog received 3 courses of weekly gemcitabine and piroxicam, as previously described, experiencing a clinical improvement after the second cycle. Hematological toxicity consisted of VCOG-CTCAE grade 1 myelosuppression (mild); other adverse events were not described. At this time, a planning CT scan for subsequent radiation treatment was performed and the dog was irradiated with the same protocol as described before. The CTV and PTV included the entire urethra; the bladder was not irradiated.
After the sixth fraction, symptoms further improved, with the dog having some pollakiuria towards the end of urination. Because of the difficulty in distinguishing whether these clinical symptoms were due to the tumor or to acute radiation toxicity, the dog was judged as having grade 1 acute toxicity following the VRTOG scoring scheme. Two weeks after radiation therapy the dog received adjuvant single agent carboplatin chemotherapy as previously described, completing 4 cycles. Three months after completion of radiation therapy, the dog underwent follow-up CT and this confirmed a 20% reduction in tumor size. Seven months after having finished the radiation therapy protocol the dog was urinating spontaneously, but she showed discomfort and pain during urination and had a swollen hind leg. No further examinations were conducted at that time. Due to the overall poor quality of life she was euthanized by the local veterinarian. Necropsy was not permitted.
Case 4
A 15.8-kg 9-year-old spayed female Irish setter dog was admitted to our clinic with a 3-week history of stranguria and pollakiuria, not showing any improvement despite treatment with piroxicam. The dog initially underwent cystoscopy, during which it was noted to have a proximal urethral mass with trigone extension. Histological examination of the mass revealed TCC. Imaging studies (abdominal ultrasound, thoracic radiography) showed no evidence of regional or distant metastases (clinical stage T2N0M0). The dog was treated with piroxicam and weekly gemcitabine, as previously described, without any clinical improvement. Chemotherapy-related side effects were scored as VCOG grade 1 hematologic toxicity and VCOG grade 2 gastrointestinal toxicity. A follow-up abdominal ultrasound after 2 cycles of chemotherapy revealed stable disease.
Because of lack of improvement, medical therapy was interrupted and an abdominal CT scan was performed for radiation therapy planning. Radiation was performed as described. The CTV and PTV included the entire urethra plus 83.4% and 97.5% of the bladder volume, respectively. After fraction number 7, clinical symptoms improved remarkably with the dog having some pollakiuria at the end of urination. Again, radiation toxicity was judged as grade 1 following the VRTOG scoring scheme. Adjuvant carboplatin administered 4 times as previously described was well-tolerated. Dog 4 showed stable disease on the follow-up CT scan performed 3 mo after completion of radiation therapy, as well as on regular follow-up ultrasound examinations up to 221 d after diagnosis. The dog was lost to follow-up and no further data could be obtained.
Discussion
Defining the optimal treatment in dogs with muscle-invasive TCC of the urinary tract is difficult. Most dogs with this disease are deemed unsuitable for surgery due to trigone location or advanced tumor stage (17). Based on published data, the absolute survival benefit for chemotherapy-treated dogs is modest at best, strongly supporting the need for the development of more effective regimens. In the dogs of this report, we described a multimodal approach consisting of neoadjuvant chemotherapy, external-beam radiation therapy, and adjuvant chemotherapy as a bladder-preserving strategy. Treatment differed between dogs; however, we were able to show that this multimodal strategy consisting of chemotherapy, non steroidal anti-inflammatory drugs, and radiation therapy is well-tolerated and should be explored further.
None of the dogs of this report qualified for surgical resection because of locally advanced disease. Neoadjuvant chemotherapy was administered with the goal of reducing tumor size, thereby facilitating the local treatment procedure, radiation therapy, because of the possibility of increased sparing of surrounding normal structures from being irradiated. Due to the documented tolerability and efficacy, gemcitabine was chosen as the induction agent (8). In the 3 dogs receiving neoadjuvant gemcitabine, chemotherapy was administered without major toxicity. Furthermore, 2 of these 3 dogs experienced improvement of symptoms, which is in agreement with previously reported data (8). Dog 2 was referred after having failed systemic mitoxantrone and was anuric at presentation. Tumor obstruction of the urinary tract raised concern and it was decided to place a prepubic cystostomy tube and irradiate this patient without administering any neoadjuvant gemcitabine.
The dogs herein received different numbers of chemotherapy courses before starting radiation therapy. Dog 1 was irradiated when symptoms increased again, dog 3 started radiation therapy as soon as symptoms improved, and dog 4 received initial irradiation after 2 cycles of gemcitabine because a clinical amelioration was not achieved using chemotherapy only. Future trials should explore the best time to start radiation therapy after administering neoadjuvant chemotherapy.
Radiation therapy (external beam and intraoperative radiation therapy) is not routinely used as part of the treatment of canine bladder neoplasia. Median survival times using different radiation strategies range from 4 to 15 mo (12,18,19). Depending on the protocol used, side effects such as pollakiuria, urinary incontinence, cystitis, stranguria, and hydronephrosis can occur. High single doses administered intraoperatively caused severe late radiation toxicity resulting in euthanasia in up to 36% of dogs with TCC (19). Using fractionated radiation protocols, the dose that can be delivered to the bladder is limited by the potential occurrence of late radiation effects, including fibrosis and bladder shrinkage. An attempt to use a radio-chemotherapy protocol in dogs with TCC failed to show a benefit of adding coarse-fractionated radiation over medical treatment alone (12). In human medicine, there is compelling evidence to support the presence of a radiation dose-response curve in TCC (20) and therefore escalating the dose would be desirable.
