Abstract
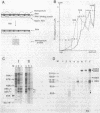
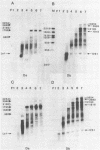
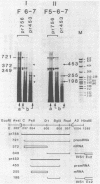
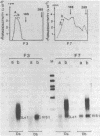
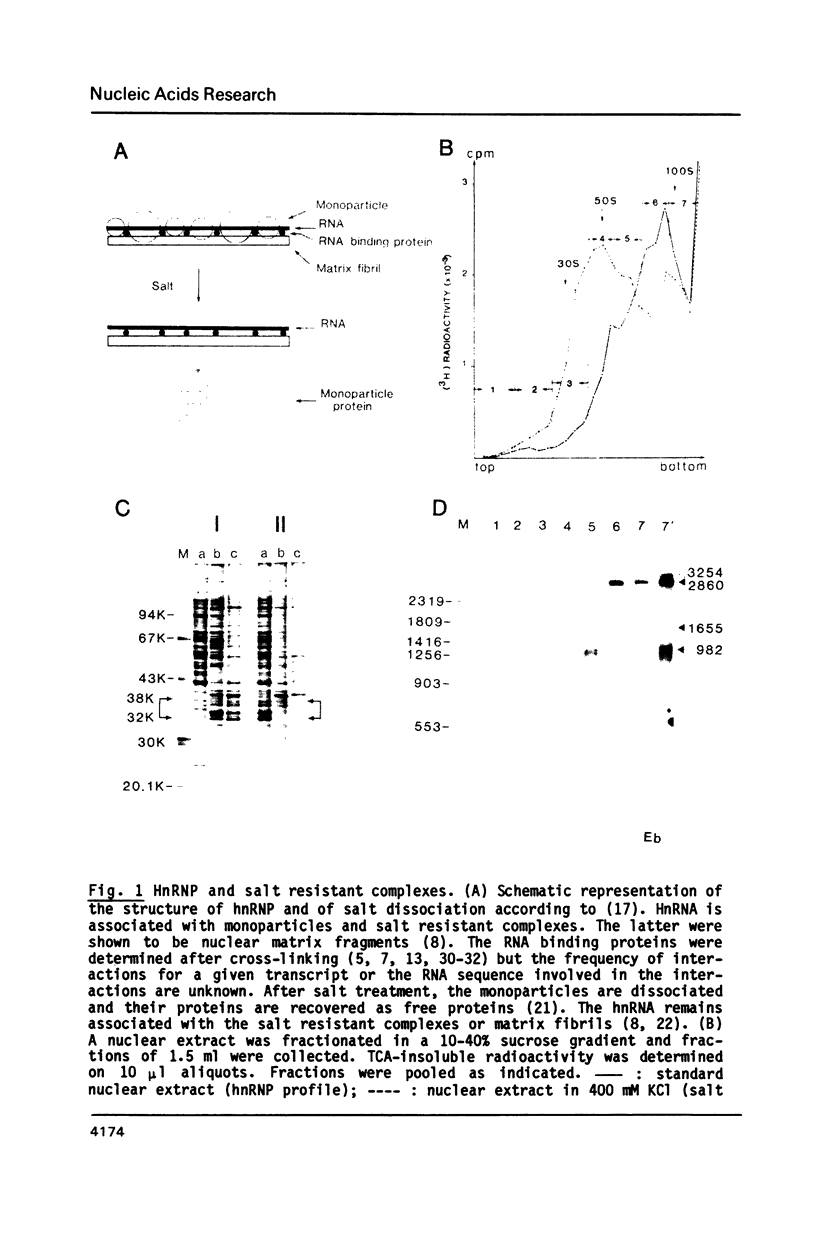
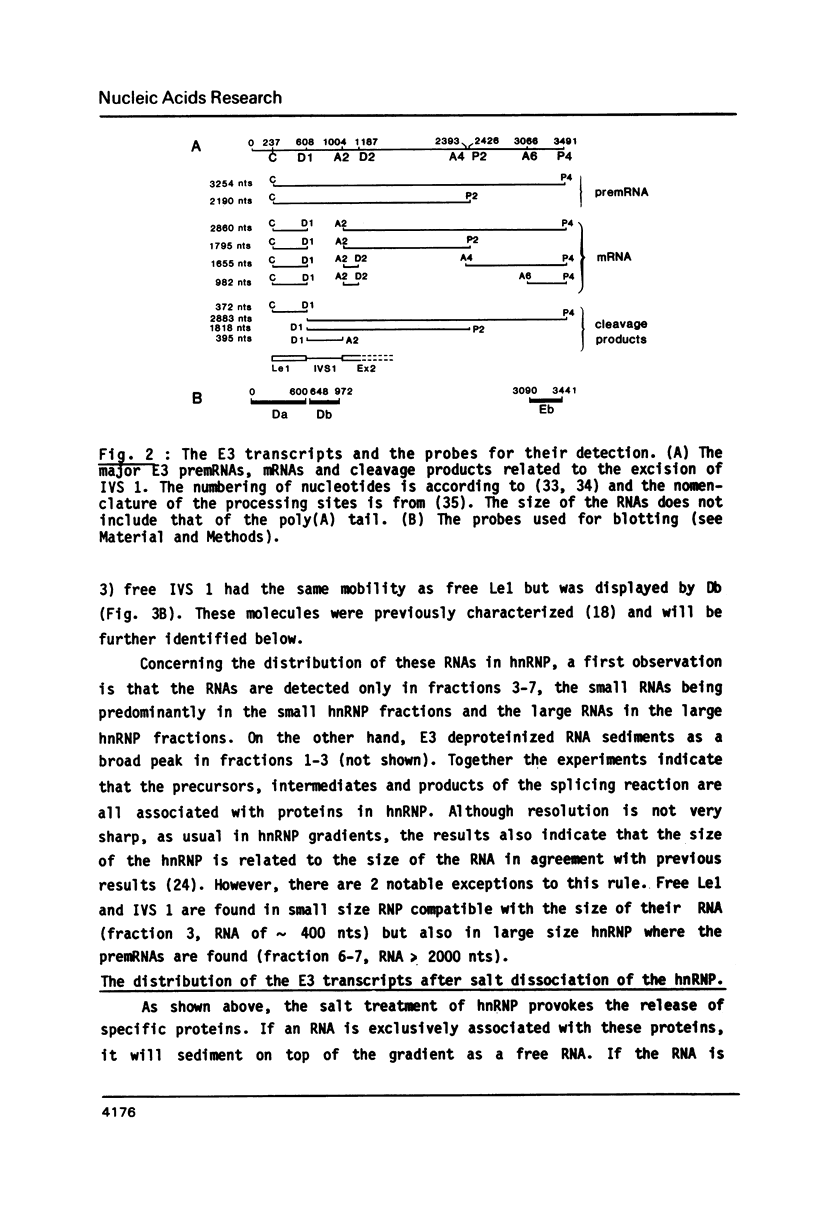
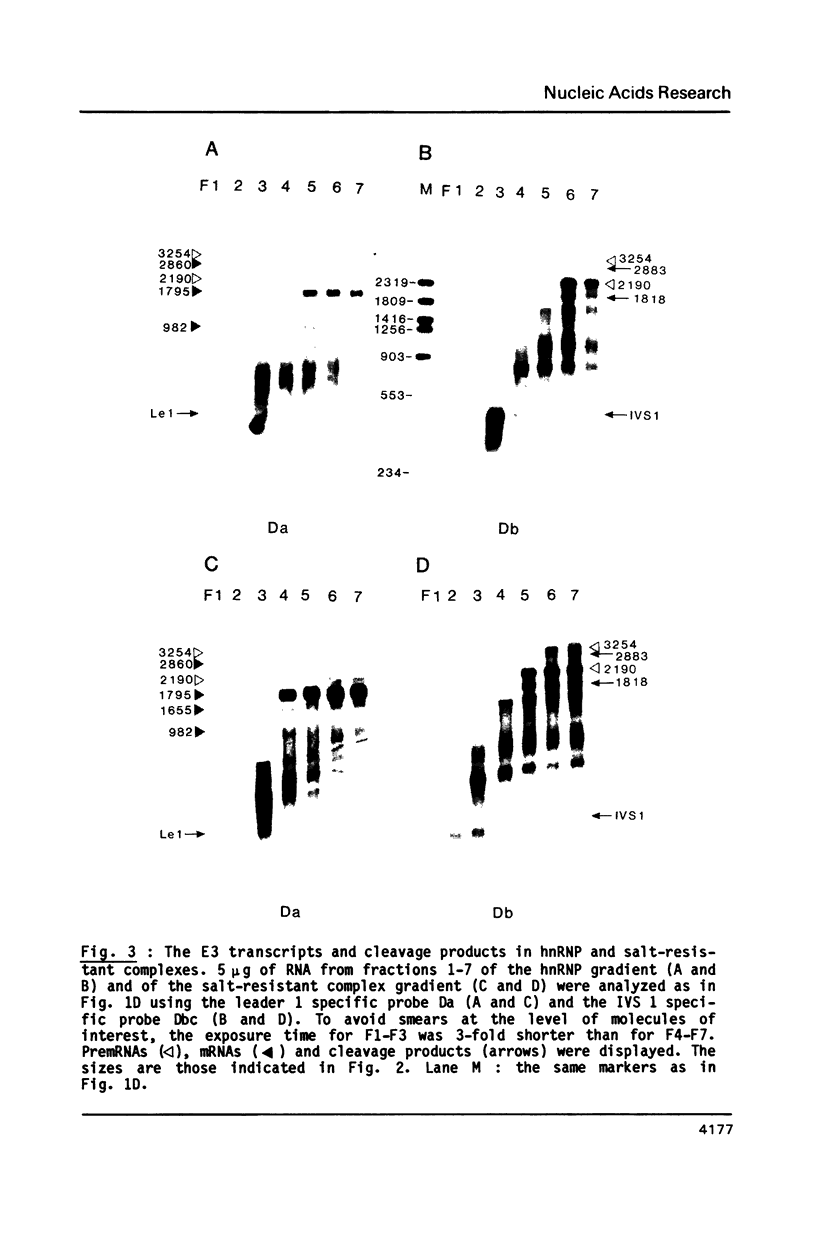
To elucidate the function of hnRNP in the splicing of premRNA, we studied the distribution of the transcripts from the early region 3 of adenovirus-2 in hnRNP from infected HeLa cells. In addition to premRNAs and mRNAs, we detected excised IVS 1 and the products of cleavage of premRNA at the 5' splice site of IVS 1 (free leader 1 and IVS 1-exon 2). All these molecules were present in hnRNP and persisted in the salt-resistant complexes after a 400 mM KCl treatment. None of them was exclusively part of the salt dissociable monoparticles. The precursors, intermediates and products of the splicing reaction were associated with both monoparticles and salt resistant complexes. This eliminates the possibility that one of the classes of RNP is exclusively involved in one of the steps of RNA processing. Whereas the size of hnRNP was related to that of its RNA for most