Genome-wide expression profiling identifies core elements of a transcriptional network that is regulated rapidly and reciprocally by light and vegetational shade signals, via a phytochrome transcription factor transduction interface. This signaling hub functions continuously to control early seedling and juvenile plant growth and development in response to the prevailing light environment.
Abstract
Plants respond to shade-modulated light signals via phytochrome (phy)-induced adaptive changes, termed shade avoidance. To examine the roles of Phytochrome-Interacting basic helix-loop-helix Factors, PIF1, 3, 4, and 5, in relaying such signals to the transcriptional network, we compared the shade-responsive transcriptome profiles of wild-type and quadruple pif (pifq) mutants. We identify a subset of genes, enriched in transcription factor–encoding loci, that respond rapidly to shade, in a PIF-dependent manner, and contain promoter G-box motifs, known to bind PIFs. These genes are potential direct targets of phy-PIF signaling that regulate the primary downstream transcriptional circuitry. A second subset of PIF-dependent, early response genes, lacking G-box motifs, are enriched for auxin-responsive loci, and are thus potentially indirect targets of phy-PIF signaling, mediating the rapid cell expansion induced by shade. Comparing deetiolation- and shade-responsive transcriptomes identifies another subset of G-box–containing genes that reciprocally display rapid repression and induction in response to light and shade signals. These data define a core set of transcriptional and hormonal processes that appear to be dynamically poised to react rapidly to light-environment changes via perturbations in the mutually antagonistic actions of the phys and PIFs. Comparing the responsiveness of the pifq and triple pif mutants to light and shade confirms that the PIFs act with overlapping redundancy on seedling morphogenesis and transcriptional regulation but that each PIF contributes differentially to these responses.
INTRODUCTION
The phytochrome (phy) family of sensory photoreceptors (phyA to phyE in Arabidopsis thaliana) constantly monitor the environment for informational light signals and direct plant growth and developmental adaptations to the prevailing conditions throughout the life cycle (Rockwell et al., 2006; Schafer and Nagy, 2006; Quail, 2010). These phy-directed responses include the induction of seed germination, seedling deetiolation (the transition from skotomorphogenic to photomorphogenic development), shade avoidance, and floral induction (Franklin and Quail, 2010; Strasser et al., 2010). Light signal perception involves photoconversion of the phy molecule from its inactive Pr to its active Pfr conformer, which then initiates an intracellular transduction process that culminates in the altered expression of nuclear genes that control the overt photomorphogenic responses.
Current data indicate that the transduction process that initiates seedling deetiolation in dark-grown plants involves rapid translocation of the light-activated photoreceptor molecule from the cytoplasm to the nucleus (Nagatani, 2004), where it interacts physically with a subset of members of the basic helix-loop-helix (bHLH) transcription factor family, termed Phytochrome-Interacting Factors (PIFs), inducing transcriptional responses in target genes (Castillon et al., 2007; Jiao et al., 2007; Bae and Choi, 2008; Leivar and Quail, 2011). The striking constitutively photomorphogenic-like phenotype of quadruple pif1 pif3 pif4 pif5 (pifq) mutant seedlings grown in complete darkness (Leivar et al., 2008a) has provided compelling evidence that these PIF family members function collectively, in a partially redundant or overlapping fashion, to constitutively promote skotomorphogenesis (repress photomorphogenesis) in young dark-grown seedlings (Leivar et al., 2008a; Shin et al., 2009). Additional evidence shows that photoactivated phy reverses this promotion/repression, upon initial exposure to light, by inducing rapid degradation of the PIF molecules, via the ubiquitin-proteasome system (Bauer et al., 2004; Park et al., 2004; Shen et al., 2005, 2008; Oh et al., 2006; Nozue et al., 2007; Shen et al., 2007; Lorrain et al., 2008). This process involves rapid, phy-induced phosphorylation of the interacting bHLH protein in the nucleus, as a prelude to ubiquitin-proteasome system–mediated proteolysis (Al-Sady et al., 2006, 2008; Shen et al., 2007, 2008; Lorrain et al., 2008).
In fully deetiolated, light-grown seedlings, although PIF levels have been strongly decreased, they are not reduced to zero. The evidence indicates instead that a lower constant level of the protein is established under prolonged, continuous red light (Rc) irradiation or under continuous white light (WLc), such as during the daylight period for seedlings grown under day/night cycles (Monte et al., 2004; Nozue et al., 2007). A considerable number of studies have shown that monogenic and various higher order pif mutant seedlings display light-hypersensitive phenotypes (shorter hypocotyls and larger cotyledons than the wild type) at the completion of deetiolation when grown in prolonged, Rc or WLc (several days) (Huq and Quail, 2002; Kim et al., 2003; Fujimori et al., 2004; Huq et al., 2004; Monte et al., 2004; Oh et al., 2004; Khanna et al., 2007; Leivar et al., 2008b; Lorrain et al., 2008). Despite interpretive complications raised by the discovery of a mutually negative feedback loop between the PIF proteins and the phyB photoreceptor (Khanna et al., 2007; Monte et al., 2007; Al-Sady et al., 2008; Leivar et al., 2008b, 2012), these data suggest that the PIF levels present continue intrinsically to promote skotomorphogenic-like growth and development at a strongly reduced level in the light (de Lucas et al., 2008; Lorrain et al., 2008; Leivar and Quail, 2011). Return of seedlings to darkness results in reaccumulation of higher levels of PIF protein, and the rate of this reaccumulation is strongly accelerated by a short, terminal pulse of far-red light (so-called end-of-day far-red [EOD-FR] treatment) before return to darkness (Monte et al., 2004; Shen et al., 2005; Nozue et al., 2007; Leivar et al., 2008a). These data indicate that photoactivated phy continues to function in the light, and early postirradiation darkness, to sustain the repression of PIF levels and that this repression is relieved rapidly upon step function removal of Pfr by the far-red (FR) pulse and further incubation in the absence of phy photoactivation (Monte et al., 2004; Shen et al., 2005).
A qualitatively similar, but quantitatively less robust, reduction in Pfr levels than for the end-of-day FR pulse treatments is induced in green plants growing in normal white light (WL) upon exposure to the FR-enriched light generated by vegetational shade (Child and Smith, 1987; Smith and Whitelam, 1997; Franklin, 2008). Light filtered through, or reflected from, neighboring vegetation is depleted in red (R), but not FR, photons to a greater or lesser extent, depending on the density and proximity of this vegetation. This results in a quantitatively variable reduction in the ratio of R-to-FR light (variably lower R:FR ratio) compared with open sunlight. This shade signal drives the phy photoequilibrium back toward the inactive Pr conformer, thus decreasing the levels of the active Pfr conformer in the cell, despite the maintenance of sustained irradiation. Plants react to this signal with a suite of growth and developmental responses, termed the shade avoidance syndrome (SAS) (or shade avoidance response), which include accelerated extension growth rates in hypocotyls, internodes (detectable within 5 to 10 min) and petioles, retarded expansion rates in cotyledons, and retarded chloroplast development (Child and Smith, 1987; Smith and Whitelam, 1997; Franklin, 2008). Experimentally, FR-enriched light is frequently provided by FR supplementation of otherwise unchanged irradiation with WLc. This protocol selectively alters the R:FR ratio without altering the photosynthetically active radiation available to the plant. Although not directly mimicking true vegetational shade (which also reduces R levels and, thus, PAR), this strategy allows assessment of the participation of the phy system in the response, in the absence of additional effects due to reduced photosynthesis (Smith and Whitelam, 1997; Franklin, 2008) and/or blue light signaling through cryptochrome 1 (Keller et al., 2011). Here, we use the term “simulated shade” (Smith and Whitelam, 1997) to refer to such FR supplementation of WLc (also called a low R:FR ratio in the literature; Salter et al., 2003; Franklin, 2008; Lorrain et al., 2008) unless otherwise indicated.
There is evidence that PIF4 and PIF5 function in the shade-induced response. The abundance of these proteins increases rapidly in WL-grown wild-type seedlings upon exposure to simulated vegetative shade, and pif4, pif5, and pif4 pif5 double mutants exhibit a reduced acceleration of hypocotyl elongation in response to this signal compared with the wild type (Lorrain et al., 2008). Conversely, PIF4 and PIF5 overexpressors display close to constitutively long hypocotyls and petioles, with consequent reduction in residual capacity for shade-responsiveness. Together with the observation that the pif4 pif5 mutations suppress the shade avoidance-like long-hypocotyl phenotype of the phyB mutant in WLc (Lorrain et al., 2008), these data indicate that these two PIFs act intrinsically to promote the SAS in fully green plants.
Transcriptome analysis of the deetiolation process in wild-type and pifq mutant seedlings has defined the transcriptional network regulated by the PIF family (Leivar et al., 2009) and has documented the pleiotropic function of these factors in implementing phy control of target gene expression during normal light-induced seedling development (Leivar et al., 2009; Lorrain et al., 2009; Shin et al., 2009). The data show that, of the alterations in gene expression induced in dark-grown seedlings in the genetically imposed absence of PIF1, 3, 4, and 5 in the pifq mutant, the large majority of changes are normally evoked by light in the wild type (via the phy system) during the transition to the fully deetiolated state in prolonged irradiation. The broad spectrum of responsive genes identified is consistent with the multifaceted biochemical, cellular, and morphogenic changes that encompass this switch in developmental direction from heterotrophic to autotrophic growth.
Comparison of the most rapidly light-responsive genes defined in the wild type with those altered by PIF absence in the pifq mutant in the dark has identified a subset (designated Class 7) that are potential direct targets of these bHLH factors (Leivar et al., 2009). The rapidly light-repressed genes in this class are particularly enriched in a variety of transcription factor–encoding genes, suggesting that the PIFs function to directly regulate and amplify the downstream transcriptional network that executes the phy-regulated, deetiolation developmental program. Consistent with this suggestion, PIFs 1, 3, 4, and 5 have all been shown to have inherent transcriptional activation activity (Huq et al., 2004; Al-Sady et al., 2008; de Lucas et al., 2008; Shen et al., 2008; Hornitschek et al., 2009). Similarly, several studies using quantitative PCR (qPCR)-based chromatin immunoprecipitation (ChIP) assays of preselected gene promoters have provided initial evidence of in vivo binding of each of the PIFs to potential target genes in seeds or developing seedlings (Oh et al., 2007, 2009; Shin et al., 2007; de Lucas et al., 2008; Feng et al., 2008; Moon et al., 2008; Hornitschek et al., 2009; Toledo-Ortiz et al., 2010), although evidence of association with G-box or E-box motif-containing sequences was lacking in several cases.
The question of the potentially differential or overlapping contributions of the individual PIF family members to the overall molecular phenotype of the developing seedling has been addressed in a limited number of expression profiling studies with single and double pif mutants. Microarray analysis of dark-grown monogenic pif3 mutant seedlings identified only 14 genes that were differentially expressed, in statistically significant and twofold (SSTF) fashion, between the mutant and the wild type (Monte et al., 2004; Leivar et al., 2009), although the increased number of statistically differentially expressed genes defined by lowering the fold change threshold from 2 to 1.5, in a recent reanalysis of these data, revealed additional targets of PIF3 regulation (Sentandreu et al., 2011). A similar study with pif1 defined only two genes as statistically different in expression between the mutant and the wild type, and these differences were less than twofold (Moon et al., 2008). These findings are consistent with the weak or absent visible morphogenic phenotypes in these mutants in the dark (Huq et al., 2004; Leivar et al., 2008a, 2009; Shin et al., 2009; Stephenson et al., 2009; Sentandreu et al., 2011). Profiling of a pif4 pif5 double mutant grown in darkness identified 113 genes that were misregulated in the mutant compared with the wild type (Lorrain et al., 2009). The degree of overlap of these genes with those misregulated in the pifq mutant suggests that PIF4 and PIF5 together, additively or redundantly, contribute significantly to the combined regulatory activities of the PIF1, 3, 4, 5 quartet (Sentandreu et al., 2011).
Two previous studies analyzed the rapid genome-wide transcriptional responses elicited by vegetative shade in light-grown wild-type seedlings using Affymetrix ATH1 microarrays and defined a subset of genes that respond rapidly (within 1 h) to the shade signal (Sessa et al., 2005; Carabelli et al., 2007; Tao et al., 2008). In addition, an initial examination of the potential role of the PIFs in regulating these responses has been reported (Hornitschek et al., 2009). Using qPCR analysis of two selected rapidly shade-responsive genes, PHYTOCHROME INTERACTING FACTOR 3-LIKE1 (PIL1) and XYLOGLUCAN ENDOTRANSGLYCOSYLASE7 (XTR7), as markers, these authors showed that pif4, pif5, and pif4 pif5 double mutants displayed reduced shade-induced elevation of expression compared with the wild type. These results indicate that PIF4 and PIF5 act positively to promote these shade-responsive transcriptional changes. Consistent with this conclusion, ChIP analysis indicates that PIF5 binds in vivo to the G-box–containing regions of the promoters of the marker genes PIL1, XTR7, and LONG HYPOCOTYL IN FAR-RED (HFR1) (Hornitschek et al., 2009).
Here, we broadened the question of the functional roles of the PIF bHLH factors in shade avoidance to include PIF1 and PIF3, in addition to PIF4 and PIF5, by examining the shade-induced gene expression changes in the pifq mutant compared with the wild type, and expanded the expression analysis to be genome-wide using microarray profiling. In addition, we explored the question of whether the rapidly shade-induced, PIF-dependent transcriptional responses represent incipient reversal of those triggered in dark-grown seedlings at the inception of deetiolation by initial exposure to light. To begin to dissect the relative contributions of the individual PIF family members to the cumulative regulated transcription of individual target genes, we also assayed the relative expression levels of selected rapid response genes in the various triple pif mutant combinations compared with the pifq quadruple mutant.
RESULTS
Simulated Vegetative Shade Induces Rapid Increases in PIF3 Levels in Light-Grown Seedlings under Dichromatic Irradiation Conditions
Early experiments under dichromatic (Rc ± continuous far-red [ChIP]) irradiation conditions showed that Rc light–grown seedlings exposed to supplemental ChIP displayed rapid (within 5 to 10 min) and robust reaccumulation of the PIF3 protein, in the absence of changes in the corresponding transcript levels (see Supplemental Figure 1 and Supplemental Analysis 1 online). Therefore, these data indicate that PIF3 levels are regulated dynamically by photoactivated phy, in deetiolated seedlings, in response to simulated shade, at the protein rather than the gene expression level.
Multiple PIF Family Members Are Necessary for Normal Responsiveness of WL-Grown Seedlings to Simulated Vegetative Shade
Examination of WL-grown, fully green seedlings showed that they likewise reaccumulate endogenous PIF3 in response to simulated vegetative shade (WLc with supplemental ChIP) (Figure 1A). Wild-type seedlings exposed to such simulated shade conditions display the long hypocotyls and petioles characteristic of the shade avoidance response, but this response is attenuated in the pifq mutant (Figures 1B and 1C). The extent of this reduction in responsiveness is significantly, albeit moderately, greater in the pifq mutant than in the pif4 pif5 double mutant, indicating that PIF1 and/or PIF3 contribute to this response, in addition to that previously reported for PIF4 and PIF5 (Lorrain et al., 2008), but that the magnitude of this contribution is quantitatively less than that of PIF4 and PIF5.
