Abstract
Clinical stage I testicular nonseminomatous germ cell tumours (NSGCTs) are highly curable. Following orchidectomy a risk-adapted approach using active surveillance (AS), nerve-sparing retroperitoneal lymph node dissection (nsRPLND) and primary chemotherapy is recommended by the current guidelines. Clinical stage I is defined as negative or declining tumour markers to their half-life following orchidectomy and negative imaging studies of the chest, abdomen and retroperitoneum. Active surveillance can be performed in low-risk and in high-risk NSGCTs with an anticipated relapse rate of about 15% and 50%. The majority of patients will relapse with good and intermediate prognosis tumours which have to be treated with three to four cycles chemotherapy. About 25–30% of these patients will have to undergo postchemotherapy retroperitoneal lymph node dissection (PC-RPLND) for residual masses. Primary chemotherapy with one or two cycles of cisplatin (Platinol), etoposide and bleomycin (PEB) is a therapeutic option for high-risk clinical stage I NSGCT associated with a recurrence rate of only 2–3% and a minimal acute and long-term toxicity rate. nsRPLND, if performed properly, will cure about 85% of all high-risk patients with clinical stage I NSGCT without the need for chemotherapy. PC-RPLND forms an integral part of the multimodality treatment in patients with advanced testicular germ cell tumours (TGCTs). According to current guidelines and recommendations, PC-RPLND in advanced seminomas with residual tumours is only indicated if a positron emission tomography (PET) scan performed 6–8 weeks after chemotherapy is positive. In nonseminomatous TGCT, PC-RPLND is indicated for all residual radiographic lesions with negative or plateauing markers. Loss of antegrade ejaculation represents the most common long-term complication which can be prevented by a nerve-sparing or modified template resection. The relapse rate after PC-RPLND is around 12%, however it increases significantly to about 45% in cases with redo RPLND and late relapses. Patients with increasing markers should undergo salvage chemotherapy. Only select patients with elevated markers who are thought to be chemorefractory might undergo desperation PC-RPLND if all radiographically visible lesions are completely resectable. PC-RPLND requires a complex surgical approach and should be performed in experienced, tertiary referral centres only.
Keywords: testicular cancer, germ cell tumour, retroperitoneal lymph node dissection, retroperitoneal lymphadenectomy, postchemotherapy RPLND
Introduction
Retroperitoneal lymph node dissection (RPLND) remains an integral part of the treatment of patients with nonseminomatous germ cell tumours (NSGCTs) of the testis. Although its indication in clinical stage I NSGCT has been reduced significantly over the last decade, it is still quite frequently performed in the US as compared with Europe. In patients with residual lesions following systemic chemotherapy for advanced disease, postchemotherapy retroperitoneal lymph node dissection (PC-RPLND) is one of the major components for curative treatment.
It is the aim of the current manuscript to critically review the current recommendations with regard to the role of RPLND in the management of clinical stage I nonseminomas and residual tumours following systemic chemotherapy.
Clinical stage I NSGCTs
The majority of patients with NSGCTs present with stage I disease [Bhardwa et al. 2005]. The standard treatment options of patients with clinical stage I disease remains controversial since patients have an excellent survival with RPLND, active surveillance or primary chemotherapy. The controversy remained over the last two decades since about 30% of all patients will harbour occult microscopic retroperitoneal lymph node metastases which cannot be reliably detected by modern imaging studies, tumour markers or molecular approaches. With RPLND the staging reliability is most accurate, however, about 70% are operated unnecessarily and 10% will develop systemic metastases with need for salvage chemotherapy. With primary chemotherapy approximately 50–70% of the patients are overtreated and might be exposed to unnecessary long-term complications. Active surveillance on the other hand is clearly indicated in low-risk disease with a recurrence rate of only 15%. In patients with high-risk disease the relapse rate varies between 35% and 55% and makes intensive salvage chemotherapy necessary.
Definition of clinical stage I
Clinical stage I is defined by negative imaging studies of the chest, the abdomen and the small pelvis. Furthermore, in order to verify clinical stage I disease elevated markers should be followed postorchidectomy until normalization. Patients without marker normalization or those in whom markers do not decline according to their half-life after orchidectomy do not have stage I disease.
Staging procedures
Recommendations concerning staging investigation are frequently based on low-level evidence rather than on the results of prospective phase III studies.
Computerized tomography (CT) of the chest, abdomen and pelvis are required as initial staging investigations with the mandatory application of oral and intravenous contrast media [Krug et al. 1999; Barentsz et al. 2006]. For the evaluation of the lung and mediastinum, chest CT scan is more sensitive than plain X-ray films [White et al. 1999; Meyer and Conces, 2002]. However, it should be noted that pulmonary/pleural nodules of <1 cm can represent a false-positive finding in CT scans [Meyer and Conces, 2002]. Furthermore, CT scans of the abdomen and pelvis might give false-negative results in up to 30% of cases due to difficulties in the interpretation of lymph nodes based on morphology and size alone [Krug et al. 1999]. Therefore, the differentiation between clinical stages I and IIA might be unreliable. A detailed description of the location, number and size of lymph nodes should be provided in the radiology report. Magnetic resonance tomography (MRT) scans of the abdomen and pelvis do not provide additional information and should be restricted to patients to whom intravenous contrast media cannot be given [Hogeboom et al. 1993]. Based on available data, positron emission tomography (PET) has not been conclusively demonstrated to improve sensitivity over staging by doing CT scanning alone [de Wit et al. 2008; Huddart et al. 2007]. Not even in high-risk stage I patients was PET sensitive enough to predict early metastatic disease in a statistically significant proportion of patients. In the prospective trial TE22 of the MRC, 111 high-risk clinical stage I NSGCT underwent 18F-fluorodeoxyglucose (FDG) PET/CT within 8 weeks after orchidectomy of whom 88 (79%) and 23 (21%) were PET-negative and PET-positive, respectively [Huddart et al. 2007]. A total of 87 PET-negative patients proceeded to active surveillance and within a median follow up of 12 months, 33 (37.9%) relapsed. Since the relapse rate among PET-negative patients is fairly high, it can be concluded that 18F-FDG PET/CT scanning is not sensitive enough to identify patients at low risk of relapse among clinical stage I NSGCT. PET scans are not recommended outside clinical trials as part of routine initial staging procedures.