molecules, the small molecules free leader 1 and excised IVS 1 were found in the large hnRNP containing premRNA as well as in small size hnRNP. A probable interpretation of these results is that the cleavage products are associated with the premRNP complex immediately after cleavage and are then released in the form of an individual RNP.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abulafia R., Ben-Ze'ev A., Hay N., Aloni Y. Control of late simian virus 40 transcription by the attenuation mechanism and transcriptionally active ternary complexes are associated with the nuclear matrix. J Mol Biol. 1984 Feb 5;172(4):467–487. doi: 10.1016/s0022-2836(84)80018-2. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Aloni Y. Processing of SV40 RNA is associated with the nuclear matrix and is not followed by the accumulation of low-molecular-weight RNA products. Virology. 1983 Mar;125(2):475–479. doi: 10.1016/0042-6822(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Brody E., Abelson J. The "spliceosome": yeast pre-messenger RNA associates with a 40S complex in a splicing-dependent reaction. Science. 1985 May 24;228(4702):963–967. doi: 10.1126/science.3890181. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Choi Y. D., Adam S. A. Characterization of heterogeneous nuclear RNA-protein complexes in vivo with monoclonal antibodies. Mol Cell Biol. 1984 Jun;4(6):1104–1114. doi: 10.1128/mcb.4.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Assembly of nuclear ribonucleoprotein particles during in vitro transcription. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1469–1473. doi: 10.1073/pnas.79.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendewey D., Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985 Aug;42(1):355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- Fuchs J. P., Jacob M. Fractionation of constituents of ribonucleoproteins containing heterogeneous nuclear ribonucleic acid. Biochemistry. 1979 Sep 18;18(19):4202–4208. doi: 10.1021/bi00586a026. [DOI] [PubMed] [Google Scholar]
- Gallinaro-Matringe H., Stevenin J., Jacob M. Salt dissociation of nuclear particles containing DNA-like RNA. Distribution of phosphorylated and nonphosphorylated species. Biochemistry. 1975 Jun 3;14(11):2547–2554. doi: 10.1021/bi00682a039. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. The status of small nuclear RNA in the ribonucleoprotein fibrils containing heterogeneous nuclear RNA. Biochim Biophys Acta. 1981 Jan 29;652(1):109–120. doi: 10.1016/0005-2787(81)90214-8. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Puvion E., Kister L., Jacob M. Nuclear matrix and hnRNP share a common structural constituent associated with premessenger RNA. EMBO J. 1983;2(6):953–960. doi: 10.1002/j.1460-2075.1983.tb01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski P. J., Seiler S. R., Sharp P. A. A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell. 1985 Aug;42(1):345–353. doi: 10.1016/s0092-8674(85)80130-6. [DOI] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. M., Chae C. B. Different RNA patterns of globin and non-globin 40S heterogeneous nuclear RNA-protein complexes in chicken reticulocyte nuclei. Nucleic Acids Res. 1983 Oct 25;11(20):7057–7068. doi: 10.1093/nar/11.20.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Galibert F. Nucleotide sequence of the EcoRI E fragment of adenovirus 2 genome. Nucleic Acids Res. 1981 Mar 11;9(5):1229–1240. doi: 10.1093/nar/9.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Gattoni R., LeMoullec J. M., Jacob M., Stévenin J. The orderly splicing of the first three leaders of the adenovirus-2 major late transcript. Nucleic Acids Res. 1982 Feb 25;10(4):1215–1229. doi: 10.1093/nar/10.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Gattoni R., Stévenin J. High specificity of the cDNA-RNase assay to detect accurate splicing in vitro. DNA. 1984 Aug;3(4):331–338. doi: 10.1089/dna.1.1984.3.331. [DOI] [PubMed] [Google Scholar]
- Kucherer C., Marty L., Blanchard J. M. Presence of the pre-mRNA for the 72k DNA binding protein in hnRNP from early adenovirus-2 infected HeLa cells. Biochem Biophys Res Commun. 1982 Mar 30;105(2):603–609. doi: 10.1016/0006-291x(82)91477-2. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Mariman E., Hagebols A. M., van Venrooij W. On the localization and transport of specific adenoviral mRNA-sequences in the late infected HeLa cell. Nucleic Acids Res. 1982 Oct 11;10(19):6131–6145. doi: 10.1093/nar/10.19.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K., Maxwell E. S., Puvion E., Scherrer K. The nuclear matrix of duck erythroblasts is associated with globin mRNA coding sequences but not with the major proteins