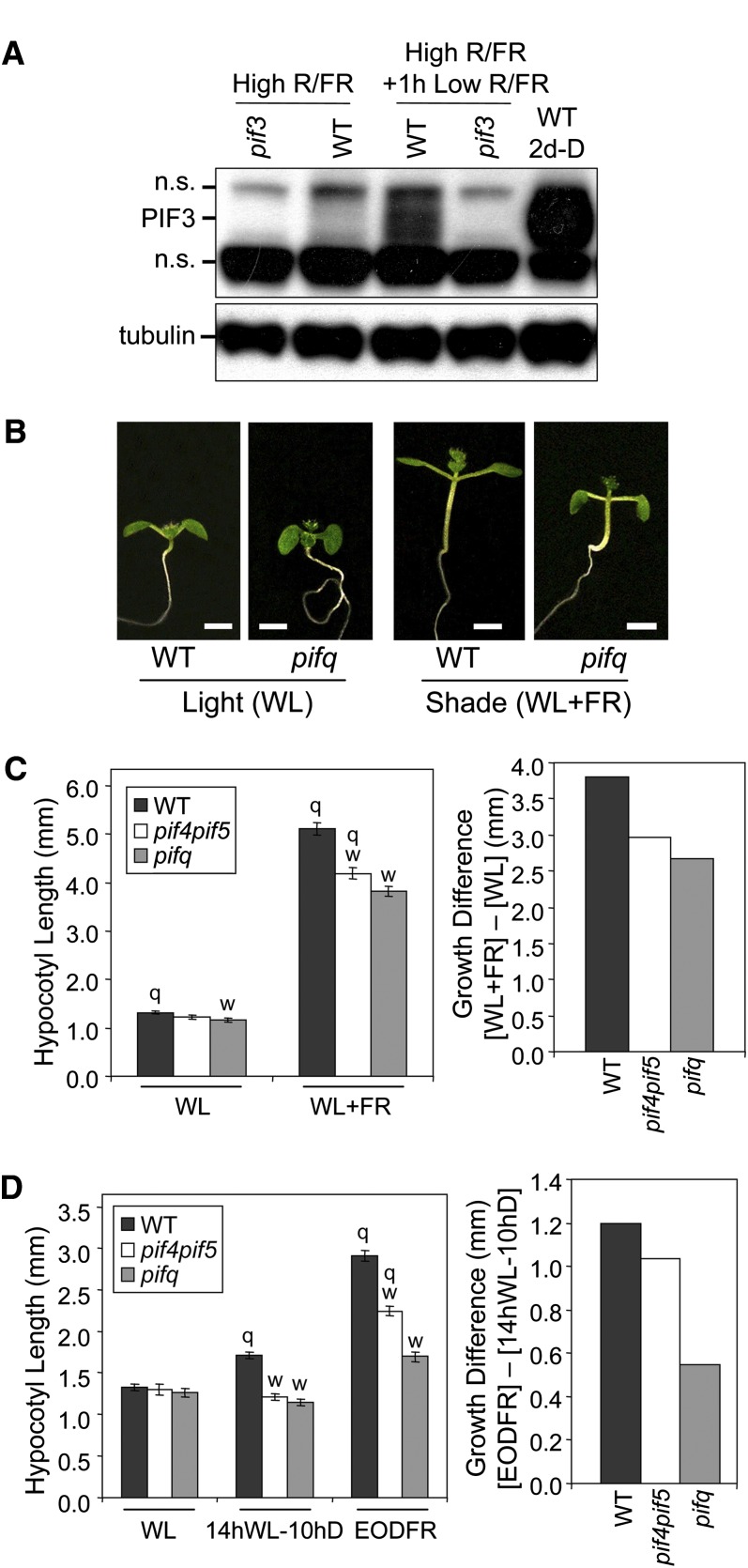
Figure 1.
Multiple PIF Family Members Are Necessary for Normal Responsiveness to Simulated Vegetative Shade in WLc-Grown Seedlings.
(A) Endogenous PIF3 levels rise in response to ChIP supplementation in WLc-grown seedlings. Two-day-old wild-type (WT) seedlings were grown in darkness (D) or WLc (20 μmol m−2 s−1) (high R:FR ratio 6.48) from germination onward, and the latter were (high R/FR + 1 h low R/FR ratio 0.006) or were not (high R/FR) exposed to supplemental ChIP for 1 h before immunoblot analysis of PIF3 protein levels. A pif3 null mutant was included as a negative control, and tubulin was used as a loading control. n.s., nonspecific.
(B) Visible phenotypes of wild-type and pifq mutant seedlings grown in WLc (20 μmol m−2 s−1) for 2 d from germination onward and then for an additional 5 d in WLc, either with (WL+FR, Low R/FR) or without (WL, High R/FR) supplemental ChIP. Bars = 2mm.
(C) Quantification of hypocotyl lengths for wild-type, pif4 pif5 double, and pifq quadruple mutant seedlings grown as in (B). Left panel: Absolute hypocotyl lengths for light (WL)- and shade (WL+FR)-grown seedlings. Right panel: Differential shade responsiveness ([WL+FR] – [WL]) of wild-type, pif4 pif5, and pifq seedlings.
(D) Quantification of hypocotyl lengths for wild-type, pif4 pif5, and pifq mutant seedlings in EOD-FR conditions. Seedlings were grown in WL (20 μmol m−2 s−1) for 2 d and then transferred for five additional days to constant WLc (WL) or to diurnal cycles of 14 h of WL followed by 10 h of darkness with (EOD-FR) or without (14 hWL-10 hD) a terminal saturating pulse of FR administered prior to the 10-h dark treatment. Left panel: Absolute hypocotyl lengths. Right panel: Differential EODFR responsiveness ([EODFR] – [14 hWL-10 hD]) of wild-type, pif4 pif5, and pifq seedlings.
(C) and (D) Left panels: Data represent mean values and se (bars) from at least 20 seedlings. Statistically significant differences from the wild type (w) and/or the pifq mutant (q) values, defined by the Student’s t test (P ≤ 0.05), are indicated for each light treatment.
[See online article for color version of this figure.]
A response pattern similar to shade avoidance is known to be observed in seedlings subjected to so-called EOD-FR treatments, where seedlings growing under diurnal light-dark cycles are administered a terminal FR light pulse at the end of each light period to remove Pfr for the duration of the subsequent dark period. Figure 1D shows that this treatment accelerates the growth rate in the wild type, as expected, but that this acceleration is progressively reduced in the pif4 pif5 double and pifq mutant seedlings. This result indicates that, in this case, PIF1 and/or PIF3 function robustly together with PIF4 and/or PIF5 to promote hypocotyl elongation during the diurnal dark period in a manner that is accelerated by the absence of Pfr (Figure 1D, right panel).
Definition of Rapid Shade-Induced Transcriptional Responses Genome-Wide
To examine the molecular phenotype of the shade avoidance response and to define genes most likely to be early, if not direct, targets of phy signaling through the PIF1, 3, 4, 5 quartet, we performed a transcriptome analysis of simulated shade–induced changes in expression in wild-type and quadruple pifq mutant seedlings. For this purpose, RNA was extracted after transfer of 2-d, WLc-grown seedlings (WL0) to supplemental ChIP light for 1 (FR1), 3 (FR3), and 24 (FR24) h or retention in WLc for an additional 24 h (WL24). The primary data and all statistical parameters generated in this analysis for all 22,000 genes on the Affymetrix ATH1 microarray have been deposited at the Gene Expression Omnibus (GSE28297) and for all genes discussed here (defined in Supplemental Figure 2 online) are presented in Supplemental Data Set 1. This analysis identified a total of 169 rapidly shade-responsive genes that were induced (131 genes; see Supplemental Data Set 2 online) or repressed (38 genes; see Supplemental Data Set 3 online) within 1 h of ChIP supplementation (FR1) in the wild-type in an SSTF manner compared with the WLc-grown control before ChIP treatment (WL0) (SSTF@FR1 genes; see Supplemental Figure 2 online). A comparison of these data with those of two formally similar, but experimentally different, earlier studies (Carabelli et al., 2007; Tao et al., 2008) is presented in Supplemental Analysis 2, Supplemental Figure 3, and Supplemental Data Sets 2 to 8 online together with analysis of the shade-induced expression changes at FR3 in wild-type seedlings (SSTF@FR3; see Supplemental Figure 2 online).
Identification of PIF-Dependent Rapidly Shade-Responsive Genes
To assess the potential role of the PIF proteins in the rapid shade-induced expression changes observed in this study, we identified those genes that exhibited statistically significant differences in transcript abundance between wild-type and pifq seedlings following 1 h of ChIP supplementation (PIF-dependent genes in Supplemental Figures 2 and 4 online). The procedure used in this analysis (detailed in Supplemental Analysis 3, Supplemental Figures 2 to 5, and Supplemental Data Sets 9 to 17 online) defined a total of seven classes of genes (A to G), of which Classes A and B combined (Classes A+B) comprised those designated as displaying rapid (at FR1), statistically significant, PIF-dependent shade responsiveness (see Supplemental Figure 4 online). A total of 123 such Class A+B genes (103 induced, Supplemental Data Set 9 online; 20 repressed, Supplemental Data Set 10 online) were identified. This genome-wide pattern of rapidly shade-responsive gene expression provides strong evidence of the involvement of the PIF quartet in early shade signaling.
Functional Dichotomy between Genes with and without Promoter-Located G-Box Motifs
The magnitude of the contribution of the four PIFs to the shade responsiveness of each shade-induced gene was defined as the fold induction ratio (FIR) (Monte et al., 2004; Tepperman et al., 2004; see Methods). This parameter quantitatively measures the magnitude of the change in expression induced by the simulated shade treatment at FR1 in the wild type (wild-type fold induction [FI]) compared with the pifq mutant (pifq FI), expressed as a ratio (wild-type FI/pifq FI). A ratio of 1.0 signifies no detectable contribution of PIF1, 3, 4, and 5 to the shade-induced response, independently of whether the underlying response to shade is intrinsically large or small for that gene. Deviations from 1.0 provide a quantitative measure of the robustness of the PIF quartet contribution to the shade response.
The 103 shade-induced PIF-dependent genes at FR1 of Classes A and B (see Supplemental Figure 4B and Supplemental Data Set 9 online) were arrayed in rank order of FIR value at 1 h of supplemental ChIP and further divided into subsets according to the range of those values, as depicted in Figure 2A. Genes at the left extreme of this array are those exhibiting the strongest dependence on the four PIFs for ChIP-induced expression, whereas those toward the right exhibit the least PIF quartet dependence. We define those genes with FIR values >1.5 as being moderately to robustly dependent on the four PIFs for shade responsiveness (Table 1) and those with FIR values <1.5 as being marginally to minimally dependent on these PIFs for shade responsiveness (see Supplemental Data Set 18 online). Several genes previously well documented as rapidly shade-induced marker genes, including ARABIDOPSIS THALIANA HOMEOBOX PROTEIN2 (ATHB2), XTR7, HFR1, PHY RAPIDLY REGULATED1 (PAR1), and INDOLE-3-ACETIC ACID INDUCIBLE29 (IAA29) (Roig-Villanova et al., 2006, 2007; Carabelli et al., 2007; Lorrain et al., 2008), are identified here as being robustly dependent on the PIF quartet for this rapid shade responsiveness (Table 1; see Supplemental Data Set 18 online).
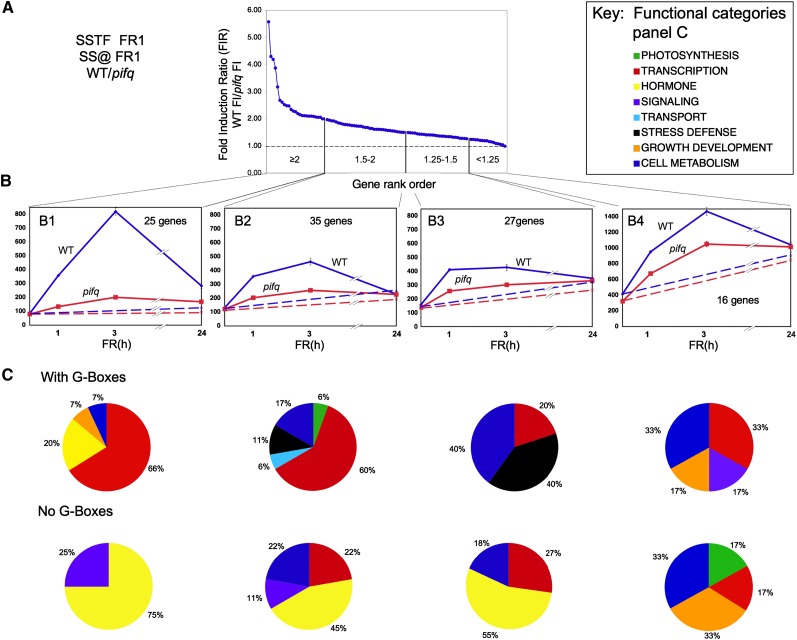
Figure 2.
Transcription Factor–Encoding and Auxin-Responsive Genes Dominate the Rapid, PIF-Dependent, Shade-Induced Gene Set.
(A) Shade-induced genes arrayed in rank order of relative responsiveness to 1 h of ChIP supplementation in the wild type (WT) compared with pifq mutant seedlings. The curve depicts the distribution of the 103 genes defined statistically both as SSTF induced by 1 h of ChIP in the wild type and as significantly dependent on the PIF quartet for that ChIP responsiveness, arrayed in order of descending FIR of the wild type/pifq. Vertical lines divide the array into bins ([B1] to [B4]) according to FIR value (range shown within each bin).
(B) Time course of mean expression of all genes in each bin over the 24-h shade exposure period. Blue, the wild type; red, pifq. Solid lines, shade exposed (WLc+ChIP); dashed lines, not shade exposed (i.e., retained in WLc). Numbers of genes in each bin are indicated. Error bars indicate se.
(C) Distribution of rapidly shade-induced genes among functional categories, expressed as a percentage of the total annotated genes within each bin, after division into groups with or without G-box motifs in their promoters. Functional category color code is shown in (A), right. Genes with unknown function were eliminated from the analysis.
Table 1.