The approach of multimodality, organ-conservation protocols seems appealing and neoadjuvant chemotherapy was expected to also reduce the radiation volume to keep the potential side effects to a minimum. The other rationale for having a smaller target volume was to be able to escalate the dose to increase local tumor control probability. Also, adding a partial bladder boost to deliver a higher dose to the portion of the bladder at highest risk, while limiting the whole bladder dose, might increase local tumor control. Especially for bladder neoplasia it remains questionable what volume should be defined as CTV, whole bladder/urethra versus partial bladder radiation therapy (gross/tumor-bearing volume/region of the bladder plus margin). Another issue of concern while irradiating bladder tumors is the difficulty to reproduce the same position/volume of bladder and rectum leading to large geometrical uncertainties. Accordingly, we tried to have the bladder emptied as much as possible for the radiation therapy-planning CT scan as well as the individual radiation therapy sessions. Ideally, before each radiation therapy fraction, image-guided radiation therapy using either 2D orthogonal setup images or 3D cone beam CT (CBCT) is used to ensure positioning accuracy.
External-beam radiation therapy was well-tolerated by all dogs and none of them had major complications. Furthermore, clinical symptoms markedly improved in all dogs. Dog 2, anuric at presentation, started urinating on its own during radiation therapy, and it was therefore possible to remove the cystostomy tube. None of the dogs in the present study developed clinical signs suggestive of bladder fibrosis; voiding function during the follow-up period was satisfactory in all patients. In patient 3, we can not clearly distinguish between tumor recurrence versus radiation-induced late side effects as a potential cause of discomfort during (spontaneous) urination. In this dog, the bladder was not included in the radiation field since the TCC was considered limited to the urethra. No gastrointestinal complications were reported. According to 2 studies that examined toxicity while irradiating the pelvic region, fraction size > 3 Gy was associated with a higher risk of late effects (21,22). We used 3.3 Gy per fraction while limiting the total dose received to < 40 Gy. Still, we are not able to exclude the occurrence of late side effects due to the short follow-up time.
All dogs received systemic adjuvant chemotherapy consisting of carboplatin. The rationale for post-radiation chemotherapy was to eradicate micrometastatic disease. Overall, the adjuvant treatment was well-tolerated, with no occurrence of hematologic or non-hematologic toxicity.
Concerning treatment response, dogs 1, 2, and 3 experienced a reduction (10 to 50%) of their tumor size based on comparison of the pre- and post-radiation therapy CT scans, while the 4th dog had stable disease. Ultrasound is a common diagnostic imaging tool for assessing tumor response within the urinary tract; however, it is often difficult to accurately quantify tumor size reduction due to bladder volume changes (14,15). This is the first report describing the usefulness of CT in assessing tumor response in urinary tract neoplasia. To ensure accuracy while comparing CT scans, dogs were always positioned in the same recumbence (sternally) and the bladder was completely emptied.
Two dogs died of causes unrelated to TCC after 364 and 574 d, and 1 dog died, probably due to the disease, after 275 d, having been clinically in remission up to that date. One dog was lost to follow-up after being clinically stable for 221 d. Although complete remissions were not achieved, stabilization of the tumor and prevention of metastatic disease improved the quality of life and prolonged survival. None of the dogs developed distant metastasis during the treatment or follow-up period. Clinically occult, asymptomatic metastases could not be completely ruled out in the 2 deceased dogs, since autopsy was not permitted by the owners. With regards to the dogs that were still alive, the short follow-up may have contributed to the low metastatic rate. Conversely, neoadjuvant and/or adjuvant chemotherapy may have reduced the incidence of distant metastases by controlling occult micrometastatic disease.