of 40S nuclear RNP. Exp Cell Res. 1981 Dec;136(2):435–445. doi: 10.1016/0014-4827(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Scherrer K. Characterization of pre-messenger-RNA-containing nuclear ribonucleoprotein particles from avian erythroblasts. Eur J Biochem. 1979 Sep;99(2):225–238. doi: 10.1111/j.1432-1033.1979.tb13249.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson R. I., van Eekelen C., Philipson L. Non-random localization of ribonucleoprotein (RNP) structures within an adenovirus mRNA precursor. Nucleic Acids Res. 1982 May 25;10(10):3053–3068. doi: 10.1093/nar/10.10.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
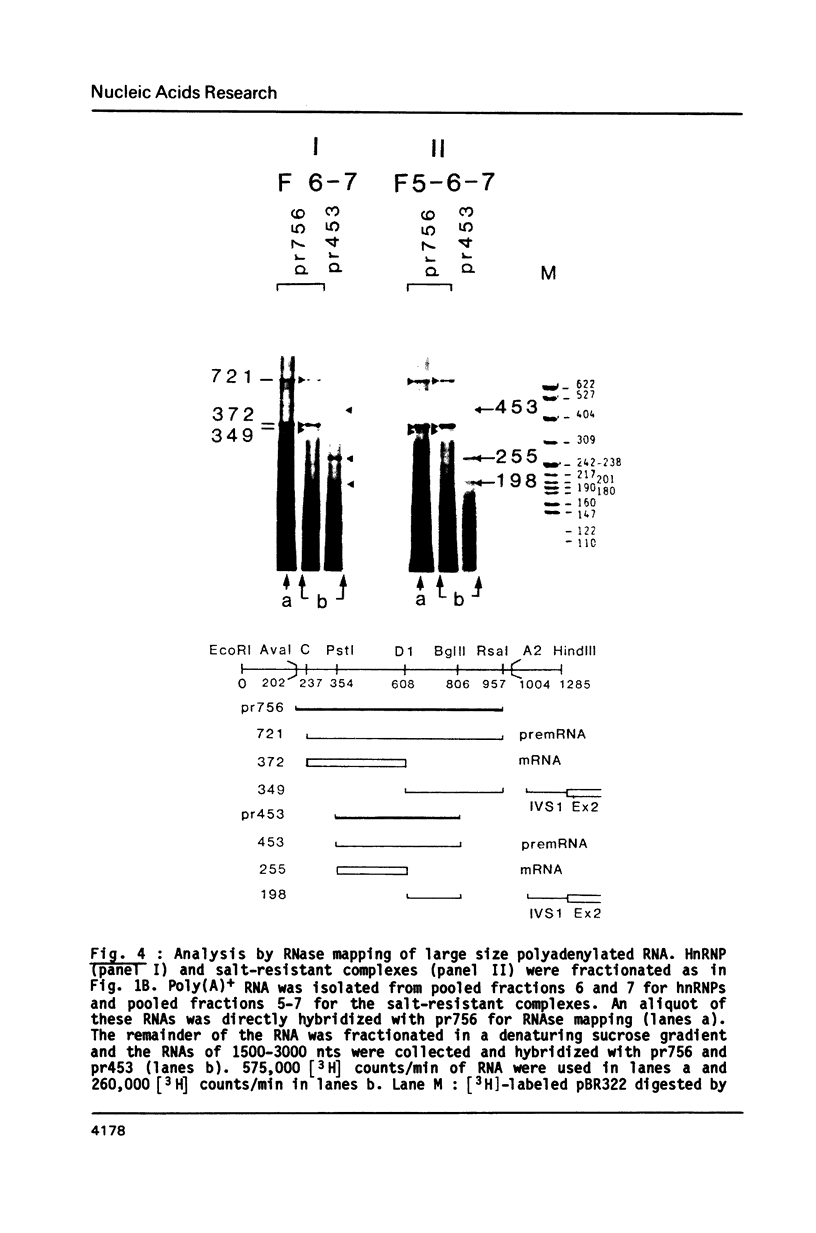
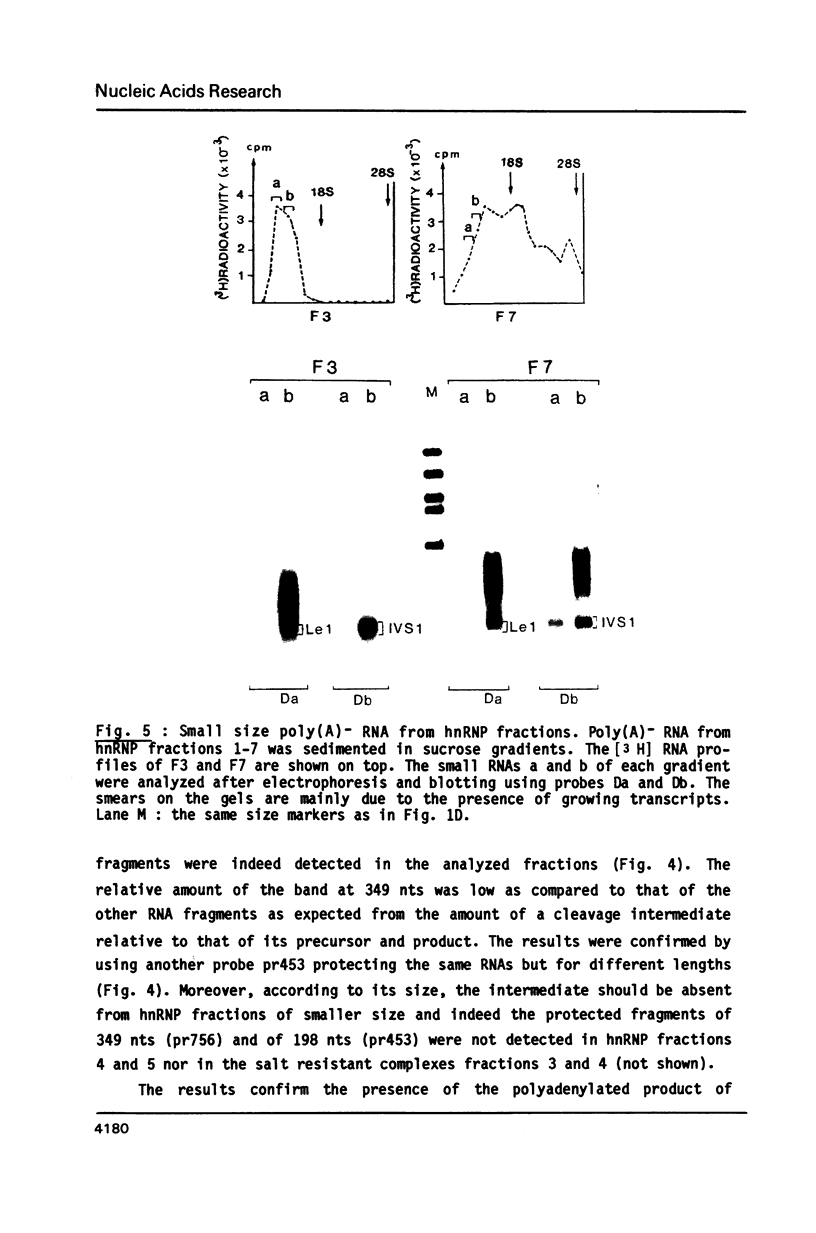
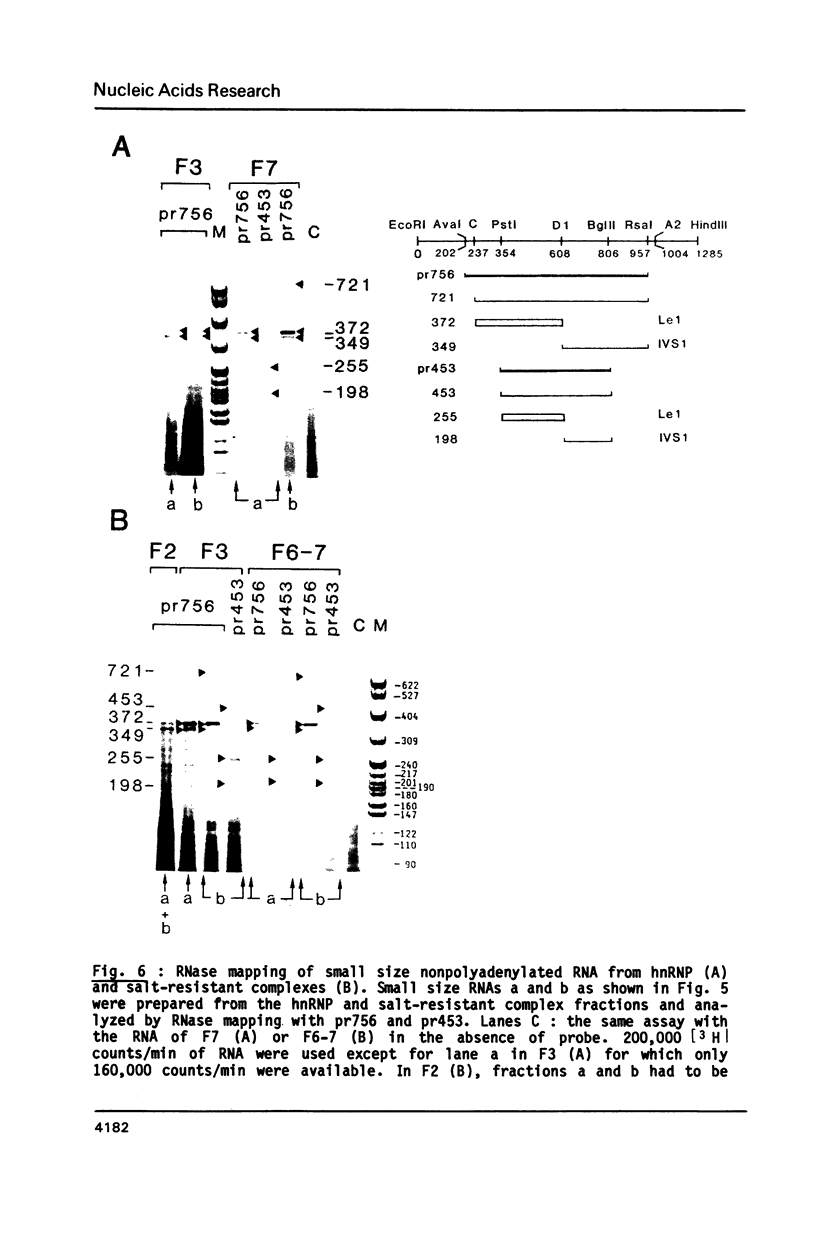
- Sittler A., Gallinaro H., Jacob M. In vivo splicing of the premRNAs from early region 3 of adenovirus-2: the products of cleavage at the 5' splice site of the common intron. Nucleic Acids Res. 1986 Feb 11;14(3):1187–1207. doi: 10.1093/nar/14.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenin J., Mandel P., Jacob M. Forme particulaire du dRNA géant dans les noyaux de cerveau de rat. Bull Soc Chim Biol (Paris) 1970 Sep 15;52(7):703–720. [PubMed] [Google Scholar]
- Stålhandske P., Persson H., Perricaudet M., Philipson L., Pettersson U. Structure of three spliced mRNAs from region E3 of adenovirus type 2. Gene. 1983 May-Jun;22(2-3):157–165. doi: 10.1016/0378-1119(83)90099-9. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Keohavong P., Jacob M. Mild nuclease treatment as a probe for a non-random distribution of adenovirus-specific RNA sequences and of cellular RNA in nuclear ribonucleoprotein fibrils. J Mol Biol. 1982 Mar 5;155(3):185–205. doi: 10.1016/0022-2836(82)90001-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Shepherd J. H., Mulvihill E. R., Palmiter R. D. Isolation of a nuclear ribonucleoprotein fraction from chick oviduct containing ovalbumin messenger RNA sequences. J Mol Biol. 1981 Aug 5;150(2):143–160. [PubMed] [Google Scholar]
- van Eekelen C., Ohlsson R., Philipson L., Mariman E., van Beek R., van Venrooij W. Sequence dependent interaction of hnRNP proteins with late adenoviral transcripts. Nucleic Acids Res. 1982 Nov 25;10(22):7115–7131. doi: 10.1093/nar/10.22.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]