Genes Displaying Rapid Responsiveness to Shade in a Robustly to Moderately PIF-Dependent Manner (Classes A and B combined [see Supplemental Figure 4B online] with a FIR >1.5; Figure 2)
| Locus | TAIR Annotation | Wild-Type FIa | pifq FIb | FR1 FIRc |
| Genes with G-Boxes in Promoter (44 Genes) | ||||
| Transcription | ||||
| AT4G32280 | IAA29 | 25.72 | 10.20 | 2.52 |
| AT4G16780 | ATHB-2 | 13.34 | 3.10 | 4.30 |
| AT1G02340 | HFR1 | 8.47 | 1.52 | 5.58 |
| AT2G42870 | PAR1 | 5.98 | 2.29 | 2.61 |
| AT3G15540 | IAA19 | 5.37 | 2.56 | 2.10 |
| AT5G39860 | PRE1 | 4.09 | 2.30 | 1.78 |
| AT4G01250 | WRKY22 | 3.84 | 2.21 | 1.73 |
| AT3G23030 | IAA2 | 3.71 | 2.37 | 1.57 |
| AT1G69570 | Dof-type Zn finger | 3.34 | 1.52 | 2.19 |
| AT1G18400 | BEE1 | 2.74 | 1.71 | 1.60 |
| AT5G15160 | bHLH 134 | 2.57 | 1.21 | 2.12 |
| AT1G14920 | GAI | 2.45 | 1.45 | 1.68 |
| AT1G01260 | bHLH 13 | 2.36 | 1.03 | 2.28 |
| AT1G69690 | TCP15 | 2.33 | 1.45 | 1.61 |
| AT3G57800 | bHLH 60 | 2.32 | 1.30 | 1.79 |
| AT5G43700 | IAA4 | 2.20 | 1.44 | 1.52 |
| AT5G28300 | Trihelix DNA binding protein | 2.15 | 1.03 | 2.09 |
| AT2G46270 | GBF3 | 2.14 | 1.27 | 1.68 |
| AT5G53980 | ATHB52 | 2.05 | 1.27 | 1.61 |
| AT1G69010 | BIM2 | 2.04 | 0.99 | 2.07 |
| AT4G30410 | Transcription factor | 2.03 | 1.18 | 1.73 |
| Hormone | ||||
| AT3G03830 | SAUR28 | 7.83 | 3.16 | 2.48 |
| AT4G13790 | SAUR25 | 5.79 | 1.38 | 4.19 |
| AT1G75450 | CKX5 | 2.68 | 1.08 | 2.48 |
| AT3G59900 | ARGOS | 2.30 | 1.22 | 1.88 |
| Cellular metabolism | ||||
| AT5G54490 | PBP1 | 3.06 | 1.77 | 1.73 |
| AT4G25260 | Invertase/pectin methylesterase inhibitor | 2.42 | 1.18 | 2.05 |
| AT1G04180 | FMO family protein | 2.37 | 1.53 | 1.55 |
| AT3G54510 | ERD protein-related | 2.27 | 1.18 | 1.92 |
| Growth/development | ||||
| AT4G14130 | XTR7 | 3.59 | 1.59 | 2.26 |
| Photosynthesis/chloroplast | ||||
| AT5G02020 | SISR | 2.14 | 1.42 | 1.50 |
| Stress/defense | ||||
| AT4G28240 | Wound-responsive protein | 2.25 | 1.43 | 1.57 |
| AT5G63650 | SNRK2.5 | 2.07 | 1.18 | 1.75 |
| Transport | ||||
| AT5G66110 | Metal ion binding | 2.08 | 1.15 | 1.80 |
| Unknown | ||||
| AT5G12050 | Unknown protein | 9.25 | 5.01 | 1.85 |
| AT3G29370 | Unknown protein | 5.48 | 2.55 | 2.15 |
| AT5G02580 | Unknown protein | 3.65 | 1.15 | 3.17 |
| AT5G50335 | Unknown protein | 3.25 | 1.55 | 2.10 |
| AT3G55840 | Unknown protein | 3.15 | 1.72 | 1.83 |
| AT5G66590 | Unknown protein | 2.65 | 1.38 | 1.93 |
| AT1G21050 | Unknown protein | 2.60 | 1.33 | 1.95 |
| AT3G29575 | Unknown protein | 2.31 | 1.09 | 2.12 |
| AT5G57760 | Unknown protein | 2.31 | 1.12 | 2.05 |
| AT3G50900 | Unknown protein | 2.05 | 1.27 | 1.61 |
| Genes with No G-Boxes in Promoter (16 Genes) | ||||
| Transcription | ||||
| AT5G44260 | Zn finger (CCCH-type) | 2.60 | 1.72 | 1.51 |
| AT3G60390 | HAT3 | 2.41 | 1.47 | 1.65 |
| Hormone | ||||
| AT5G18060 | SAUR23 | 11.26 | 4.20 | 2.68 |
| AT1G29490 | SAUR68 | 6.92 | 1.79 | 3.87 |
| AT1G29440 | SAUR63 | 4.74 | 2.41 | 1.97 |
| AT4G38850 | SAUR15 | 4.20 | 2.10 | 2.00 |
| AT1G29460 | SAUR65 | 3.65 | 2.09 | 1.75 |
| AT2G21200 | SAUR7 | 3.24 | 2.04 | 1.58 |
| AT1G02400 | GA2OX6 | 2.43 | 1.43 | 1.70 |
| Cellular metabolism | ||||
| AT5G02540 | SDR | 4.40 | 2.49 | 1.77 |
| AT4G27280 | Calcium binding EF hand | 3.33 | 2.22 | 1.50 |
| Signaling | ||||
| AT5G14470 | GHMP kinase-related | 3.23 | 2.08 | 1.55 |
| AT3G16800 | PP2C | 2.32 | 0.99 | 2.34 |
| Unknown | ||||
| AT2G28400 | Unknown protein | 2.24 | 1.37 | 1.64 |
| AT1G36940 | Unknown protein | 2.16 | 1.34 | 1.61 |
| AT4G35720 | Unknown protein | 2.05 | 0.97 | 2.10 |
Genes in boldface type were previously identified as shade responsive (Carabelli et al., 2007; Tao et al., 2008). TAIR, The Arabidopsis Information Resource.
FI in the wild type after exposure to 1 h of simulated shade signal.
FI in pifq after exposure to 1 h of simulated shade signal.
The time-course curves for the mean expression of the four FIR subsets of genes graphically depict the gradation in dependence on the PIF quartet for rapid responsiveness to shade (Figure 2B). The data show the very weak early shade responsiveness in the pifq mutant of the 25 genes with a FIR >2 at 1 h of ChIP compared with the wild type (Figure 2B1), and the declining differential in wild-type and pifq expression levels in shade-treated seedlings across the other gene subsets (Figure 2B, panels left [B1] to right [B4]). It is also notable that the percentage of genes that contain G-box motifs in their promoters within these subsets declines from left to right as follows: FIR >2, 80%; FIR 1.5 to 2, 69%; FIR 1.25 to 1.5, 41%; FIR 1.0 to 1.25, 50% (Figure 2B).
These PIF-dependent genes within each FIR subset were divided into G-box–containing and non-G-box–containing groups and assigned to functional categories. The percentage of the annotated genes falling into each category is displayed in Figure 2C. It is notable that the G-box–containing genes (Figure 2C, top) are enriched overall for genes encoding putative or established transcription-related factors (52%), compared with the non-G-box–containing group (Figure 2C, bottom) (24%). However, most strikingly, the G-box–containing, high-FIR genes are particularly enriched in such transcription factor–encoding genes: FIR >2 group, 67%, and FIR 1.5 to 2 group, 61% (Figure 2C, two top left panels) (Table 1; see Supplemental Data Set 18 online). Conversely, the non-G-box–containing group is preferentially enriched in hormone-related genes (38%) compared with the G-box–containing set (10%). Again, this enrichment is prominent for the higher FIR genes in the non-G-box–containing gene-set: FIR >2, 67%; FIR 1.5 to 2, 50%; and FIR 1.25 to 1.5, 55% compared with FIR 1 to 1.25, 0%.
Also very striking, auxin-responsive genes (including SMALL AUXIN UP RNA (SAUR) 1, 7, 9, 15, 23, 25, 28, 62, 63, 64, 65, 66, 67, and 68; Table 1; see Supplemental Data Set 18 online) dominate this hormone category, making up 88% of the total number of genes overall in this category and 92% of the non-G-box–containing subset. Because as noted above, the majority of hormone-related, shade-induced genes are non-G-box containing, it follows that the majority of these auxin-related genes are non-G-box containing. Furthermore, in our functional classification scheme, we chose to classify the AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) genes as transcription related, given the known function of their encoded proteins in regulating other genes in response to auxin. Five of these genes, IAA2, IAA3, IAA4, IAA19, and IAA29, are identified here as being G-box–containing, shade-induced genes, with four of the five displaying moderate to robust dependence on the PIF quartet for induction (Table 1; see Supplemental Data Set 18 online). This observation both enhances the apparent strong involvement of auxin in early phy-PIF–induced shade responses and suggests a possible dichotomy within the auxin-related gene set, whereby the regulatory AUX/IAA genes provide potential direct targets for the PIFs through their G-box motifs, and the SAUR/auxin-responsive group may be indirect targets, given that all but two lack G-box motifs in their promoters. It is also notable that an additional subset of seven rapidly shade-induced genes (albeit in a non-PIF–dependent manner) (Classes D and E in Supplemental Figure 4B online) are auxin related (IAA1, IAA5, IAA34, SAUR10 [At2G18010], SAUR46 [At2G37030], GH3.3, and GH3.4) (see Supplemental Data Set 19 online). These represent 41% of the total number of genes in this category.
Collectively, these data identify a subset of transcription factor–encoding genes as strong candidate direct targets of rapid phy-PIF–mediated shade signaling, including several AUX/IAA genes involved in transcriptional regulation in the auxin response pathway, and document similarly rapid, but perhaps indirect, induction of a suite of auxin-responsive genes (Table 1). These results suggest that the phy-PIF pathway may act directly to induce or modulate auxin signaling as a major conduit by which rapid growth responses to shade (known to occur within minutes) are triggered.
Rapidly Shade-Elicited Transcriptional Responses Represent Selective Incipient Reversal of Rapidly Light-Triggered Transcriptional Responses Initiated during Deetiolation
To examine whether and to what extent the rapid, PIF-regulated changes in gene expression evoked by shade represent incipient reversal of the initial changes triggered upon first exposure of dark-grown seedlings to light at the onset of deetiolation, we performed a meta-analysis that merged our present shade microarray data with those of our previous deetiolation microarray study (Leivar et al., 2009). The data for this analysis are in Supplemental Data Set 20 online. This analysis provides a direct comparison between the genome-wide changes triggered by Rc light at the initiation of the deetiolation process and those induced by ChIP light enrichment at the initiation of the shade avoidance process. Here, we focused on the rapidly (1 h) shade-induced genes.
Overall, 64% of early (1 h ChIP), shade-induced genes (131 genes of the shade response Classes A, B, D, and E in Supplemental Figure 2B online) overlap with genes that are repressed by light exposure during deetiolation. Conversely, genes displaying rapid (within 1 h) repression in response to Rc light during deetiolation (deetiolation Classes 3, 6, and 7; Leivar et al., 2009) are strongly enriched in early shade-induced genes compared with classes where light-imposed repression was slow or absent during deetiolation (deetiolation Classes 1, 2, and 4; see Supplemental Figure 6 online). The data for the individual deetiolation class genes are summarized in Figure 3 and Supplemental Figures 7 to 10 online (see Supplemental Data Sets 21 to 35 online for associated gene lists). Section A of these figures contains a Venn diagram showing the extent of overlap between the dark-to-light repressed genes (deetiolation) and the 103 rapid, PIF-dependent light-to-shade induced genes at FR1 (Classes A and B; see Supplemental Figure 4B online) for each deetiolation gene class defined by Leivar et al. (2009). Section B displays the time courses of the mean expression level of all genes within each Venn sector, after dividing the genes between those with (three top panels) and without (three bottom panels) G-box motifs in their promoters. Within each of the six panels in Section B, the curves in the left subpanel show the light-repressed gene time courses (dark-to-light) derived from the data of Leivar et al. (2009), and those in the right subpanel (light-to-shade) show the shade-induced time courses derived from this study. Section C shows the functional category distribution of the genes within each respective panel in Section B of the figure.
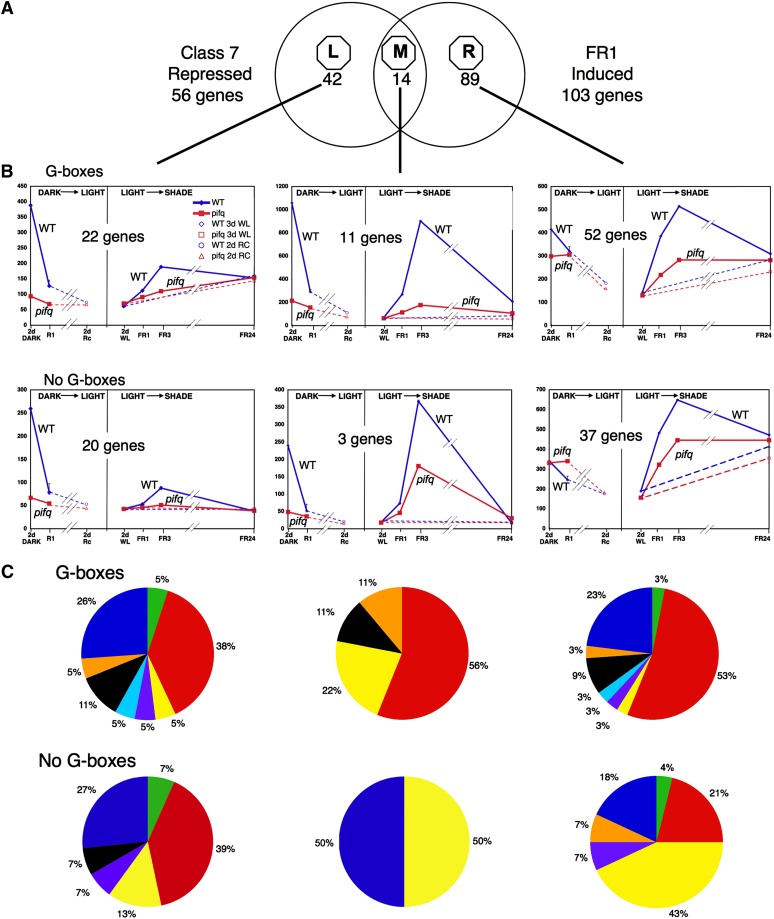
Figure 3.
Merging Light-Repressed and Shade-Induced Transcriptome Data Identifies Genes Responding Rapidly and Reciprocally to Light and Shade Signals.
(A) Venn diagram depicting the overlap between the rapidly (R1 h) dark-to-light–repressed PIF-dependent (deetiolation Class 7) genes (Leivar et al., 2009) and the rapidly light-to-shade–induced PIF-dependent (FR1, Class A+B; see Supplemental Figure 4B online) genes (this study). Sectors are designated as left (L), middle (M), and right (R), and the number of genes in each is indicated.