Prognostic risk factors
Infiltration of venous blood vessels or lymphatic infiltration (vascular invasion [VI]) by the primary tumour are the most important prognostic indicators for occult metastases and must be assessed in all patients [Pont et al. 1990; Read et al. 1992; Heidenreich et al. 1998b; Albers et al. 2003b; Perrotti et al. 2004]. Without adjuvant treatment 48% of the patients with VI will develop metastases while only 14–22% of those without will relapse (EBM IIB: 67). Based on these data, VI alone does not represent a valuable prognostic risk factor for a risk adapted approach since it will result in an unnecessary overtreatment rate of about 50%. The proliferation rate as well as the percentage of embryonal carcinoma (ECA) in relation to the total tumour volume are further prognostic indicators [Heidenreich et al. 1998b; Albers et al. 2003b]. The combination of absence of VI and a percentage of ECA <45% correctly identified 91.5% of all patients with true pathological stage I disease [Heidenreich et al. 1998b]. On the other hand, the presence of VI and a percentage of ECA >80% correctly predicted pathological stage IIA/B disease in 88% of the patients. Based on these data, the German Testicular Cancer Study Group performed a prospective study in which 200 patients with clinical stage I NSGCT were assigned to RPLND and risk factors were assessed prospectively [Albers et al. 2003b]. The combination of absence of VI, percentage of ECA <50% and a MIB-1 proliferating index <20% correctly identified pathological stage II disease with an 86.5% accuracy. If none of the prognostic risk factors were present, the risk of occult retroperitoneal disease was 16% and patients were classified as low risk. The risk of lymph node metastases was 65% if at least VI and percentage ECA >50% were present and the patients were classified as high risk. In another small prospective evaluation, Perotti and colleagues tested a prediction model in which patients with a percentage of ECA >80% and/or the presence of VI were assigned to a high risk of occult metastatic disease [Perotti et al. 2004]. The authors correctly predicted final pathological stage II disease in 67% when only one prognostic factor was present.
The combination of imaging studies, pathohistological evaluation and immunhistochemical staining might improve the prediction of the final pathological stage of the disease. Localization and size of lymph nodes in conjunction with a low volume of ECA, absence of vascular invasion and a low MIB-1 proliferation rate might give important information with regards to the probability of lymph node metastases. In a retrospective analysis of 91 clinical stage 1 NSGCT who underwent nerve-sparing RPLND (nsRPLND), 40 out of 41 patients were correctly classified as low-risk tumours for metastases [Leibovitch et al. 1995a, 1998]. Patients with lymph nodes <1 cm in diameter which are located in the primary landing zone, a low volume of ECA harbours a risk of <10% of occult retroperitoneal lymph node metastases and might be best managed by active surveillance.
Treatment of patients with nonseminoma clinical stage I
If treatment is performed correctly, the cure rate of patients with clinical stage I NSGCT should be 99% regardless of the management chosen. Basically, three treatment options with the same high cure rate but significant differences in frequency and type of treatment-associated toxicities might be offered to the patient: active surveillance, primary chemotherapy with one or two cycles of cisplatin (Platinol), etoposide and bleomycin (PEB) and nsRPLND. When choosing a risk-adapted approach in clinical stage I NSGCT one has to reflect that all of the patients will be long-term survivors so that long-term side effects of treatment should be minimal or nonexistent. Therefore, it is the aim of ongoing research to minimize treatment and toxicities without comprising therapeutic efficacy. According to the most recommendations of the European Germ Cell Cancer Consensus Group Conference (EGCCCG) low-risk patients should be primarily offered active surveillance [Krege et al. 2008], whereas systemic chemotherapy with two cycles of PEB represents the treatment of choice for high-risk patients. Currently, a prospective, randomized phase III trial is ongoing comparing the oncological efficacy and the treatment-associated side effects of two cycles of PEB versus one cycle of PEB in clinical stage I high-risk NSGCT. Reflecting the published new data, both therapeutic approaches might be challenged.
Retroperitoneal lymph node dissection
According to the EGCCCG and the European Association of Urology (EAU) guidelines, active surveillance and primary chemotherapy with two cycles of PEB represent the treatment options of choice in patients with low-risk and high-risk NSGCT, respectively [Krege et al. 2008; Albers et al. 2005]. In patients unwilling to undergo a surveillance strategy or adjuvant chemotherapy, nsRPLND may be performed. Owing to focus of the review article, we only concentrate on the issue of primary RPLND and discuss the pros and cons of the two conservative treatment options.