Although treatment was not consistent among dogs with regards to neoadjuvant treatment (drug and length of time it was given), all dogs received the same radiation therapy protocol, adjuvant chemotherapy and non-steroidal anti-inflammatory drugs. Treatment was well-tolerated, showing that combining systemic chemotherapy with local external-beam radiation therapy may be feasible. On the other hand, it is difficult to interpret the contribution of each component of this multimodal approach to antitumor activity. Results of this study did not allow the conclusion that this protocol is superior concerning survival times than using medical treatment alone. Nevertheless, the data suggest that the approach described in this report is worth further investigation, possibly using slight modifications and a completely standardized overall treatment combination. Future studies with a larger population of dogs undergoing this approach should be performed to assess whether this strategy may be successful in dogs with locally advanced TCC and poor prognosis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003;17:136–144. doi: 10.1892/0891-6640(2003)017<0136:ctcc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Moore AS, Cardona A, Shapiro W, Madewell BR. Cisplatin (cisdiamminedichloroplatinum) for treatment of transitional cell carcinoma of the urinary bladder or urethra: A retrospective study of 15 dogs. J Vet Intern Med. 1990;4:148–152. doi: 10.1111/j.1939-1676.1990.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 3.Chun R, Knapp DW, Widmer WR, Glickman NW, DeNicola DB, Bonney PL. Cisplatin treatment of transitional cell carcinoma of the urinary bladder in dogs: 18 cases (1983–1993) J Am Vet Med Assoc. 1996;209:1588–1591. [PubMed] [Google Scholar]
- 4.Knapp DW, Glickman NW, Widmer WR, et al. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother Pharmacol. 2000;46:221–226. doi: 10.1007/s002800000147. [DOI] [PubMed] [Google Scholar]
- 5.Greene SN, Lucroy MD, Greenberg CB, Bonney PL, Knapp DW. Evaluation of cisplatin administered with piroxicam in dogs with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 2007;23:1056–1060. doi: 10.2460/javma.231.7.1056. [DOI] [PubMed] [Google Scholar]
- 6.Chun R, Knapp DW, Widmer WR, et al. Phase II clinical trial of carboplatin in canine transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 1997;11:279–283. doi: 10.1111/j.1939-1676.1997.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Boria PA, Glickman NW, Schmidt BR, et al. Carboplatin and piroxicam therapy in 31 dogs with transitional cell carcinoma of the urinary bladder. Vet Comp Oncol. 2005;3:73–80. doi: 10.1111/j.1476-5810.2005.00070.x. [DOI] [PubMed] [Google Scholar]
- 8.Marconato L, Zini E, Lindner D, Suslak-Brown L, Nelson V, Jeglum KA. Toxic effects and antitumor response of gemcitabine in combination with piroxicam treatment in dogs with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 2011;238:1004–1010. doi: 10.2460/javma.238.8.1004. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RD, Hankes GH. Ureterocolonic anastomosis in a dog with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 1987;190:1427–1429. [PubMed] [Google Scholar]
- 10.Stone EA, Withrow SJ, Page RL, Schwarz PD, Wheeler SL, Seim HB., 3rd Ureterocolonic anastomosis in ten dogs with transitional cell carcinoma. Vet Surg. 1988;17:147–153. doi: 10.1111/j.1532-950x.1988.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 11.Stone EA, George TF, Gilson SD, Page RL. Partial cystectomy for urinary bladder neoplasia: Surgical technique and outcome in 11 dogs. J Small Anim Pract. 1996;37:480–485. doi: 10.1111/j.1748-5827.1996.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 12.Poirier VJ, Forrest LJ, Adams WM, Vail DM. Piroxicam, mitoxantrone, and coarse fraction radiotherapy for the treatment of transitional cell carcinoma of the bladder in 10 dogs: A pilot study. J Am Anim Hosp Assoc. 2004;40:131–136. doi: 10.5326/0400131. [DOI] [PubMed] [Google Scholar]
- 13.Veterinary Cooperative Oncology Group. Veterinary cooperative oncology group — common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 14.Geisse AL, Lowry JE, Schaeffer DJ, Smith CW. Sonographic evaluation of urinary bladder wall thickness in normal dogs. Vet Radiol Ultrasound. 1997;38:132–137. doi: 10.1111/j.1740-8261.1997.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 15.Hume C, Seiler G, Porat-Mosenco Y, Caceres A, Shofer F, Sorenmo K. Cystosonographic measurements of canine bladder tumours. Vet Comp Oncol. 2010;8:122–126. doi: 10.1111/j.1476-5829.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 16.Ladue T, Klein MK Veterinary Radiation Therapy Oncology Group. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound. 2001;42:475–476. doi: 10.1111/j.1740-8261.2001.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 17.Henry CJ. Management of transitional cell carcinoma. Vet Clin North Am Small Anim Pract. 2003;33:597–613. doi: 10.1016/s0195-5616(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 18.Withrow SJ, Gillette EL, Hoopes PJ, McChesney SL. Intraoperative irradiation of 16 spontaneously occurring canine neoplasms. Vet Surg. 1989;18:7–11. doi: 10.1111/j.1532-950x.1989.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker M, Breider M. Intraoperative radiotherapy of canine bladder cancer. Vet Radiol. 1987;28:200–204. [Google Scholar]
- 20.Morrison R. The results of treatment of cancer of the bladder — A clinical contribution to radiobiology. Clin Radiol. 1975;26:67–75. doi: 10.1016/s0009-9260(75)80017-1. [DOI] [PubMed] [Google Scholar]
- 21.Arthur JJ, Kleiter MM, Thrall DE, Pruitt AF. Characterization of normal tissue complications in 51 dogs undergoing definitive pelvic region irradiation. Vet Radiol Ultrasound. 2008;49:85–89. doi: 10.1111/j.1740-8261.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CR, McNiel EA, Gillette EL, Powers BE, LaRue SM. Late complications of pelvic irradiation in 16 dogs. Vet Radiol Ultrasound. 2002;43:187–192. doi: 10.1111/j.1740-8261.2002.tb01668.x. [DOI] [PubMed] [Google Scholar]