(B) Time courses of the mean expression levels of all genes within each Venn sector, after dividing the genes between those with (top graphs) and without (bottom graphs), G-box motifs in their promoters. Curves in the left subpanel of each graph box show the light-repressed gene time courses (from Leivar et al., 2009), and those in the right subpanel the rapidly shade-induced genes identified here. Blue, the wild type; red, pifq. Solid lines: Light (Rc)- or shade (WLc+ChIP)-exposed. Left subpanels: Break indicates noncontinuity of time course between 1 h R (R1) and 2 d R (Rc); right subpanels, dashed lines, not shade-exposed (i.e., retained in WLc). Error bars indicate se. WT, the wild type.
(C) Functional category distribution of the genes within each respective graph box in (B). Color code as in Figure 2.
We focus the initial analysis here on the Class 7 rapidly light-repressed (deetiolation) genes, as these appear to have an expression pattern in dark-grown and deetiolating seedlings that is consistent with being direct targets of phy-induced, PIF-mediated regulation (Figure 3). The middle Venn diagram sector (designated Meta-analysis Class M) represents those genes that display robust, rapid, reciprocal responsiveness to the onset of the light (Rc) and shade (supplemental ChIP) signals, respectively (Figure 3A, Table 2; see Supplemental Data Set 21 online). This is visually apparent from direct comparison of the respective wild-type curves (Figure 3B, center panels, Class M). The data also show the robust dependence on the PIF proteins for the elevated levels of expression of these genes both in the dark and the shade. The left-hand Venn sector (designated Meta-analysis Class L) comprises genes that display robust, rapid repression of expression in the wild type by the initial light signal but subsequently markedly weaker and slower induction in the shade (Figure 3B, left panels) (see Supplemental Data Set 22 online). However, this minimal shade response does retain apparently substantial PIF dependence, most obviously at the FR3 time point. The right-hand Venn sector (designated Meta-analysis Class R) represents genes that lack robust, rapid light-imposed repression but display rapid and robust PIF-dependent shade induction (Figure 3B, right panels) (see Supplemental Data Set 23 online). The prominence of the transcription factor genes across both the light-repressed and shade-induced subsets is reinforced by this analysis (Figure 3C).
Table 2.
M Class Genes Displaying Rapid, Robust, Reciprocal Responsiveness to Both Light and Simulated Shade Signals (Figure 3)
| Locus | TAIR Annotation | Light Repression in the Wild Typea | Shade Induction in the Wild Typeb | FR1 FIRc |
| Genes with G-Boxes in Promoter (11 Genes) | ||||
| Transcription | ||||
| AT1G69690 | TCP15 | 0.45 | 2.33 | 1.61 |
| AT3G15540 | IAA19 | 0.36 | 5.37 | 2.10 |
| AT4G16780 | ATHB-2 | 0.11 | 13.34 | 4.30 |
| AT4G32280 | IAA29 | 0.11 | 25.72 | 2.52 |
| AT5G53980 | ATHB52 | 0.10 | 2.05 | 1.61 |
| Hormone | ||||
| AT1G75450 | CKX5 | 0.34 | 2.68 | 2.48 |
| AT4G13790 | SAUR25 | 0.24 | 5.79 | 4.19 |
| Growth/development | ||||
| AT4G14130 | XTR7 | 0.37 | 3.59 | 2.26 |
| Stress/defense | ||||
| AT5G63650 | SNRK2.5 | 0.29 | 2.07 | 1.75 |
| Unknown | ||||
| AT5G02580 | Unknown | 0.13 | 3.65 | 3.17 |
| AT5G57760 | Unknown | 0.14 | 2.31 | 2.05 |
| Genes with No G-Boxes in Promoter (Three Genes) | ||||
| Hormone | ||||
| AT4G36110 | SAUR9 | 0.24 | 5.16 | 1.37 |
| Cellular Metabolism | ||||
| AT5G02540 | SDR | 0.20 | 4.40 | 1.77 |
| Unknown | ||||
| AT4G35720 | Unknown | 0.16 | 2.05 | 2.10 |
Genes in boldface type were previously identified as shade responsive (Carabelli et al., 2007). TAIR, The Arabidopsis Information Resource.
Mean fold repression of expression in wild-type Arabidopsis seedlings exposed to 1 h Rc (R1) over the level in dark control seedlings (D1).
Mean fold induction of expression in wild-type Arabidopsis seedlings exposed to 1 h of simulated shade (WL+ChIP) over the level in WL control seedlings (WL0).
FIR of shade induction in wild-type versus pifq seedlings after 1 h of simulated shade (FI/WT)/(FI/pifq).
Overall, the data reveal dichotomies in the pattern of regulation within each of the PIF-dependent, rapidly light-repressed (Class 7; Leivar et al., 2009) and rapidly shade-induced (Classes A and B; see Supplemental Figure 4B online) gene sets. The subset common to both gene sets (Figure 3A, Class M), exhibiting dual, reciprocal light shade responsiveness, suggests that they are targets of reversible regulation by PIF-mediated phy signaling. The G-box–containing group within this subset appears to be the most likely to be direct targets of this signaling pathway (Table 2; see Supplemental Data Set 21 online). However, a larger subset of rapidly light-repressed genes does not exhibit rapid reciprocal PIF-dependent induction by shade (Figure 3, Class L), suggesting perhaps either an intrinsically low transcription rate coupled with a high turnover rate or a unidirectional change in the mode of transcriptional regulation of these genes during deetiolation (see Supplemental Data Set 22 online). Conversely, a large subset of rapidly shade-induced genes does not exhibit rapid reciprocal repression upon the initial dark-to-light transition of dark-grown seedlings (Figure 3, Class R). These genes thus appear to have newly acquired the capacity to respond to phy-PIF signaling during or after deetiolation (see Supplemental Data Set 23 online).
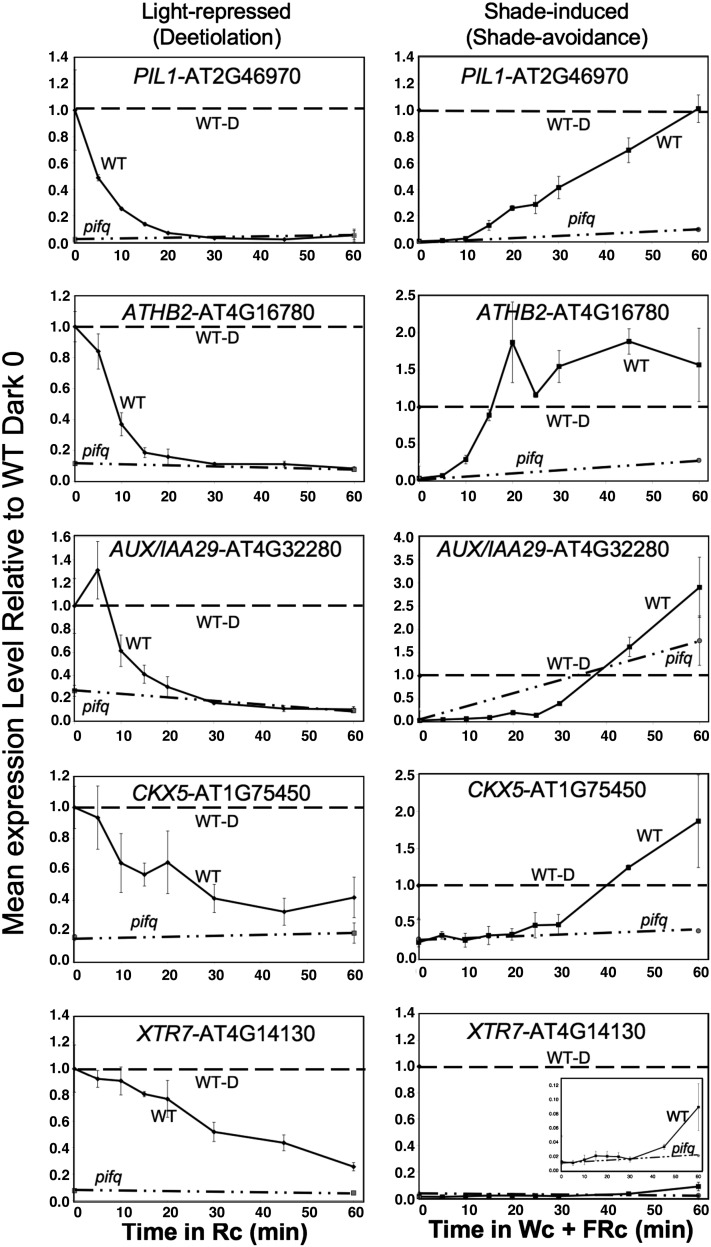
To examine the speed of the transcriptional responses elicited by the light and shade signals, selected genes displaying reciprocal responsiveness (Class M in Figure 3) were subjected to more detailed time-course analysis by qPCR over the first 60 min of exposure to each signal. The data are compared directly for each gene in Figure 4 and Supplemental Figure 11 online, normalized to the level of expression in 2-d dark-grown seedlings grown in parallel in each case. The data for the shade-induced expression increases were obtained in this study, whereas those for the light-imposed expression decreases were obtained in our previous study (Leivar et al., 2009) or in additional unpublished experiments. In addition to validating the microarray results (see Supplemental Figure 5 online), these data identify two subclasses of early-response genes. One subclass, which includes PIL1, ATHB2, IAA29, and CYTOKININ OXIDASE5 (CKX5), displays rapid and robust, PIF-dependent shade induction to levels equivalent to, or exceeding, those of dark-grown seedlings within this 60-min period (Figure 4). This subclass comprises candidates for being direct targets of phy-PIF signaling and having key roles in central functions that initiate switching between skotomorphogenic and photomorphogenic developmental pathways. The other subclass, which includes XTR7, SAUR25, and AT5G02580, although displaying the twofold, statistically significant, PIF-dependent shade induction that defines this class (see insets), exhibits quantitatively less robust recovery in expression levels over the first 1 h of shade exposure compared with the levels originally present in dark-grown seedlings (Figure 4; see Supplemental Figure 11 online).
Figure 4.
Selected G-Box–Containing, Class M Genes Display Rapid, Robust, PIF-Dependent, Reciprocal Transcriptional Responses to Light and Shade Signals.
Wild-type (WT) (solid curves) and pifq mutant (dot-dashed curves) seedlings were grown either for 2 d in the dark and then exposed to Rc (left panels) or for 2 d in WLc and then exposed to WLc+ChIP (simulated shade) (right panels) for increasing periods from 0 to 60 min. Expression of the indicated genes was determined by qPCR, and PROTEIN PHOSPHATASE2A (PP2A) was used as a normalization control. Data are presented relative to the mean of 2-d dark-grown control seedlings (WT-D) in each case set at unity (horizontal dashed curve) and represent the mean and se of three independent biological replicates. Deetiolation data for PIL1, ATHB2, and IAA29 are from Leivar et al. (2009); the remainder is from this study.
Morphogenic and Expression Analysis of pif Mutant Combinations Reveals Differential Contributions of Individual PIFs to Skotomorphogenesis and Shade Avoidance
Given the evidence that PIF1, PIF3, PIF4, and PIF5 (the PIF quartet) appear to contribute collectively to the maintenance of the etiolated state in dark-grown seedlings (Leivar et al., 2008a; Shin et al., 2009), that PIF1 appears to dominate the regulation of seed germination (Oh et al., 2004), and that PIF4 dominates the hypocotyl elongation induced by warm temperature (Koini et al., 2009; Stavang et al., 2009), we wished to assess the individual contributions of these PIFs to the skotomorphogenic and shade avoidance morphological and molecular responses. To initiate this process, we analyzed pif1, pif3, pif4, and pif5 single mutants (to assess the effect of the absence of each one of the PIFs in the presence of the others) and the pif triple mutant combinations pif1 pif3 pif4, pif1 pif3 pif5, pif1 pif4 pif5, and pif3 pif4 pif5 (to assess the effect of the presence of each one of the PIF quartet separately in the absence of the other three by comparison to pifq). pif1 pif3 and pif4 pif5 double mutants were also included as controls for dark and shade phenotypes (Leivar et al., 2008a; Lorrain et al., 2008, 2009).
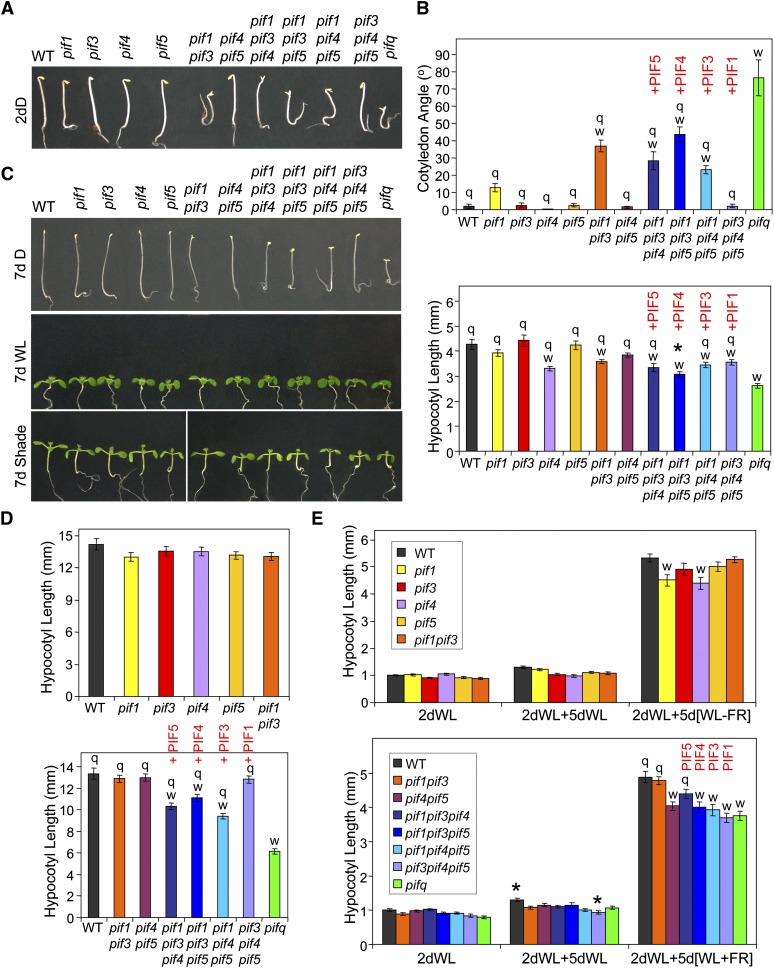
Phenotypic analysis of 2-d-old (Figures 5A and 5B) and 4-d-old (see Supplemental Figure 12A online) dark-grown seedlings suggest that PIF1 has the most prominent role in maintaining appressed cotyledons in etiolated seedlings but that it does this in a partially redundant manner with PIF3, PIF4, and PIF5. This conclusion comes from at least three observations: (1) Although the pif1 single mutant shows only a minimal, nonstatistically significant cotyledon separation phenotype in the dark, higher order pif mutant combinations that include the pif1 mutation show an increased degree of cotyledon separation compared with those that retain the wild-type PIF1 locus. (2) The wild-type PIF1 gene is the only PIF member able to fully complement the pifq cotyledon separation phenotype, since the pif3 pif4 pif5 triple mutant behaves like the wild type. (3) PIF3, PIF4, and PIF5 genes show more partial functions in maintaining appressed cotyledons in darkness in the absence of the other PIFs, as inferred from comparing pif1 pif4 pif5, pif1 pif3 pif5, and pif1 pif3 pif4 triple mutants with pifq.