Primary RPLND is still widely practiced in the United States for high-risk patients although less so in Canada and Europe [Yoon et al. 2005]. RPLND provides accurate staging information regarding retroperitoneal lymph node status. With proper selection of patients for RPLND, two-thirds have low-burden pathologic stage (pS) II disease, and almost 90% will be cured by surgery only [Debono et al. 1997; Stephenson et al. 2005]. The rationale for primary nsRPLND for patients with clinical stage I NSGCT is based on the evidence that it represents the primary metastatic site in more than 80% of patients and that it is also the most frequently involved site of chemoresistant mature teratoma which holds the potential of malignant transformation and late relapse if left unresected [Jewett et al. 1988; Donohue et al. 1993; Baniel et al. 1995]. Virtually all patients who relapse after primary RPLND are chemotherapy naïve and eventually cured by usually three cycles of cisplatin-based chemotherapy. Only a minority of clinical stage I NSGCT harbours occult systemic metastatic disease and might be better managed by inductive systemic chemotherapy. RPLND simplifies follow up and makes it more liberal. Subsequent retroperitoneal relapse is rare, and abdominal imaging can be restricted to one baseline CT a few months after surgery. With the introduction of the nerve-spearing technique along with various modified templates, antegrade ejaculation rates of 90–100% have been reported, with significantly reduced morbidity and virtually unknown mortality [Donohue et al. 1990, 1993; Nicolai et al. 2010]. However, opponents of nsRPLND argue that up to 75% of patients with clinical stage I NSGCT managed by primary RPLND will undergo unnecessary treatment. However, this only holds true if a non-risk-adapted strategy is chosen in every single clinical stage I patient. Recently, patients selection factors on outcome after primary RPLND have been reported and the application of these parameters might allow a risk-adapted indication RPLND [Heidenreich et al. 2003]. The authors analysed a cohort of 453 patients with clinical stage I to IIB NSGCT who underwent RPLND between 1989 and 2002. Of those, 308 (68%) and 122 (27%) presented with clinical stage I and clinical stage IIA disease, respectively. Interestingly the frequency of clinical stage I patients increased significantly over time from 65% to 76% (p = 0.03) in the years 1989 to 1998 and 1999 to 2002, respectively, which might be the result of improved imaging studies. Whereas the frequency of mature teratoma remained fairly constant in the two time periods (22% versus 21%) the number of patients with low volume disease (pN1) increased significantly from 40% to 64% (p = 0.01) so that adjuvant chemotherapy could be spared in more patients. A total of 217 (70%) patients of the 308 clinical stage I NSGCTs demonstrated true pathological stage I disease after RPLND. The 4-year progression-free probability in this cohort was 97%; the risk of systemic progression decreased from 14% before 1999 to 1.3% after 1999 suggesting an improved risk stratification for systemic disease based on the selection criteria developed after critical analysis of the first patient cohort being treated between 1989 and 1999. For clinical stage I NSGCT elevated postorchiectomy tumour markers appear to be associated with a significantly increased risk of progression which was as high as 72%. The question, however, remains if patients with ECA predominance and/or lymphovascular invasion should undergo RPLND or primary chemotherapy due to an anticipated high risk of systemic relapse following locoregional surgical treatment. Stephenson and colleagues analysed the outcome of 267 patients with clinical stage I and clinical stage IIA NSGCTs with one or two of the aforementioned risk factors who underwent nsRPLND [Stephenson et al. 2005]. ECA and VI were present in 31% of the patients and ECA without VI was identified in 10% whereas 58% demonstrated VI without ECA. A total of 129 (66%) patients with clinical stage I and 26 (37%) with clinical stage IIA had pathological stage I disease. A total of 112 patients demonstrated lymph node metastases and 60 (54%) and 52 (46%) demonstrated pN1 and pN2 disease, respectively. The presence of both risk factors was associate with a significantly higher risk of retroperitoneal metastases (54% versus 37%, p = 0.009), however the risk to harbour pN2 disease was not significantly increased. Patients with pathological stage I were followed actively and did not receive adjuvant chemotherapy whereas 22% and 83% of patients with pN1 and pN2 disease received adjuvant cytotoxic treatment with two cycles, respectively. All patients remained disease free during the complete follow-up period. A total of 16% of pS II patients had teratoma in the retroperitoneum which would not have been eliminated by primary chemotherapy. A total of 211 patients did not receive adjuvant chemotherapy and 26 (12.3%) patients experienced relapse with four recurrences developing in the retroperitoneum due to a modified template resection. The 5-year progression-free survival probability including a full bilateral template would be 90%. All relapsing patients could be salvage by four cycles of etoposide and cisplatin (EP) chemotherapy. Summarizing the data of the total cohort of 267 patients, 80 (29.9%) clinical stage I/IIA high-risk patients received either adjuvant or salvage chemotherapy. If only high-risk clinical stage I NSGCTs are considered, an estimated 89% would have been free of progression 5 years after chemotherapy.
In a similar approach, Nicolai and colleagues reviewed their experience of primary RPLND with no adjuvant chemotherapy in a cohort of 322 consecutive clinical stage I NSGCTs who were followed for a median time of 17 years [Nicolai et al. 2010]. A total of 262 (81.4%) patients were staged as pS I whereas 41 (12.7%) and 19 (5.9%) patients demonstrated pS IIA and IIB, respectively; 50 patients (15.5%) developed a recurrence with 96% occurring in the first 2 years of follow up. The majority of relapses (n = 44) were located outside the retroperitoneum, whereas six and four relapses developed in the retroperitoneum and in the contralateral testis. The four testicular relapses have to be considered as de novo tumours resulting from pre-existing testicular intraepithelial neoplasia and should be counted as true recurrences. A total of 271 (84.1%) patients of the total cohort did not experience relapsing disease including 68.3% of the patients with pS IIA/B. Based on multivariate analysis, the presence of VI, percentage of ECA >50%, presence of lymph node metastases increased the probability of relapses by a factor of 2.7, 3.5 and 2.9, respectively.
Rassweiler and colleagues assessed the role of laparoscopic RPLND in the management of clinical stage I NSGCTs reviewing the literature comprising a total of more than 800 patients [Rassweiler et al. 2008]. Whereas no significant differences with regard to complications could be observed when compared with open RPLND, it became evident that more than 90% of patients with positive lymph nodes underwent adjuvant chemotherapy making laparoscopic RPLND to a mere staging surgery. However, the laparoscopic approach is feasible is highly specialized centres, the curative potential of this approach still has to be evaluated.
Although rare with an incidence of only 2–5%, clinical stage I mature teratoma of the testis harbour a risk of about 16% [Leibovitch et al. 1995b; Heidenreich et al. 1997] for retroperitoneal lymph node metastases. The majority of these patients will demonstrate teratomatous elements in the retroperitoneal lymph node metastases, so that nsRPLND represents the treatment of choice.
When discussing nsRPLND as primary treatment option in patients with clinical stage I NSGCT, potential surgery-related complications have to be considered. Quite recently, the German Testicular Cancer Study Group evaluated the outcome of 239 clinical stage I NSGCTs who underwent nsRPLND [Heidenreich et al. 2003]. Minor complications and major complications were observed in 14.2% and in 5.4%, respectively. Antegrade ejaculation could be preserved in 93.3% of the patients and the frequency of ejaculation correlated significantly with the experience of the single surgeon. A total of 14 (0.8%) patients developed relapses with the majority (n = 11) being located in the extraperitoneal areas.
In summary, nsRPLND seems to cure about 85–90% of patients with high-risk clinical stage I NSGCT (Table 1). Whereas 67% of low-risk NSGCTs are overtreated due to true pS I in 70% of the patients and a low systemic recurrence rate of 3%, high-risk patients might benefit from surgery.