Figure 5.
Phenotypic Analysis of pif Mutant Combinations Provides Evidence for Differential Contributions of Individual PIF Quartet Members to Skotomorphogenesis and Shade Avoidance.
Wild-type (WT) and pif mutants were analyzed phenotypically for cotyledon separation and/or for hypocotyl length in seedlings grown in darkness (D), in WLc, or in WL supplemented with ChIP (shade, WL+FR). Light conditions were as in Figure 1A.
(A) Visible phenotypes of wild-type and pif mutant seedlings grown in the dark for 2 d (2dD).
(B) Quantification of cotyledon angle (top) and hypocotyl length (bottom) phenotypes of 2-d-old dark grown wild-type and pif mutant seedlings.
(C) Visible phenotypes of wild-type and pif mutant seedlings grown either in the dark for 7 d (7 d D), in WLc for 7 d (7 d WL), or in WL for 2 d and then in WL+FR for five additional days (7 d Shade).
(D) Quantification of hypocotyl length phenotype of 7-d-old dark-grown wild-type and pif mutant seedlings. pif single mutants are shown at the top, whereas pif4 pif5 double, pif triple, and pifq mutants are shown at the bottom. Wild-type and pif1 pif3 mutants were included in both experiments.
(E) Quantification of hypocotyl length phenotype of wild-type and pif mutant seedlings grown in WLc from germination onward (2dWL) or for 2 days in WLc and then for an additional 5 d either in WLc (2dWL+5dWL) or in WL with supplemental ChIP (2dWL+5d[WL+FR]). pif single mutants are shown at the top, whereas pif4 pif5 double, pif triple, and pifq mutants are shown at the bottom. Wild-type and pif1 pif3 mutants were included in both experiments.
Graphical data in (B), (D), and (E) represent mean values and se (bars) from at least 20 seedlings. Statistically significant differences from the wild type (w) and/or the pifq mutant (q) values, defined by Tukey’s multiple comparison test (P ≤ 0.05), are indicated for each treatment. An asterisk indicates statistically significant differences from the pifq mutant by Student’s t test (P ≤ 0.05), shown only for values that were not defined as “q” by the Tukey’s test. PIF1, PIF3, PIF4, and PIF5 red labels on top of the triple pif mutant graphs are intended to highlight the PIF gene that is present as a wild-type copy in the corresponding triple mutant.
Overall, the hypocotyl elongation phenotype of young 2-d-old dark-grown seedlings broadly mirrors the cotyledon separation phenotype, in that elongation declines progressively with the absence of increasing numbers of PIF family members (cf. single, double, and triple mutants with pifq; Figures 5A and 5B, bottom). On the other hand, the contributions of each individual PIF to elongation in the absence of the other three (see the triple mutants) appear to be quantitatively more similar to each other than for the cotyledon phenotype in these seedlings (Figure 5B). In 7-d dark-grown seedlings, PIF1 emerges as the most active in complementing the pifq short-hypocotyl phenotype (cf. pif3 pif4 pif5 to pifq), although relatively strong contributions are also observed for PIF3, PIF4, and PIF5 (cf. pif1 pif4 pif5, pif1 pif3 pif5, and pif1 pif3 pif4 to pifq) (Figures 5C and 5D). Similar effects (although perhaps less pronounced) were also seen in 4-d-old dark-grown hypocotyls (see Supplemental Figure 12B online). These apparent quantitative differences between young and older seedlings might reflect developmental changes in PIF activity or perhaps the greater variability and smaller dynamic range of differences in hypocotyl length between the wild type and pifq in the 2-d-old than in the older seedlings.
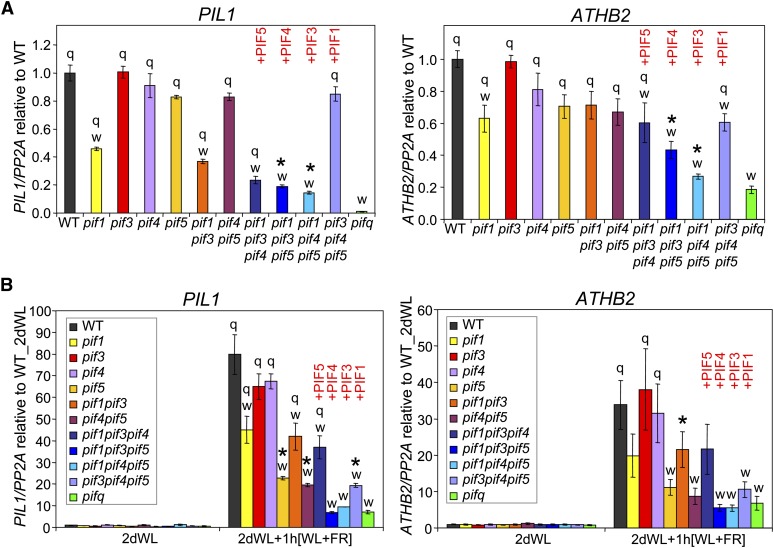
Expression analysis of established PIF-dependent genes (PIL1 and ATHB2) in the dark shows an expression profile pattern that is broadly consistent with the morphological phenotypes of the various pif mutant combinations tested above (Figure 6A). Among the pif single mutants, pif1 shows the most robust PIL1 misexpression in 2-d-old dark-grown seedlings (Figure 6A), and PIF1 is also by far the most active gene among the PIF quartet in complementing the low-PIL1 misexpression phenotype of the pifq mutant (cf. pif3 pif4 pif5 to pifq). A similar pattern is observed for ATHB2 expression (Figure 6A), although in this case the role of PIF1 in inducing ATHB2 in the dark is quantitatively more similar to the other PIFs, and relatively strong contributions of PIF5 and, to a lesser extent, PIF4 are also observed (cf. pif3 pif4 pif5, pif1 pif3 pif5, and pif1 pif3 pif4 to pifq). The data thus suggest that PIF1 is the most active player among the PIF quartet in promoting the expression of selected PIF-dependent genes during skotomorphogenesis, a function also exerted by the other PIF members in a partially redundant manner.
Figure 6.
PIF1 and PIF5 Differentially Dominate Promotion of Expression of Selected M Class Genes during Skotomorphogenesis and Initiation of Shade Avoidance.
(A) ATHB2 and PIL1 gene expression in 2-d-old dark-grown seedlings. Data are presented relative to the wild-type (WT) mean, which was set at unity.
(B) ATHB2 and PIL1 gene expression in seedlings grown for 2 d in WLc (2dWL) or seedlings grown for 2 d in WL and then treated with WL supplemented with ChIP for 1 h (2dWL+1 h[WL+FR]). Light conditions were as in Figure 1A. Data are presented relative to the mean of WT-WL set at unity.
ATHB2 and PIL1 gene expression were measured by qPCR in wild-type and pif mutant seedlings grown under the indicated conditions, and PP2A was used as a normalization control as described (Shin et al., 2007). Data represent the mean and se of at least three independent biological replicates. Statistically significant differences from the wild type (w) and/or the pifq mutant (q) values, defined by Tukey’s multiple comparison test (P ≤ 0.05), are indicated for each treatment. An asterisk indicates statistically significant differences from the pifq mutant by Student’s t test (P ≤ 0.05), shown only for values that were not defined as “q” by the Tukey’s Test. PIF1, PIF3, PIF4, and PIF5 red labels on top of the triple pif mutant graphs are intended to highlight the PIF gene that is present as a wild-type copy in the corresponding triple mutant.
Phenotypic analysis of hypocotyl elongation responses to simulated vegetational shade of the various pif mutant combinations is presented in Figures 5C and 5E. The pif1, pif3, pif4, and pif5 single and pif1 pif3 double mutants show variable minor-to-absent hypocotyl phenotypes in response to long-term simulated shade, as documented in two independent experiments (Figure 5E, top; see Supplemental Figure 13 online). These data alone would thus suggest only marginal contributions of the individual PIF quartet members to the long-term hypocotyl elongation responses to simulated shade (i.e., in the presence of the other members of the PIF quartet). However, more consistently reproducible phenotypes are observed for pif4 pif5, pif3 pif4, and pif3 pif5 double mutants compared with the wild type (Figure 5E, bottom; Lorrain et al., 2008; Leivar et al., 2012), suggesting that a certain degree of redundancy exists among these PIFs in regulating shade avoidance responses. The relatively smaller difference in hypocotyl shade responsiveness between the pif4 pif5 and pifq mutants in this experiment than in Figure 1C falls short of statistical significance, reinforcing the conclusion that the PIF1-PIF3 pair contribute quantitatively very moderately to the collective activity of the quartet, in controlling this response, compared with PIF4 and PIF5 combined. However, combination of the pif mutations into higher order triple and quadruple pif mutants provides interesting insight into the relative activities of the individual PIFs (Figure 5E, bottom). In contrast with the triple pif mutant analysis in 7-d-old dark-grown seedlings (Figures 5C and 5D), PIF1 does not dominate complementation of the pifq hypocotyl phenotype in response to long-term simulated shade, in the absence of the other three PIFs. Instead, PIF5 is the only PIF quartet member that individually displays significant promotive activity in the absence of the other three (cf. pif1 pif3 pif4 to pifq in Figure 5E, bottom).
Expression analysis of representative M-class genes, PIL1 and ATHB2, in the various pif mutant combinations, in response to simulated shade, shows that PIF5 is the most prominent contributor to the rapid induction of these genes in response to shade, with PIF1 being the next strongest (Figure 6B). This prominent role of PIF5 is inferred from the robust reduction in shade-induced expression of PIL1 and ATHB2 in the pif5 single mutant compared with the wild type, which is in contrast with the relatively more modest effect of pif1 and the marginal-to-absent effects of pif3 and pif4 monogenic mutations (Figure 6B). Conversely, in good agreement with these data, the PIF5 gene is the most active in complementing the loss-of-shade–induced PIL1 gene expression phenotype of pifq (cf. pif1 pif3 pif4 to pifq), followed by a relatively less pronounced effect of the PIF1 gene (cf. pif3 pif4 pif5 to pifq). A similar tendency is observed for ATHB2, albeit in a nonstatistically significant manner in this analysis (Figure 6B).
DISCUSSION
Previously we defined the PIF-regulated transcriptional network that promotes skotomorphogenesis in dark-grown seedlings and that responds rapidly to PIF removal by phy-induced proteolysis at the inception of deetiolation upon first exposure to light (Leivar et al., 2009). Here, we identified a set of genes that respond rapidly to the shade signal, in a PIF-dependent manner, and uncovered a dichotomy within this set suggesting that transcription factor–encoding genes may dominate those that are direct PIF targets, whereas auxin-responsive genes may be indirect targets. In addition, we defined a further core subset of these genes that display diametrically opposed, rapid responsiveness to initial light and shade exposure. These data thus identify a subset of genes that apparently remain continuously under active, reversible, and potentially direct transcriptional regulation by phy-PIF signaling throughout seedling deetiolation and subsequent vegetative growth and development of the fully green plant. Moreover, we provided morphogenic and marker gene expression evidence that individual members of the PIF quartet contribute differentially to the collective action of these factors in exerting this regulation during skotomorphogenesis and shade avoidance, suggesting that they may provide selective channeling of the phy-mediated light signal to defined sectors of the transcriptional network.
Overall, the shade-induced expression profiles in wild-type seedlings observed here overlap significantly with those in previous similar studies (Devlin et al., 2003; Carabelli et al., 2007; Tao et al., 2008) (see Supplemental Figure 3 online). In particular, a considerable fraction of the genes responding within 1 h to the shade stimulus in our study (Table1; see Supplemental Data Set 18 online) have also been documented previously as rapidly shade responsive (Steindler et al., 1999; Salter et al., 2003; Roig-Villanova et al., 2006, 2007; Carabelli et al., 2007; Lorrain et al., 2008; Tao et al., 2008; Kozuka et al., 2010). This study expands this list of rapid-response genes and identifies those that require one or more members of the PIF quartet (PIF1, 3, 4, and/or 5) for normal, full responsiveness to the signal (Figure 2; see Supplemental Figure 4 online). We show that endogenous PIF3 levels increase rapidly in response to shade-induced reduction in phy Pfr levels (Figure 1; see Supplemental Figure 1 online), similarly to that reported for PIF4 and PIF5 proteins overexpressed ectopically in transgenic lines (Lorrain et al., 2008) and consistent with the involvement of these PIFs in the stimulation of enhanced axis elongation and other responses that make up the SAS (Smith and Whitelam, 1997; Franklin, 2008; Lorrain et al., 2008) (Figure 1C). Shade-induced genes (see Supplemental Figure 4B online) dominate the rapidly responsive class of loci (see Supplemental Figure 4A online) and have thus been the focus of our study.
Our analysis identifies those genes that are most robustly dependent on the PIFs for shade induction (FIR >1.5) (Figures 2B1 and 2B2, Table 1). Several of these genes have been identified previously as being rapidly shade responsive in wild-type seedlings, including ATHB2, XTR7, HFR1, PAR1, and IAA29 (Steindler et al., 1999; Roig-Villanova et al., 2006, 2007; Carabelli et al., 2007; Lorrain et al., 2008; Hornitschek et al., 2009) (Table 1; see Supplemental Data Set 18 online). Our data show that genes with G-box–containing promoters are enriched among the robustly PIF-dependent loci (69 to 81% of genes with FIR >1.5 after 1 h of simulated shade) (Table 1; see Supplemental Data Set 18 online). Because PIF proteins have been shown to bind sequence specifically to the core G-box motif (Martínez-García et al., 2000; Huq and Quail, 2002; Huq et al., 2004; Shin et al., 2007; de Lucas et al., 2008; Leivar et al., 2008b; Moon et al., 2008; Hornitschek et al., 2009; Oh et al., 2009), this strong positive correlation is consistent with the possibility that those genes exhibiting robust PIF-regulated expression may be direct targets of transcriptional regulation by promoter-bound PIF proteins. The available ChIP analysis of targeted G-box–containing promoters, such as XTR7, HFR1,and PIL1, supports this suggestion (Shin et al., 2007; de Lucas et al., 2008; Moon et al., 2008; Hornitschek et al., 2009; Oh et al., 2009; Toledo-Ortiz et al., 2010). Moreover, comparison of the G-box–containing and non-G-box–containing genes within this set (Figures 2B1 and 2B2) reveals an interesting functional dichotomy. The G-box–containing subset is enriched for transcription factor–encoding genes (61 to 67%), suggesting a function in the primary transcriptional network that regulates downstream genes in the phy signaling cascade (Figure 2C, top). Conversely, the non-G-box–containing subset are enriched for hormone-related genes (50 to 67%), especially auxin-responsive genes (Figure 2C, bottom), reinforcing the established notion that auxin is strongly involved in the rapid growth responses (≤10 min) observed upon shade exposure (Smith and Whitelam, 1997; Steindler et al., 1999; Tanaka et al., 2002; Vandenbussche et al., 2003; Carabelli et al., 2007; Roig-Villanova et al., 2007; Franklin, 2008; Tao et al., 2008; Keuskamp et al., 2010; Kozuka et al., 2010; Cole et al., 2011; Grebe, 2011). However, closer inspection of the auxin-responsive gene set suggests a further dichotomy and level of complexity. There appears to be a possible dual track of rapid PIF regulation of the auxin signaling pathway: (1) A potentially indirect track stimulating bioactive auxin activity (perhaps via synthesis, transport, or activation of inactive conjugates) or signaling, with resultant induction of several SAUR genes with no G-boxes in their promoters (Figure 2C, bottom, Table 1; see Supplemental Data Set 18 online); and (2) a potentially direct track involving direct transcriptional activation of AUX/IAA genes by promoter-bound PIF proteins, represented by the G-box–containing genes IAA2, IAA4, IAA19, and IAA29 (Figure 2C, top, Table 1; see Supplemental Data Set 18 online). As the AUX/IAA genes are negative regulators of auxin signaling (reviewed in Mockaitis and Estelle, 2008), it is possible that PIF promotion of their expression functions to precede and/or augment rapid auxin-induced accumulation of the transcripts of these genes to prevent an over-response to the increased auxin and/or to generate a high capacity for rapid synthesis of the negative regulatory AUX/IAA proteins upon any subsequent reduction in auxin activity. Collectively, these data suggest that initiation or modulation of auxin signaling is at least one of the major circuits that the PIFs target initially in response to the shade signal, consistent with the role of this hormone in implementing rapid shade-induced growth responses (Tao et al., 2008; Cole et al., 2011). PIF modulation of auxin signaling might also operate in other conditions where growth is induced, as proposed for diurnal conditions (Nozue et al., 2011) and as recently demonstrated for PIF4 in response to high temperature (Koini et al., 2009; Franklin et al., 2011). Intriguingly, however, whereas Franklin et al. (2011) observe PIF4-dependent, high-temperature-induced increased expression of the auxin biosynthetic genes TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 and CYTOCHROME P450 CYP79B2, we do not detect significant shade responsiveness of these genes. This observation might suggest that the phys and high-temperature signal through the PIFs to the auxin system via different pathways.
It is notable that bHLH factor genes (PIL1, HFR1, PAR1, BES1-INTERACTING MYC-LIKE PROTEIN2 [BIM2], BR ENHANCED EXPRESSION1 [BEE1], AT5G15160, AT1G01260, and AT3G57800) dominate the G-box–containing, robustly PIF-dependent, transcription factor–encoding gene set (Figure 2C, top, Table 1; see Supplemental Data Set 18 online). This suggests that these PIF-related factors may have evolved as variants from the same gene family recruited to function in the primary transcriptional network regulated by the PIFs in response to shade. The presence of homeodomain- (ATHB2 and ATHB52) and a TEOSINTE BRANCHED1/CYCLOIDEA (TCP)-encoding (TCP/PCF15 [TCP15]) gene in this group suggests early divergence of downstream targets in the transcriptional cascade. It is notable that several of these transcription factors have already been shown to have regulatory roles in the SAS, including ATHB2 (Steindler et al., 1999), PIL1 (Salter et al., 2003; Roig-Villanova et al., 2006), HFR1 (Sessa et al., 2005; Hornitschek et al., 2009), and PAR1 (Roig-Villanova et al., 2007). This is consistent with the PIFs acting to induce a transcriptional regulatory network that implements or fine-tunes the response to the shade signal. As BIM2 and BEE1 have been implicated in the brassinosteroid signaling pathway (Friedrichsen et al., 2002; Yin et al., 2005), these genes are potential regulatory nodes for PIF and brassinosteroid crosstalk (Sorin et al., 2009; Kozuka et al., 2010; Keller et al., 2011; Keuskamp et al., 2011) in response to shade. The presence of CKX5 (a cytokinin oxidase–encoding gene) and GA2OX6 (a gibberellin-2 oxidase) (Figure 2C, top; see Supplemental Data Set 18 online) suggests that cytokinin and gibberellin levels (two other proposed shade-regulatory hormones; Carabelli et al., 2007; Djakovic-Petrovic et al., 2007; Stamm and Kumar, 2010), in addition to auxin levels, may be early targets of PIF-mediated shade regulation. It is notable that GIBBERELLIC ACID INSENSITIVE (GAI), a repressor of the gibberellin pathway proposed to constrain shade avoidance responses (Djakovic-Petrovic et al., 2007), is induced by the shade signal in a robustly PIF-dependent manner. Since DELLA proteins, including GAI, have been proposed to constrain growth by inhibiting the PIFs (de Lucas et al., 2008; Feng et al., 2008), the data suggest that the PIFs induce a complex negative-feedback regulatory loop, possibly to modulate the response to the shade signal. Direct PIF regulation of cell wall metabolism, possibly related to the rapid growth responses to shade, is also suggested by the presence of XTR7/XTH15 (a xyloglucan transglycosylase–encoding gene; Rose et al., 2002) in this group. This is consistent with the observation that xth15 knockout mutants show reduced shade-induced growth responses (Sasidharan et al., 2010). Collectively, the data focus attention on a subset of genes that provide potential targets for future investigations aimed at securing more detailed insight into central cellular processes involved in the initiation of the shade avoidance response.
Certain facets of the SAS, such as accelerated hypocotyl and internode cell elongation rates and retarded chloroplastogenesis (Franklin, 2008), represent changes in growth and development in the direction of those that preexist in the etiolated seedling before light exposure. Our comparison of the transcriptome changes induced by light and shade at the inception of deetiolation and shade avoidance, respectively, has provided evidence (1) of a core subset of genes that respond rapidly and reciprocally to the production of, and reduction in, high Pfr levels upon initial light and shade exposure, respectively; (2) that require the PIF quartet for normal, maximal expression levels in both dark-grown and shade-exposed seedlings; and (3) whose promoters contain G-box motifs capable of direct recruitment of the PIF factors (Figure 3, Class M genes, and Table 2). The data suggest that these genes are direct targets of sustained, continuous modulation by the phy-PIF signaling system, whereby the PIFs constitutively promote their expression in an ongoing manner, and photoactivated phy quantitatively abrogates this activity by inducing controlled PIF proteolysis in response to impinging light signals. Therefore, these genes seem likely to comprise a central, primary transcriptional network module responsible for rapidly redirecting adaptive responses to the fluctuating light environment as the plant progresses through the life cycle. Consistent with this view, 13 out 14 of these class M genes (Table 2) have been shown in a recently published study to be upregulated during the growing phase at night in seedlings growing under diurnal conditions (Nozue et al., 2011), and among those, 10 of them require PIF4 and/or PIF5 for their expression.
Our comparison of the skotomorphogenic and shade-induced, visible and molecular phenotypes of monogenic, triple, and quadruple pif mutants in dark-grown and shade-exposed seedlings provides evidence that the individual PIF1, 3, 4, and 5 proteins contribute, qualitatively and quantitatively to different extents, to the collective activity of this PIF quartet in regulating growth and development. The data show that PIF1 dominates promotion of cotyledon appression in dark-grown seedlings, with PIF3, 4, and 5 contributing to a lesser extent (Figure 5B, top). These data are consistent with previous reports (Leivar et al., 2008a; Shin et al., 2009), although in one case the analysis was incomplete since it only involved a selected subset of pif mutant combinations (Leivar et al., 2008a) and in the other the appression data were not quantified by these authors (Shin et al., 2009). A similar, although less dramatic, pattern is observed for the hypocotyl length phenotype, where PIF1 again appears to dominate, especially in 7-d dark-grown seedlings (Figure 5D). Conversely, none of the PIF quartet appears to dominate promotion of shade-induced hypocotyl elongation, although if anything, PIF5 appears to show a more significant contribution in the absence of the other three PIF members (Figure 5E). By comparison, PIF4 has been shown to strikingly dominate high-temperature-induced elongation growth (Koini et al., 2009; Stavang et al., 2009; Franklin et al., 2011), PIF1 and PIF3 have been reported to dominate promotion of agravitropism in dark-grown seedlings (Shin et al., 2009), PIF1 dominates regulation of seed germination (Oh et al., 2006), and PIF3 plays a prominent role in promoting seedling growth under diurnal light-dark conditions (Soy et al., 2012) in conjunction with PIF4 and PIF5 (Nozue et al., 2007).
Marker gene expression analysis reveals a more complex picture, whereby the pattern of individual PIF involvement is different between skotomorphogenesis and shade avoidance. Whereas PIF1 dominates promotion of PIL1 expression in dark-grown seedlings (with PIF3, 4, and 5 contributing less, but at comparable levels to each other), PIF5 dominates shade-induced promotion of PIL1 expression (with PIF1, 3, and 4 contributing less) (Figure 6). ATHB2 displays a similar general pattern of differential regulation in response to darkness and shade, except that PIF5 appears to have a significantly more prominent role in promoting this gene’s expression in dark-grown seedlings in the absence of the other three PIF members, where its activity is comparable to that of PIF1. These data suggest that the different PIF family members may function to differentially distribute phy-regulated signaling across different sectors of the transcriptional network. In addition, the residual responsiveness of the pifq mutant to shade here, as well as to light during deetiolation (Leivar et al., 2008a, 2009), is suggestive of residual factors other than PIF1, 3, 4, and 5, that, like these PIFs, act continuously to promote facets of skotomorphogenic-like gene expression and development. Such factors might include additional PIF family members, such as PIF6, PIF7, and/or PIF8 (Leivar and Quail, 2011), or other potential regulators. Finally, emerging evidence of the central position of the PIF family at the nexus of multiple convergent signaling pathways (Leivar and Quail, 2011) indicates that further detailed definition of the functional roles of these factors in various cellular processes will be of considerable interest.
METHODS
Plant Materials, Seedling Growth, and Measurements
Except for pif1 pif4 pif5 and pif4 pif5, all the pif mutant combinations used in this study were described elsewhere (Leivar et al., 2008a) and were generated using pif1-1 (Huq et al., 2004), pif3-3 (Monte et al., 2004), pif4-2 (Leivar et al., 2008b), and pif5-3 (Khanna et al., 2007) single mutants. pif1 pif4 pif5 and pif4 pif5 were obtained by crossing pif1 pif3, pif4-2, and pif5-3 as described (Leivar et al., 2008a).
Seeds were sterilized and plated in germination medium without Suc as described (Leivar et al., 2009) and were then stratified for 5 d at 4°C in darkness. For phenotypic analysis under simulated shade conditions, seeds were transferred to WLc (19 μmol m−2 s−1, R/FR ratio of 6.48) for 2 d at 21°C and then grown for five additional days under the same WL fluence rate with (WL-FR, R/FR ratio of 0.006) or without (WL) supplemental ChIP.
For dark-grown seedlings, seeds were treated with a 3-h WL pulse to synchronize germination, followed by a 5-min FR pulse to avoid pseudo-dark effects as reported (Leivar et al., 2008a, 2009). Alternatively, a 5-min R pulse followed by a 3-h dark incubation and a terminal 5-min FR pulse was used (Leivar et al., 2008a, 2009). Seeds were then grown in the dark at 21°C for the indicated time.
Hypocotyl length and cotyledon opening were measured from at least 20 seedlings as described (Monte et al., 2003; Leivar et al., 2008a). Data were analyzed using Tukey’s multiple comparison test (GraphPad Prism software) and/or Student’s t test as indicated.
For the experiments performed under dichromatic R+FR simulated-shade conditions, seeds were induced to germinate directly in Rc (10 μmol m−2 s−1), and seedlings were grown for 3 d in Rc. Seedlings were then maintained in the same fluence rate of Rc supplemented with ChIP (low R/FR ratio of 0.1 or 0.02 as indicated) for the specified time.
Protein Extraction and Immunoblot and β-Glucuronidase Assays
Protein extracts of Arabidopsis thaliana seedlings and immunodetection of PIF3 in R+FR and WL+FR were done as described (Al-Sady et al., 2006; Leivar et al., 2008b). Briefly, protein extracts of Arabidopsis seedlings were prepared (Al-Sady et al., 2006; Leivar et al., 2008b), and total protein was quantified using a Protein DC kit (Bio-Rad). β-Mercaptoethanol was added to the samples before loading, and then equal amounts of protein were subjected to polyacrylamide gel electrophoresis. Immunodetection of PIF3 was performed using affinity-purified anti-PIF3 antisera (Al-Sady et al., 2006), and a mouse monoclonal antibody against α-tubulin (Sigma-Aldrich) was used as a loading control. Anti-rabbit-horseradish peroxidase and anti-mouse-horseradish peroxidase were used as secondary antibodies (Promega), and ECL or ECL plus chemiluminescence kits (Amersham) were used for detection. Alternatively, alkaline phosphatase–coupled anti-rabbit Antiserum (Promega) was used as a secondary antibody for detection of PIF3 (Al-Sady et al., 2006). β-Glucuronidase (GUS) activity assays with 35S:GUS:PIF3 transgenics were performed as described (Monte et al., 2004).
Gene Expression Analysis by qPCR and RNA Gel Blots
For qPCR experiments, ~250 seedlings were grown for 2 d in either darkness or WLc as indicated above. Two-day-old WLc-grown seedlings were then exposed to WL+FR as described above, and samples were harvested at the indicated times. Quantitative RT-PCR analysis was performed as described (Leivar et al., 2009). Briefly, an RNeasy Plus Plant Mini kit (Qiagen) was first used to extract RNA. Then, 1 μg of total RNA was treated with DNase I (Invitrogen) to further eliminate genomic DNA, and first-strand cDNA synthesis was done using the Super-Script First Strand cDNA synthesis for RT-PCR kit (Invitrogen) and oligo(dT20) as a primer. cDNA was treated with RNase H, and samples were then diluted 1:30 with water. Ten microliters of the diluted samples were used for real-time PCR (MyIQ single-color real-time PCR detection system; Bio-Rad). Eva-green (Biotium) was used for detection, and 0.1% Tween 20, 0.1 mg/mL BSA, and 5% DMSO were added to the PCR mix as described (Khanna et al., 2007). Each qPCR assay was repeated at least two times, and the mean expression values from these technical replicates were used for further calculations. Gene expression was measured from at least three biological replicates, and PP2A (AT1G13320) was used as a normalization control as described (Shin et al., 2007; Leivar et al., 2009). Normalized gene expression was represented relative to the wild type set as a unity. Primer sequences for qPCR of PP2A (AT1G13320), PIL1 (AT2G46970), HAT4/ATHB2 (AT4G16780), AUX/IAA29 (AT4G32280), SAUR25 (AT4G13790), and Unknown (AT5G02580) were described elsewhere (Leivar et al., 2009). Primer sequences for CKX5 (AT1G75450) were as follows: CKX5_F (5′-ACCGTCCACCCTTCCGAC-3′) and CKX5_R (5′-CAGGTGACTTCAGCATACCGAA-3′). Primer sequences for XTR7 (AT4G14130) were as follows: PLR145_F (5′-CGGCTTGCACAGCCTCTT-3′) and PLR146_R (5′-TCGGTTGCCACTTGCAATT-3′). Data were analyzed using Tukey’s Multiple Comparison Test (GraphPad Prism software) and/or a Student’s t test as indicated.