Table 1.
Therapeutic burden associated with the different treatment strategies considering the relapse rates given in the most recent series with risk – adapted management calculated per 100 patients.
| Treatment | Therapy at relapse | RPLND/pt | CHT/ pt | Interventions/pt | |||||
|---|---|---|---|---|---|---|---|---|---|
| Surveillance | Pathological stage | Relapse | IGCCCG | CHT | Total number of cycles | ||||
| Low risk | 14% | 12 good 2 intermediate |
12 × 3 2 × 4 |
44 | 0.04 | 0.44 | 0.48 | ||
| High risk | 53% | 45 good 8 intermediate |
45 × 3 8 × 4 |
167 | 0.14 | 1.67 | 1.81 | ||
| nsRPLND | Low risk | PSI = 72% PSII = 28% |
8% | 8 good | 8 × 3 | 24 | 1.0 | 0.24 | 1.24 |
| High risk | PSI = 67% PSII = 33%* |
3% 3% |
3 good 3 good |
3 × 3 8 × 2 2 × 3 |
31 | 1.0 | 0.31 | 1.31 | |
| Primary CHT |
High riska | 1.5% | 2 good | 100 × 2 2 × 3 |
206 | 0.01 | 2.06 | 2.07 | |
| High riskb | 3.2% | 3 good | 100 × 1 3 × 3 |
109 | 0.02 | 1.09 | 1.11 | ||
25/33 patients are pN1 with no need for adjuvant chemotherapy, 8/33 patients are pN2 with the need for two cycles of adjuvant chemotherapy.
Standard approach with two cycles of cisplatin (Platinol), etoposide and bleomycin (PEB).
Minimized approach with one cycle of PEB.
Active surveillance has the lowest therapeutic burden for low-risk patients; active surveillance and primary chemotherapy according to the standard have the highest therapeutic burden for high-risk patients.
RPLND, retroperitoneal lymph node dissection; pt, patient; CHT, chemotherapy; nsRPLND, nerve-sparing RPLND; IGCCCG, .
Postchemotherapy RPLND
Surgical resection of postchemotherapy residual retroperitoneal lymph nodes or residual visceral metastatic deposits represents an integral part of the multimodality treatment for patients with advanced testicular cancer undergoing systemic chemotherapy [Krege et al. 2008; Albers et al. 2005]. The rationale for PC-RPLND is to remove persistent retroperitoneal lymph nodes that may contain mature teratoma in approximately 30–40% and vital cancer in about 10–20% of the patients [Krege et al. 2008; Albers et al. 2004, 2005; Beck and Foster, 2006; Oldenburg et al. 2003].
In NSGCTs, PC-RPLND is currently indicated in men with normalized or plateauing serum tumour markers and residual disease [Krege et al. 2008; Albers et al. 2005]. In patients with residual lesions <1 cm, there is an increased risk of residual teratoma, if teratoma was present in the initial histology so that these patients are further candidates for PC-RPLND [Albers et al. 2004b]. The rationale to resect even small residual masses with mature teratomas lies in their disposition for progressive local growth, their risk of malignant transformation and their risk of late relapse. Residual masses with viable germ cell tumour elements reflect chemoresistance and these lesions will definitely progress when left in situ despite second-line or salvage chemotherapy [Oldenburg et al. 2003]. Patients with normalized serum tumour markers and complete resolution of all metastatic disease do not need to undergo PC-RPLND since only 3–5% of these men will relapse when undergoing active surveillance [Gerl et al. 1997]. In men with residual disease after primary chemotherapy for advanced seminomas PC-RPLND is only indicated if the residual mass is >3 cm in diameter and demonstrates positive findings in the FDG PET scan. In all other cases the masses should not necessarily be resected, but should be closely followed by imaging investigations and tumour marker determinations [Krege et al. 2008].
Although PC-RPLND is a routine surgical intervention in experienced centres its treatment-associated complications might be substantial, since PC-RPLND will require additional surgical procedures of adjacent organs in about 25% of the cases [Krege et al. 2008].
PC-RPLND in advanced seminomas
Following primary cisplatin-based chemotherapy, viable cancer can be demonstrated in about 12–30% of men with residual masses >3 cm and in less than 10% in those with residual masses <3 cm in diameter (Table 2). Following guideline-adapted cytotoxic protocols, however, the incidence of viable cancer in residual seminomatous masses has decreased to 20% irrespective of their size [Flechon et al. 1979; Friedman et al. 1985; Schultz et al. 1989; Fosså et al. 1987; Ravi et al. 1994; Puc et al. 1996; Mosharafa et al. 2003]. Adhering to the former recommendation to resect all residual masses >3 cm diameter would result in an overtreatment rate of 80% without any therapeutic benefit for the patient reducing PC-RPLND to a mere invasive staging procedure. Furthermore, surgical resection residual seminomatous elements is technically challenging due to the severe desmoplastic reaction between the regressing mass and the adjacent vascular and visceral structures. As has been shown in retrospective studies, PC-RPLND in seminomas results in a higher frequency of additional intraoperative procedures and an increased rate of postoperative complications [Mosharafa et al. 2003]. Additional nephrectomy and vascular procedures such as partial or complete resection of the vena cava and placement of aortic prosthesis are necessary in up to 38% of the patients as compared with only about 25% of men undergoing PC-RPLND for advanced NSGCTs.
Table 2.
Residual retroperitoneal masses in advanced seminomas after systemic chemotherapy: tumor size and final histology.
| Study | n | Size | n | PC-RPLND | Viable cancer |
|---|---|---|---|---|---|
| Friedman et al. [1985] | 15 | ≥ 3 cm/< 3 cm | 11/4 | 3/0 | 0 |
| Schultz et al. [1989] | 21 | ≥ 3 cm/< 3 cm | 9/12 | 1/2 | 0 |
| Fosså et al. [1987] | 16 | ≥ 3 cm/< 3 cm | 10/6 | 3/1 | 0 |
| Ravi et al. [1994] | 43 | ≥ 3 cm/< 3 cm | 25/18 | 15/4 | 3/0 |
| Puc et al. [1996] | 104 | ≥ 3 cm/< 3 cm | 30/74 | 27/28 | 6/0 |
| Flechon et al. [1979] | 60 | ≥ 3 cm/< 3 cm | 31/29 | 15/12 | 2/0 |
| Total | 259 | ≥ 3 cm/< 3 cm | 116/143 | 64/47 | 11(17%)/0 |
PC-RPLND, postchemotherapy retroperitoneal lymph node dissection
In order to better select patients who might benefit from PC-RPLND, the role of FDG PET to predict the presence of viable tumour in residual masses of advanced seminomas was prospectively evaluated. After initial positive results [De Santis et al. 2004], studies were expanded to 54 patients with 74 documented residual masses on CT ranging from 1 to 11 cm [Becherer et al. 2005]. After PET scanning the patients either underwent surgery or were followed clinically; any growing lesion was assumed to be malignant whereas regressing lesions or residual masses remaining stable for ≥24 months were considered to contain nonviable elements only. The sensitivity and specificity to detect viability with FDG PET was 80% and 100%, respectively; there was no false-positive scan and there were three false-negative PET scans. In accordance with the current recommendation of the EGCCCG, postchemotherapy as well as postradiotherapy residual masses in seminoma patients should not necessarily be resected, irrespective of their size, but should be closely followed by imaging investigations and tumour marker determinations [Krege et al. 2008; Flechon et al. 1979; Friedman et al. 1985; Schultz et al. 1989; Fosså et al. 1987; Ravi et al. 1994; Puc et al. 1996; Mosharafa et al. 2003; De Santis et al. 2004; Becherer et al. 2005; Albers et al. 2004b; Kamat et al. 1992; Hofmockel et al. 1996; Herr et al. 1997]. In patients with residual lesions <3 cm in size, the use of FDG PET scanning is optional. No resection or any other treatment modality besides further active surveillance is necessary in patients with a negative PET scan, while a positive PET scan, if performed more than 4–6 weeks after day 21 of the last chemo/radiotherapy, is a strong and reliable predictor of viable tumour tissue in patients with residual lesions. In FDG PET positive patient histology should be obtained by biopsy or resection. Further treatment should be based on the results of histology and may include observation, surgery, radiation or further chemotherapy. In patients with progressive disease after first-line chemotherapy, histology should be obtained and salvage chemotherapy given after confirmation of seminoma [Krege et al. 2008; Albers et al. 2005].
PC-RPLND in advanced NSGCTs
In patients who achieve complete remission, i.e. normalized tumour markers and no residual lesions after chemotherapy, postchemotherapy surgery is not required [Krege et al. 2008; Albers et al. 2005; Fossa et al. 1989; Toner et al. 1990]. In patients with any residual mass irrespective of size and normalization of tumour markers the residual masses should be resected [Fossa et al. 1989; Toner et al. 1990; Aprikian et al. 1994; Herr et al. 1997]. Histology of residual masses after first-line chemotherapy will be necrosis, mature teratoma and vital cancer in about 50%, 35% and 15% of patients, respectively.
In patients with residual lesions <1 cm, PC-RPLND also should be strongly considered since it has been shown in various retrospective single-centre analyses that up to 20% and 8% of the patients will harbour mature teratoma and vital cancer despite the small sized lesions [Toner et al. 1990]. There is even an increased risk of residual teratoma, if teratoma was present in the initial histology. If technically feasible, all residual masses should be resected. In persistent retroperitoneal disease, retroperitoneal surgery should include all areas of initial metastatic sites. However, this approach has been challenged by recent retrospective studies from three groups [Kollmannsberger et al. 2010; Ehrlich et al. 2010; Pfister et al. 2011]. Kollmannsberger and colleagues analysed 276 patients who underwent systemic chemotherapy for metastatic NSGCTs [Kollmannsberger et al. 2010]. A total of 161 (58.3%) achieved a complete remission which was defined by the presence of residual lesions <1 cm and all patients were followed without surgical resection. After a mean follow-up of 40 (2–128) months, relapses were observed in 6% of the patients and none of them died after appropriate salvage therapy. However, 94% of the patients belonged to the good prognosis group according to the IGCCCG classification and only 3% belonged to the intermediate and the poor risk group. In a similar approach, Ehrlich and colleagues evaluated 141 patients who were observed after systemic chemotherapy and residual lesions <1 cm [Ehrlich et al. 2010]. After a mean follow up of up to 15 years, 9% of the patients relapsed and 3% of the patients died due to testis cancer. IGCCCG risk group classification predicted the outcome best: recurrence-free survival and cancer-specific survival were 95% and 99%, respectively, in men who belonged to the good risk group whereas it dropped to 91% and 73% if the patients belonged to the intermediate and poor risk group. However, only six out of 12 relapses developed in the retroperitoneum so that only 50% of the patients would have had a potential benefit from PC-RPLND. Quite recently, the German Testicular Cancer Study Group (GTCSG) analysed the outcome of 392 patients who underwent PC-RPLND for residual lesions of any size and they correlated the final pathohistological findings with the size of the residual masses and the IGCCCG risk profile [Pfister et al. 2011]. A total of 9.4% and 21.8% of the men with residual lesions smaller than 1 cm harboured vital cancer and mature teratoma in the resected specimens, respectively. These numbers increased to 21% and 25% in patients with residual lesions of 1–1.5 cm and to 36% and 42% in men with lesions larger than 1.5 cm. The IGCCCG risk profile was not identified as an independent risk to predict the final pathohistology of small residual lesions. The GTCSG drew the conclusion that all patients with any visible residual masses should be resected in a tertiary referral centre.