For RNA gel blot analysis, total RNA extraction, loading, and hybridization conditions were as described (Monte et al., 2003). Specific probe for PIF3 was prepared as described (Monte et al., 2004).
Microarray-Based Expression Profiling: RNA Isolation, cRNA Synthesis, and Hybridizations
About 1500 Arabidopsis wild-type and pifq mutant (Leivar et al., 2008a) seeds were plated separately for each sample on germination medium without Suc at room temperature. During this procedure, the seeds were routinely exposed to WL for a total of 1.5 h after imbibition. Seeds were then stratified for 5 d at 4°C in darkness and then grown in WL (19 μmol m−2 s−1, R/FR ratio of 6.48) for 2 d at 21°C (WL0 samples). Two-day-old WL-grown seedlings were then maintained in the same fluence rate of WL supplemented with FR light (WL-FR, R/FR ratio of 0.006) for 1 (FR1), 3 (FR3), or 24 (FR24) hours before harvesting. Control seedlings were also maintained in parallel in the same fluence rate of WL for 24 h (WL24) before harvesting. Three different biological replicates of each treatment were grown separately and extracted, processed, and analyzed independently. Total RNA isolation, cRNA synthesis, and microarray hybridizations were performed as described (Monte et al., 2004) with minor variations (Leivar et al., 2009). Briefly, total RNA was prepared as described (Chang et al., 1993) followed by further purification using RNeasy columns (Qiagen). RNA target preparation was performed following the GeneChip 3′ IVT express kit (Affymetrix). ATH1 microarrays (Affymetrix) were used for gene expression detection. Hybridization and washes were performed as described by Affymetrix in the Functional Genomics Laboratory facility at the University of California at Berkeley (http://qb3.berkeley.edu/qb3/fgl/).
Microarray Data Analysis
Microarray data analysis was performed as described (Leivar et al., 2009) with minor modifications. Determination of statistical differences between genotypes and/or light treatments was made using the software analysis package AFFY (which is available as part of the Bioconductor project; www.bioconductor.org). To identify statistically significant, rapidly shade-responsive genes in the wild type, a moderated t test (Smyth, 2004) available as software analysis PLM and LIMMA AFFY packages as part of the Bioconductor project, was applied to the wild-type data, comparing gene expression at 2 d in WLc (WL0) with that after 1 h of WL+ChIP irradiation (FR1). Log scale gene expression values were calculated using a Robust Multiarray Analysis. Fold-change values between various genotypes and treatments were calculated using the mean expression value of the replicate samples. Shade-responsive genes were defined as those that varied twofold from the WL0 time point with a P value (adjusted for false discovery rate) <0.05 SSTF genes (Hu et al., 2009). Similar analyses were done at the 3- and 24-h WL+FR (FR3 and FR24) time points. Genes that were differentially expressed with statistical significance in the pifq mutant compared with the wild type (P value <0.05 versus the wild-type, adjusted for false discovery rate) were identified at each time point (FR1, FR3, and FR24). If the ratio of gene expression of the wild type/pifq at WL0 was above 1.6 or below 0.625, they were defined as a separate class of genes, already different at WL0 before shade treatment in the pifq mutant (see Supplemental Figure 4, Supplemental Data Set 12, and Supplemental Data Set 15 online) and excluded from further analysis.
To provide a quantitative estimate of the robustness of the responsiveness of each gene to the shade treatment, we calculated a mean FI value for induced genes, separately for the wild type and pifq mutant. FI is defined as the mean of the triplicate expression values in 1-h WL+ChIP-irradiated seedlings (designated FR1) divided by the mean of the triplicate expression values in the 2-d WLc (WL0)–treated seedlings:
To provide a quantitative measure of the robustness of the contribution of the PIF quartet to the shade-induced response of each gene, we calculated a FIR value for induced genes as described (Monte et al., 2004). FIR is defined as the ratio of the FI values for the wild type and pifq mutant:
To simplify the functional classification analysis, genes were assigned to single functional categories based on the gene descriptions provided by Affymetrix and TIGR404 (http://compbio.dfci.harvard.edu/tgi/) and the classification provided by Munich Information Center for Protein Sequences (http://www.helmholtz-muenchen.de/en/mips/projects/funcat/index.html) as described (Leivar et al., 2009). Gene products targeted to the chloroplast were assigned to the “photosynthesis/chloroplast” category, whereas those with predicted or established transcription or DNA binding activity were assigned to the “transcription” category. Occurrence of G-boxes in promoter regions, defined as 3 kb upstream of the transcription start site, was determined using PATMATCH (http://www.Arabidopsis.org/cgi-bin/patmatch/nph-patmatch.pl) as described (Leivar et al., 2009).
Meta-Analysis
CEL files from this study and those from our previous analysis of seedling deetiolation in the pifq mutant (Leivar et al., 2009) were combined in a single Robust Multiarray Analysis. Values derived from this analysis were used for graphic representations shown in Figure 3 and Supplemental Figures 7 to 10 online.
Accession Numbers
The microarray data reported in this article have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through Gene Expression Omnibus Series accession number GSE28297. Sequence data can be found in the Arabidopsis Genome Initiative database under accession numbers AT2G20180 (PIF1/PIL5), AT1G09530 (PIF3), AT2G43010 (PIF4), and AT3G59060 (PIF5/PIL6). Supplemental Data Sets 1 to 35 are deposited in the DRYAD repository: http://dx.doi.org/10.5061/dryad.gd031k26.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Reduction of Steady State Photoactivated-phy (Pfr) Levels in Continuous Light Induces Rapid Increases in PIF3 Abundance in Arabidopsis Seedlings.
Supplemental Figure 2. Genome-Wide Patterns of Rapid and Delayed Shade-Responsive Gene Expression in Wild-Type and pifq Mutant Seedlings.
Supplemental Figure 3. Limited Overlap of Rapidly Shade-Responsive Genes Identified Here with Those in Previous Transcriptome Analyses.
Supplemental Figure 4. Genome-Wide Patterns of Rapidly Shade-Responsive Gene Expression Establish PIF Involvement in Early Shade Signaling.
Supplemental Figure 5. qPCR Validation of Microarray Data.
Supplemental Figure 6. Genes Displaying Rapid Light-Triggered Repression during Deetiolation Are Enriched in Early Shade-Induced Genes.
Supplemental Figure 7. Merging of Deetiolation: Class 3 Light-Repressed and Rapidly Shade-Induced Transcriptome Data.
Supplemental Figure 8. Merging of Deetiolation: Class 6 Light-Repressed and Rapidly Shade-Induced Transcriptome Data.
Supplemental Figure 9. Merging of Deetiolation: Class 1 Light-Repressed and Rapidly Shade-Induced Transcriptome Data.
Supplemental Figure 10. Merging of Deetiolation: Class 4 Light-Repressed and Rapidly Shade-Induced Transcriptome Data.
Supplemental Figure 11. A Subset of G-Box–containing Class-M Genes Display SSTF but Quantitatively Minimal Shade-Induced Responsiveness Relative to Initial Expression Levels in Dark-Grown Seedlings.
Supplemental Figure 12. Phenotypic Analysis of 4-d-Old Dark-Grown Wild-Type and pif Mutant Seedlings.
Supplemental Figure 13. Quantification of the Hypocotyl Elongation Phenotype of Additional pif Single Mutants Grown under Simulated Shade.
Supplemental Analysis 1. Analysis of PIF3 Levels under Dichromatic Irradiation Conditions.
Supplemental Analysis 2. Comparison of Shade-Responsive Genes at FR1 with Previously Reported Studies and Definition of Shade-Responsive Genes at FR3.
Supplemental Analysis 3. Details of Identification of PIF-Dependent Rapidly Shade-Responsive Genes.
The following Supplemental Data Sets are deposited in the DRYAD repository: http://dx.doi.org/10.5061/dryad.gd031k26.
Supplemental Data Set 1. Expression Data and Primary Analysis for All Genes Identified in Supplemental Figure 2 Online (1381 Genes).
Supplemental Data Set 2. Expression Data and Primary Analysis for All Rapidly Induced Shade-Responsive Genes at FR1 (131 Genes).
Supplemental Data Set 3. Expression Data and Primary Analysis for All Rapidly Repressed Shade-Responsive Genes at FR1 (38 Genes).
Supplemental Data Set 4. Genes Induced Twofold or More in This Study and Either or Both Earlier Studies (Carabelli et al., 2007; Tao et al., 2008) in Response to 1 h (FR1) (131 Genes).
Supplemental Data Set 5. Expression Data and Primary Analysis for All FR3 Shade-Responsive Genes (878 Genes).
Supplemental Data Set 6. Genes Induced at FR1 1.75- to Twofold That Are SSTF Induced at FR3 (55 Genes).
Supplemental Data Set 7. Genes Repressed at FR1 1.75- to Twofold That Are SSTF Repressed at FR3 (60 Genes).
Supplemental Data Set 8. Genes Induced or Repressed 1.75- to Twofold in This Study That Overlap with Those Reported by Carabelli et al. (2007) (Six Genes).
Supplemental Data Set 9. SSTF-FR1–Induced Genes Exhibiting a Statistically Significant Difference in Expression between Wild-Type and pifq Seedlings at FR1 (103 Genes).
Supplemental Data Set 10. SSTF-FR1–Repressed Genes Exhibiting a Statistically Significant Difference in Expression between Wild-Type and pifq Seedlings at FR1 (20 Genes).
Supplemental Data Set 11. FR1 Shade-Responsive Genes Exhibiting No Statistical Differences between the Wild Type and pifq (26 Genes).
Supplemental Data Set 12. FR1 Shade-Responsive Genes with a Preexisting Difference between Genotypes at WL0 (20 Genes).
Supplemental Data Set 13. SSTF Shade-Responsive Genes at FR3 Exhibiting a Statistically Significant Difference in Expression between Wild-Type and pifq Seedlings at FR3 (579 Genes).
Supplemental Data Set 14. SSTF Shade-Responsive Genes at FR3 Displaying No Statistical Difference between Wild-Type and pifq Seedlings (249 Genes).
Supplemental Data Set 15. FR3 Shade-Responsive Genes with a Preexisting Difference between Wild-Type and pifq Seedlings at WL0 (50 Genes).
Supplemental Data Set 16. Expression Data and Primary Analysis for All Shade-Induced Genes Identified in Supplemental Figure 4B Online (626 Genes).
Supplemental Data Set 17. Expression Data and Primary Analysis for All Shade-Repressed Genes Identified in Supplemental Figure 4C Online (644 Genes).
Supplemental Data Set 18. SSTF FR1-Induced Genes Arrayed by Dependence on PIF by Fold Induction Ratio and Sorted by G-Box Presence/Absence and Functional Category (103 Genes).
Supplemental Data Set 19. Expression Data and Primary Analysis for All Non-PIF-Dependent Early Shade-Induced Genes.
Supplemental Data Set 20. Primary Data for Meta-Analysis Derived from Deetiolation and Shade Response Studies (22,207 Genes; ATH1 Array).
Supplemental Data Set 21. Deetiolation Class 7/Meta-Analysis Class M: Genes That Display Robust, Rapid, Reciprocal Responsiveness to the Onset of Light (Rc) and Shade (Supplemental ChIP) Signals, Respectively (14 Genes).
Supplemental Data Set 22. Deetiolation Class 7/Meta-Analysis Class L: Genes That Display Robust, Rapid Responsiveness to the Onset of Light (Rc) but No Reciprocal Response to Shade (Supplemental ChIP) Signals (42 Genes).
Supplemental Data Set 23. Deetiolation Class 7/Meta-Analysis Class R: Genes That Display Robust, Rapid Responsiveness to the Onset of Shade (Supplemental ChIP) Signals but Do Not Show a Reciprocal Responsiveness to the Onset of Light (Rc) (89 Genes).
Supplemental Data Set 24. Deetiolation Class 3/Meta-Analysis Class M: Genes That Display Robust, Rapid, Reciprocal Responsiveness to the Onset of Light (Rc) and Shade (Supplemental ChIP) Signals, Respectively (Eight Genes).
Supplemental Data Set 25. Deetiolation Class 3/Meta-Analysis Class L: Genes That Display Robust, Rapid Responsiveness to the Onset of Light (Rc) but No Reciprocal Response to Shade (Supplemental ChIP) Signals (37 Genes).
Supplemental Data Set 26. Deetiolation Class 3/Meta-Analysis Class R: Genes That Display Robust, Rapid Responsiveness to the Onset of Shade (Supplemental ChIP) Signals but Do Not Show a Reciprocal Responsiveness to the Onset of Light (Rc) (95 Genes).
Supplemental Data Set 27. Deetiolation Class 6/Meta-Analysis Class M: Genes That Display Robust, Rapid, Reciprocal Responsiveness to the Onset of Light (Rc) and Shade (Supplemental ChIP) Signals, Respectively (Eight Genes).
Supplemental Data Set 28. Deetiolation Class 6/Meta-Analysis Class L: Genes That Display Robust, Rapid Responsiveness to the Onset of Light (Rc) but No Reciprocal Response to Shade (Supplemental ChIP) Signals (21 Genes).
Supplemental Data Set 29. Deetiolation Class 6/Meta-Analysis Class R: Genes That Display Robust, Rapid Responsiveness to the Onset of Shade (Supplemental ChIP) Signals but Do Not Show a Reciprocal Responsiveness to the Onset of Light (Rc) (95 Genes).
Supplemental Data Set 30. Deetiolation Class 1/Meta-Analysis Class M: Genes That Display Robust Repression with Respect to Dark Controls under Continuous Light Conditions (Rc) and Rapid Induction in Response to Shade (Supplemental ChIP) Signals, Respectively (18 Genes).
Supplemental Data Set 31. Deetiolation Class 1/Meta-Analysis Class L: Genes That Display Robust Repression with Respect to Dark Controls under Continuous Light Conditions (Rc) and Do Not Respond to Shade (Supplemental ChIP) signals, respectively (322 Genes).