Considerations for the most appropriate surgical strategy
PC-RPLND is a challenging surgical procedure which requires detailed knowledge of the retroperitoneal anatomy, familiarity with surgical techniques of the vascular and intestinal structures as well as profound experience in the management of patients with testicular cancer. Depending on the size and the extent of the residual lesions, the surgeon has to modify his surgical approach to the retroperitoneal space. An abdominal midline incision from the xyphoid to the symphysis can be used in most patients with unilateral and infrahilar disease (Figure 1(a)–(c)), whereas a Chevron incision might be more suitable in those men with bilateral and suprahilar disease. About 10% of the patients demonstrate persistent retrocrural disease so that a thoracoabdominal approach (Figure 2(a) and (b)) will be best to easily and safely explore this anatomical region [Albers et al. 2004a; Fujioka et al. 1993; Skinner et al. 1982]. In particular, the thoracoabdominal approach needs surgical expertise and knowledge of the retroperitoneal anatomy in order to prevent significant complications. Although the morbidity of PC-RPLND exceeds that of primary nsRPLND, modifications of cytotoxic regimens, the surgical approach, and perioperative care have resulted in a decreased incidence of acute and long-term complications. Owing to the high treatment-related acute morbidity, however, surgery of residual masses should be performed at specialized centres only [Krege et al. 2008; Albers et al. 2005].
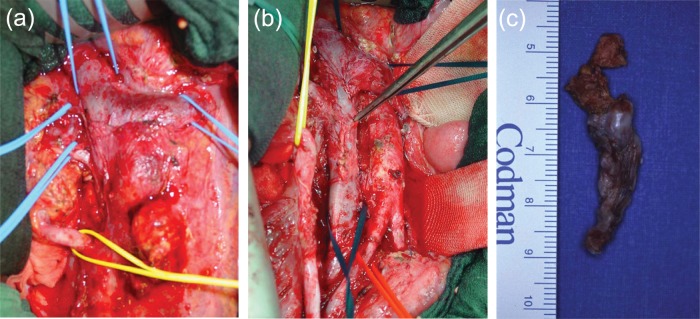
Figure 1.
(a) Retroperitoneal situs after abdominal midline incision demonstrating a large interaortacaval and paraaortal mass. Prior to the resection of the mass all major vessels have been secured. (b) The same situs demonstrating complete resection of the mass. The inferior vena cava had been opened to resect a large intracaval tumour thrombus. (c) Resected intracaval tumour thrombus demonstrating mature teratoma on pathohistological examination.
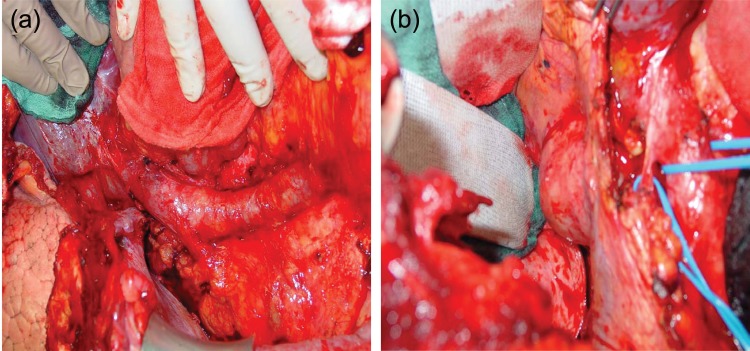
Figure 2.
(a) Intraoperative situs of a patient with a right-sided retrocrural mass after a thoracoabdominal incision: the diaphragm has been incised, the right liver lobe has been completely mobilized via a Langenbeck’s manoeuvre, the suprahilar and infrahilar aspect of the inferior vena cava is exposed. (b) The right-sided retrocrural mass is exposed and completely excised; histology revealed mature teratoma.
In patients with residual masses at multiples sites, an individual decision should be made regarding the number and extension of resections. Decisions on the extent of surgery should be based on the risk of relapse of an individual patient and on quality-of-life issues. Resection of residual tumours outside the abdomen or lung should also be considered on an individual basis, since discordant histology is found in 35–50% of patients [Hartmann et al. 1997]. Pulmonary or mediastinal residual masses harbour necrosis/fibrosis only in 90% if the retroperitoneal masses did not contain mature teratoma or viable cancer. Therefore, these lesions might be managed by surveillance and elective surgery at the time of progression. If, however, pulmonary or mediastinal nodules were found to contain necrosis/fibrosis at primary resection, about 45% of retroperitoneal residual masses will demonstrate a discordant histology so that PC-RPLND is indicated.
Preoperative imaging studies
Prior to PC-RPLND, a complete metastatic and physical evaluation including (1) CT of the chest, the abdomen and the small pelvis about 6–8 weeks after the last cycle of chemotherapy, (2) measurement of the serum tumour markers and (3) pulmonary function testing in men with an increased risk of pulmonary toxicity (four cycles PEB, age >40 years, smoking history, renal insufficiency) should be performed prior to PC-RPLND.
In particular, in patients with large residual masses, imaging studies should allow an adequate assessment of the large retroperitoneal vascular structures since involvement of the inferior vena cava (IVC) and the abdominal aorta can be expected in about 6–10% (Figure 3(a)–(c)) and 2%, respectively [Beck and Lalka, 1998; Beck et al. 2001; Christmas et al. 1998; Heidenreich et al. 1998a, 2011]. Magnetic resonance imaging represents the most appropriate imaging technique to predict infiltrations of the vessel wall and the presence of an intracaval tumour thrombus. Infiltrations of the IVC wall or IVC thrombi should be completely resected since about two thirds of the patients harbour vital cancer or mature teratoma in the infiltrating masses. Usually intraoperative reconstruction or replacement of the IVC is not necessary since chronic venous sequalae are to be expected in less than 5% of all patients [Beck and Lalka, 1998; Heidenreich et al. 1998a, 2011].
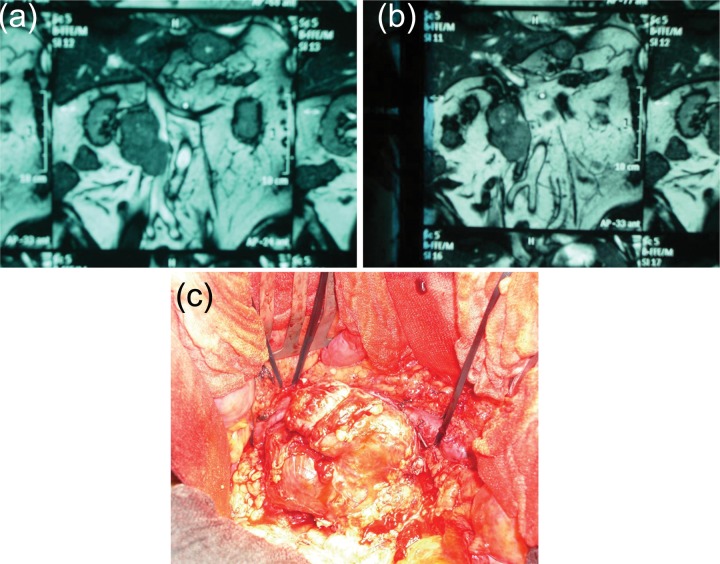
Figure 3.
(a, b) MRI of the retroperitoneum demonstrating a large paracaval mass with compression and potential infiltration of the lateral wall of the inferior vena cava. (c) Intraoperative situs of the same patient demonstrating the extension of the mass with infiltration of the wall of the IVC.
The necessity for aortic replacement is rare and usually accompanied by large residual masses involving additional adjacent structures and making additional surgical procedures necessary such as nephrectomy, IVC resection, small bowel resection and hepatic resection. In the majority of cases mature teratoma or vital carcinoma was identified in the aortic wall [Beck and Lalka, 1998; Beck et al. 2001; Christmas et al. 1998; Heidenreich et al. 1998a, 2011].
Timing of PC-RPLND
Once residual masses have been diagnosed PC-RPLND should be initiated as soon as possible with a complete resection of all retroperitoneal and intraperitoneal masses. Complete resection of residual masses is of very important prognostic significance. In a recent retrospective analysis, Sonneveld and colleagues demonstrated that about 50% of all patients with locoregional recurrences after PC-RPLND had an incomplete resection at time of first surgery [Sonneveld et al. 1998]. Hendry and colleagues retrospectively analysed the outcome of 443 patients undergoing either immediate or elective PC-RPLND once progression of the residual masses was demonstrated [Hendry et al. 2002]. A significant benefit with regard to progression-free survival (83% versus 62%, p = 0.001) and cancer-specific survival (89% versus 56%, p = 0.001) was identified for the immediate surgical approach. Incomplete resection and large size of the residual mass were identified as prognostic risk factors predicting poor outcome. Both parameters were observed more frequently in the group of patients who underwent elective PC-RPLND.
PC-RPLND: extent of surgery
The anatomical extent of PC-RPLND has been discussed controversially for many years. It has been common practice to perform a full bilateral template dissection deriving from experiences of the 1980s when most patients presented with high-volume residual disease when undergoing retroperitoneal surgery. The boundaries of a full bilateral template include the crura of the diaphragm, the bifurcation of the common iliac arteries and the ureters thereby including the primary and secondary landing zones of the right (paracaval, interaortocaval) and the left (paraaortic, preaortic) testicles. Wood and colleagues demonstrated an 8% incidence of contralateral spread among 113 patients with bulky disease undergoing full bilateral PC-RPLND after cisplatin- or carboplatin-based chemotherapy [Wood et al. 1992]. Similarly, Qvist and colleagues and Rabbani and coworkers reported a 5.7% and a 2.6% incidence of teratomatous residues outside the boundaries of a modified template dissection [Qvist et al. 1991; Rabbani et al. 1998]. Nowadays, however, systemic chemotherapy is delivered for relatively low volume retroperitoneal disease (clinical stage IIB) with most metastases being restricted to the primary landing zone of the tumour-bearing testicle. Although the potential of contralateral spread does exist especially from right to left, it is usually not common in low-volume residues questioning the appropriateness of full bilateral dissection for any residual disease. In a retrospective analysis, Aprikian and colleagues analysed the outcome of 40 patients undergoing limited or bilateral radical PC-RPLND [Aprikian et al. 1994]. A limited approach was chosen if intraoperative frozen section analysis (FSA) of the resected mass demonstrated necrosis or fibrosis, whereas a radical RPLND was used in the presence of mature teratoma or viable cancer. A total of 20% of the patients experienced recurrences (14% and 26% in the limited and radical RPLND, respectively) with none of the recurrences located in the retroperitoneum. The authors suggested the use of intraoperative FSA to trigger the most appropriate surgical approach in the clinical scenario of PC-RPLND. Herr analysed the therapeutic outcome of limited versus full bilateral PC-RPLND based on the results of FSA of the resected mass [Herr, 1997]. If FSA demonstrated necrosis, a limited RPLND was performed, in all other cases patients underwent bilateral RPLND. After a median follow up of 6 years, 14 relapses were observed with only two developing in the retroperitoneum; furthermore, six major surgical complications were observed with five after bilateral RPLND. Modified PC-RPLND was considered to be a safe surgical procedure in a well-selected group of patients with advanced testicular cancer. These early retrospective and single-centre studies indicate that a modified PC-RPLND might be a safe approach in men with limited retroperitoneal disease and right/left primary tumours with no evidence of teratoma or viable cancer on frozen section analysis of the residual mass. However, application of the modified unilateral template to PC-RPLND still is discussed controversially among tertiary referral centres based on the 3–8% incidence of mature teratoma or viable cancer in the contralateral landing zone [Ehrlich et al. 2006; Eggener et al. 2007]. Quite recently, two experienced groups, the Indiana group and the GTCSG, reported their experience of patients undergoing modified unilateral template PC-RPLND [Beck et al. 2007; Heidenreich et al. 2009]. The group at Indiana University has performed a limited PC-RPLND in 100 men with low-volume retroperitoneal disease (<5 cm) confined to the primary landing zone of the primary tumour [Beck et al. 2007]. After a mean follow-up of 32 months only four patients relapsed, all outside the boundaries of the modified and even of the bilateral template. The 2-year and 5-year disease-free survival was 95%.