Supplemental Data Set 32. Deetiolation Class 1/Meta-Analysis Class R: Genes That Do Not Display Responsiveness with Respect to Dark Controls under Continuous Light Conditions (Rc) and Are Rapidly Induced in Response to Shade (Supplemental ChIP) Signals, Respectively (85 Genes).
Supplemental Data Set 33. Deetiolation Class 4/Meta-Analysis Class M: Genes That Display Robust Repression with Respect to Dark Controls under Continuous Light Conditions (Rc) and Rapid Induction in Response to Shade (Supplemental ChIP) Signals, Respectively (11 Genes).
Supplemental Data Set 34. Deetiolation Class 4/Meta-Analysis Class L: Genes That Display Robust Repression with Respect to Dark Controls under Continuous Light Conditions (Rc) and Do Not Respond to Shade (Supplemental ChIP) Signals, Respectively (243 Genes).
Supplemental Data Set 35. Deetiolation Class 4/Meta-Analysis Class R: Genes That Do Not Display Responsiveness with Respect to Dark Controls under Continuous Light Conditions (Rc) and Are Rapidly Induced in Response to Shade (Supplemental ChIP) Signals, Respectively (92 Genes).
Supplementary Material
Acknowledgments
We thank Enamul Huq for providing pif1 pif3 seeds, Matthew Hudson for advice on statistics, the Functional Genomics Laboratory at the University of California at Berkeley for hybridizations of the microarrays, Christine Carle and Tiffany Liu for early technical work on the project, Ana Smith for making media and solutions, and Jaime Martínez-García for helpful comments on the manuscript. This work was supported by the “Comissionat per a Universitats i Recerca del Departament d’Innovació, Universitats i Empresa” fellowship of the Generalitat de Catalunya (Beatriu de Pinós Program) and Marie Curie International Reintegration Grant PIRG06-GA-2009-256420 to P.L., by grants from Marie Curie IRG-046568, Spanish “Ministerio de Ciencia e Innovación” BIO2006-09254 and BIO2009-07675, and Generalitat de Catalunya 2009-SGR-206 to E.M., and by National Institutes of Health Grant GM-47475, Department of Energy Grant DEFG03-87ER13742, and USDA Agricultural Research Service Current Research Information System Grant 5335-21000-027-00D to P.H.Q.
AUTHOR CONTRIBUTIONS
P.L. and J.M.T. designed and performed the research, contributed new analytical tools, analyzed the data, and wrote the article. M.M.C., E.M., B.A.-S., and E.E. performed the research and analyzed the data. P.H.Q. designed the research and wrote the article.
Glossary
- phy
phytochrome
- PIF
Phytochrome-Interacting Factor
- Rc
continuous red light
- WLc
continuous white light
- EOD-FR
end-of-day far-red
- R
red light
- FR
far-red light
- SAS
shade avoidance syndrome
- qPCR
quantitative PCR
- ChIP
chromatin immunoprecipitation
- SSTF
statistically significant and twofold
- FRc
continuous far-red light
- FIR
fold induction ratio
- FI
fold induction
- WL
white light
References
- Al-Sady B., Kikis E.A., Monte E., Quail P.H. (2008). Mechanistic duality of transcription factor function in phytochrome signaling. Proc. Natl. Acad. Sci. USA 105: 2232–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sady B., Ni W., Kircher S., Schäfer E., Quail P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Bae G., Choi G. (2008). Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59: 281–311 [DOI] [PubMed] [Google Scholar]
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C., Adám E., Fejes E., Schäfer E., Nagy F. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M., Possenti M., Sessa G., Ciolfi A., Sassi M., Morelli G., Ruberti I. (2007). Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev. 21: 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon A., Shen H., Huq E. (2007). Phytochrome Interacting Factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 12: 514–521 [DOI] [PubMed] [Google Scholar]
- Chang S., Puryear J., Cairney J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11: 113–116 [Google Scholar]
- Child R., Smith H. (1987). Phytochrome action in light-grown mustard: Kinetics, fluence-rate compensation and ecological significance. Planta 172: 219–229 [DOI] [PubMed] [Google Scholar]
- Cole B., Kay S.A., Chory J. (2011). Automated analysis of hypocotyl growth dynamics during shade avoidance in Arabidopsis. Plant J. 65: 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucas M., Davière J.M., Rodríguez-Falcón M., Pontin M., Iglesias-Pedraz J.M., Lorrain S., Fankhauser C., Blázquez M.A., Titarenko E., Prat S. (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- Devlin P.F., Yanovsky M.J., Kay S.A. (2003). A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol. 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic-Petrovic T., de Wit M., Voesenek L.A., Pierik R. (2007). DELLA protein function in growth responses to canopy signals. Plant J. 51: 117–126 [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A.E. (2002). Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., et al. (2008). Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.A. (2008). Shade avoidance. New Phytol. 179: 930–944 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Lee S.H., Patel D., Kumar S.V., Spartz A.K., Gu C., Ye S., Yu P., Breen G., Cohen J.D., Wigge P.A., Gray W.M. (2011). Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. USA 108: 20231–20235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.A., Quail P.H. (2010). Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen D.M., Nemhauser J., Muramitsu T., Maloof J.N., Alonso J., Ecker J.R., Furuya M., Chory J. (2002). Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 162: 1445–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T., Yamashino T., Kato T., Mizuno T. (2004). Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 45: 1078–1086 [DOI] [PubMed] [Google Scholar]
- Grebe M. (2011). Out of the shade and into the light. Nat. Cell Biol. 13: 347–349 [DOI] [PubMed] [Google Scholar]
- Hornitschek P., Lorrain S., Zoete V., Michielin O., Fankhauser C. (2009). Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 28: 3893–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Su Y.S., Lagarias J.C. (2009). A light-independent allele of phytochrome B faithfully recapitulates photomorphogenic transcriptional networks. Mol. Plant 2: 166–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E., Al-Sady B., Hudson M., Kim C., Apel K., Quail P.H. (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Huq E., Quail P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21: 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Keller M.M., Jaillais Y., Pedmale U.V., Moreno J.E., Chory J., Ballaré C.L. (2011). Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 67: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp D.H., Pollmann S., Voesenek L.A., Peeters A.J., Pierik R. (2010). Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp D.H., Sasidharan R., Vos I., Peeters A.J., Voesenek L.A., Pierik R. (2011). Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J. 67: 208–217 [DOI] [PubMed] [Google Scholar]
- Khanna R., Shen Y., Marion C.M., Tsuchisaka A., Theologis A., Schäfer E., Quail P.H. (2007). The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell 19: 3915–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Yi H., Choi G., Shin B., Song P.S., Choi G. (2003). Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15: 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koini M.A., Alvey L., Allen T., Tilley C.A., Harberd N.P., Whitelam G.C., Franklin K.A. (2009). High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 19: 408–413 [DOI] [PubMed] [Google Scholar]
- Kozuka T., Kobayashi J., Horiguchi G., Demura T., Sakakibara H., Tsukaya H., Nagatani A. (2010). Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Al-Sady B., Carle C., Storer A., Alonso J.M., Ecker J.R., Quail P.H. (2008b). The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell 20: 337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Cohn M.M., Quail P.H. (April 5, 2012). Phytochrome signaling in green Arabidopsis seedlings: Impact assessment of a mutually-negative phyB-PIF feedback loop. Mol. Plant http://dx.doi.org/10.1093/mp/sss031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. (2008a). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Quail P.H. (2011). PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Tepperman J.M., Monte E., Calderon R.H., Liu T.L., Quail P.H. (2009). Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21: 3535–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Lorrain S., Trevisan M., Pradervand S., Fankhauser C. (2009). Phytochrome interacting factors 4 and 5 redundantly limit seedling de-etiolation in continuous far-red light. Plant J. 60: 449–461 [DOI] [PubMed] [Google Scholar]
- Martínez-García J.F., Huq E., Quail P.H. (2000). Direct targeting of light signals to a promoter element-bound transcription factor. Science 288: 859–863 [DOI] [PubMed] [Google Scholar]
- Mockaitis K., Estelle M. (2008). Auxin receptors and plant development: A new signaling paradigm. Annu. Rev. Cell Dev. Biol. 24: 55–80 [DOI] [PubMed] [Google Scholar]
- Monte E., Alonso J.M., Ecker J.R., Zhang Y., Li X., Young J., Austin-Phillips S., Quail P.H. (2003). Isolation and characterization of phyC mutants in Arabidopsis reveals complex crosstalk between phytochrome signaling pathways. Plant Cell 15: 1962–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte E., Al-Sady B., Leivar P., Quail P.H. (2007). Out of the dark: How the PIFs are unmasking a dual temporal mechanism of phytochrome signalling. J. Exp. Bot. 58: 3125–3133 [DOI] [PubMed] [Google Scholar]
- Monte E., Tepperman J.M., Al-Sady B., Kaczorowski K.A., Alonso J.M., Ecker J.R., Li X., Zhang Y., Quail P.H. (2004). The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 101: 16091–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Zhu L., Shen H., Huq E. (2008). PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc. Natl. Acad. Sci. USA 105: 9433–9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A. (2004). Light-regulated nuclear localization of phytochromes. Curr. Opin. Plant Biol. 7: 708–711 [DOI] [PubMed] [Google Scholar]
- Nozue K., Covington M.F., Duek P.D., Lorrain S., Fankhauser C., Harmer S.L., Maloof J.N. (2007). Rhythmic growth explained by coincidence between internal and external cues. Nature 448: 358–361 [DOI] [PubMed] [Google Scholar]
- Nozue K., Harmer S.L., Maloof J.N. (2011). Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis. Plant Physiol. 156: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Kang H., Yamaguchi S., Park J., Lee D., Kamiya Y., Choi G. (2009). Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Kim J., Park E., Kim J.I., Kang C., Choi G. (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16: 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Yamaguchi S., Hu J., Yusuke J., Jung B., Paik I., Lee H.S., Sun T.P., Kamiya Y., Choi G. (2007). PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19: 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Yamaguchi S., Kamiya Y., Bae G., Chung W.I., Choi G. (2006). Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 47: 124–139 [DOI] [PubMed] [Google Scholar]
- Park E., Kim J., Lee Y., Shin J., Oh E., Chung W.I., Liu J.R., Choi G. (2004). Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 45: 968–975 [DOI] [PubMed] [Google Scholar]
- Quail P.H. (2010). Phytochromes. Curr. Biol. 20: R504–R507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell N.C., Su Y.S., Lagarias J.C. (2006). Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57: 837–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig-Villanova I., Bou J., Sorin C., Devlin P.F., Martínez-García J.F. (2006). Identification of primary target genes of phytochrome signaling. Early transcriptional control during shade avoidance responses in Arabidopsis. Plant Physiol. 141: 85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig-Villanova I., Bou-Torrent J., Galstyan A., Carretero-Paulet L., Portolés S., Rodríguez-Concepción M., Martínez-García J.F. (2007). Interaction of shade avoidance and auxin responses: A role for two novel atypical bHLH proteins. EMBO J. 26: 4756–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.K., Braam J., Fry S.C., Nishitani K. (2002). The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 43: 1421–1435 [DOI] [PubMed] [Google Scholar]
- Salter M.G., Franklin K.A., Whitelam G.C. (2003). Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426: 680–683 [DOI] [PubMed] [Google Scholar]
- Sasidharan R., Chinnappa C.C., Staal M., Elzenga J.T., Yokoyama R., Nishitani K., Voesenek L.A., Pierik R. (2010). Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol. 154: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer E., Nagy F. (2006). Photomorphogenesis in Plants and Bacteria. (Dordrecht, The Netherlands: Springer) [Google Scholar]
- Sentandreu M., Martín G., González-Schain N., Leivar P., Soy J., Tepperman J.M., Quail P.H., Monte E. (2011). Functional profiling identifies genes involved in organ-specific branches of the PIF3 regulatory network in Arabidopsis. Plant Cell 23: 3974–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G., Carabelli M., Sassi M., Ciolfi A., Possenti M., Mittempergher F., Becker J., Morelli G., Ruberti I. (2005). A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev. 19: 2811–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Moon J., Huq E. (2005). PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 44: 1023–1035 [DOI] [PubMed] [Google Scholar]
- Shen H., Zhu L., Castillon A., Majee M., Downie B., Huq E. (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Khanna R., Carle C.M., Quail P.H. (2007). Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145: 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Kim K., Kang H., Zulfugarov I.S., Bae G., Lee C.H., Lee D., Choi G. (2009). Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 106: 7660–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Park E., Choi G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Smith H., Whitelam G.C. (1997). The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 20: 840–844 [Google Scholar]
- Smyth G.K. (2004). Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3: Article 3
- Sorin C., Salla-Martret M., Bou-Torrent J., Roig-Villanova I., Martínez-García J.F. (2009). ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant J. 59: 266–277 [DOI] [PubMed] [Google Scholar]
- Soy J., Leivar P., González-Schain N., Sentandreu M., Prat S., Quail P.H., Monte E. (March 12, 2012). Phytochrome-imposed oscillations in PIF3-protein abundance regulate hypocotyl growth under diurnal light-dark conditions in Arabidopsis. Plant J. http://dx.doi.org/10.1111/j.1365-313X.2012.04992.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm P., Kumar P.P. (2010). The phytohormone signal network regulating elongation growth during shade avoidance. J. Exp. Bot. 61: 2889–2903 [DOI] [PubMed] [Google Scholar]
- Stavang J.A., Gallego-Bartolomé J., Gómez M.D., Yoshida S., Asami T., Olsen J.E., García-Martínez J.L., Alabadí D., Blázquez M.A. (2009). Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 60: 589–601 [DOI] [PubMed] [Google Scholar]
- Steindler C., Matteucci A., Sessa G., Weimar T., Ohgishi M., Aoyama T., Morelli G., Ruberti I. (1999). Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- Stephenson P.G., Fankhauser C., Terry M.J. (2009). PIF3 is a repressor of chloroplast development. Proc. Natl. Acad. Sci. USA 106: 7654–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B., Sánchez-Lamas M., Yanovsky M.J., Casal J.J., Cerdán P.D. (2010). Arabidopsis thaliana life without phytochromes. Proc. Natl. Acad. Sci. USA 107: 4776–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Nakamura S., Mochizuki N., Nagatani A. (2002). Phytochrome in cotyledons regulates the expression of genes in the hypocotyl through auxin-dependent and -independent pathways. Plant Cell Physiol. 43: 1171–1181 [DOI] [PubMed] [Google Scholar]
- Tao Y., et al. (2008). Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman J.M., Hudson M.E., Khanna R., Zhu T., Chang S.H., Wang X., Quail P.H. (2004). Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 38: 725–739 [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Huq E., Rodríguez-Concepción M. (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 107: 11626–11631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F., Vriezen W.H., Smalle J., Laarhoven L.J., Harren F.J., Van Der Straeten D. (2003). Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol. 133: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Vafeados D., Tao Y., Yoshida S., Asami T., Chory J. (2005). A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120: 249–259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.