It was the purpose of the GTCSG to assess the oncological necessity of full bilateral PC-RPLND in 152 patients with normalized or plateauing serum tumour markers [Heidenreich et al. 2009]. Depending on the size of the residual mass or the location of the primary testicular tumour a full bilateral template resection (n = 54) or a modified template resection (n = 98) was performed. If patients exhibited a well-defined lesion ≤2 cm modified PC-RPLND was performed, lesions >5 cm were always treated by a full bilateral PC-RPLND. Lesions 2–5 cm in diameter were approached dependent on the site of the primary and the location of the mass: interaortacaval residuals were always approached with a full bilateral PC-RPLND, whereas as paraaortic and paracaval lesions were treated by a modified PC-RPLND if the metastatic site corresponded to site of the primary; otherwise, a full bilateral PC-RPLND was initiated. There were no significant intraoperative complications; there was, however, a significant difference with regard to postoperative morbidity between bilateral and modified PC-RPLND with more complications in patients undergoing extended surgery (p < 0.001). Antegrade ejaculation was preserved in 85% of patients undergoing modified PC-RPLND (see Figure 4) whereas it could not be preserved in 75% of the cases undergoing full bilateral PC-RPLND (p = 0.02), respectively, 8 (5.2%) recurrences were observed after a mean follow up of 39 (6–105) months: one in-field relapse following modified PC-RPLND and seven recurrences outside the boundaries of full bilateral PCRPLND. Two-year disease-free survival was 78.6% and 92.8% for bilateral and modified PCRPLND, respectively. Limitations are a still short follow up, limited number of patients and retrospective nature. There was no significant correlation with extent of surgery and frequency and location of relapses.
Figure 4.
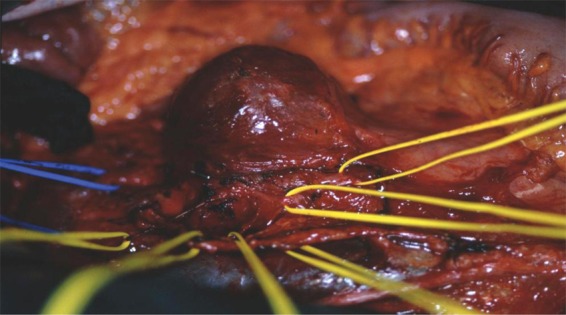
Intraoperative situs of a patient with a large paraaortic residual mass; the sympathetic nerve fibres initially running ventrally to the mass have been isolated (yellow vessel loops) and preserved, the mass was excised completely.
Based on the data presented, full bilateral PC-RPLND is not always required and it should be considered as the surgical approach of choice in patients with extensive residual masses, interaortacaval location or a location of the residual mass not corresponding to the site of the primary testis tumour. In well-defined small masses <5 cm, a modified template RTR does not interfere with oncological outcome but decreases treatment-associated morbidity.
PC-RPLND after salvage chemotherapy or previous retroperitoneal surgery
Patients who have undergone salvage chemotherapy, prior primary or PC-RPLND, those judged to be unresectable and those with disease progression prior to retroperitoneal surgery are at high risk for both a poor therapeutic outcome and an increased frequency of surgery-associated complications. The presence of any one of these poor prognostic parameters increases the risk of relapse from 12% to 45% [Krege et al. 2008; Albers et al. 2005].
The presence of residual tumour masses after salvage chemotherapy is associated with a higher frequency of viable cancer, a higher likelihood of incomplete surgical resection and a higher risk of postoperative relapse as compared with those patients undergoing PC-RPLND after first-line chemotherapy [Her et al. 1997]. Recently, Eggener and colleagues demonstrated that modern chemotherapeutic salvage regimens containing taxanes significantly reduced the presence of viable cancer from 42% to 14% (p = 0.01) when compared with earlier cisplatin-based cytotoxic regimes [Eggener et al. 2007]; the rates of teratoma in the residual tumours was similar with 31% and 33%. They found a 10-year disease-specific survival of 70% so that PC-RPLND even after multiple chemotherapy regimens is indicated if the masses appear to be completely resectable.
Although rare, a subset of patients needs repeat RPLND due to metastatic tumour recurrence after primary RPLND or PC-RPLND because of incomplete tumour resection during initial surgery [Waples and Messing, 1993; Cespedes and Peretsman, 1999; Sexton et al. 2003; McKiernan et al. 2003; Heidenreich et al. 2005]. Repeat RPLND itself represents a poor risk factor associated with a significantly lower 5-year survival rate of only 55% as compared with 86% in the group of patients undergoing adequate PC-RPLND. The long-term outcome after repeat RPLND relies on the complete resection of all residual retroperitoneal masses which will harbour viable cancer and mature teratoma in 20–25% and 35–40%, respectively. Whereas the cure rate for those with mature teratoma only approaches 100%, it decreases significantly to 44% and 20% in the presence of viable cancer and teratoma with malignant transformation, respectively. Repeat RPLND is a challenging surgical procedure associated with higher rates of adjunctive surgical procedures with ipsilateral nephrectomy and vascular procedures being the most frequent adjunctive surgeries.
Repeat RPLND often represents the last chance of cure for patients with in-field recurrences and it usually can be performed with an acceptable morbidity. Repeat RPLND will result in a long-term survival of 67–75%; if patients present with in-field recurrences and elevated markers, systemic chemotherapy followed by PC-RPLND should be initiated. In patients with negative markers, immediate RPLND should be performed since most masses will harbour mature teratoma only.
Desperation PC-RPLND
The term ‘desperation RPLND’ applies to patients with persistently elevated or increasing serum tumour markers after primary inductive chemotherapy or after salvage chemotherapy due to either intrinsic or extrinsic chemoresistance. PC-RPLND in this cohort of patients is associated with a higher frequency of adjunctive surgeries and a poorer outcome. Usually, surgery alone is felt to result in a low likelihood of cure due to widespread systemic disease. However, according to the data of various groups, the 5-year overall survival is 54–67% so that surgery might be indicated in a well-selected subset cohort of patients [Albers et al. 2000; Beck et al. 2005]. In a recent series, increasing preoperative ß-hCG, elevated AFP, redo RPLND and incomplete resection had been identified as negative risk factors associated with a poor survival. Despite elevated serum tumour markers about 45–50% of all patients harbour mature teratoma or necrosis/fibrosis in the surgical specimen resulting in a high cure rate. Patients with elevated but declining serum tumour markers and patients who had received first-line chemotherapy only had the highest likelihood to demonstrate teratoma or necrosis in the resected specimen. On the other hand, patients with incomplete resection demonstrate a poor prognosis and most likely do not benefit from extensive surgery. It is of utmost importance to identify those patients with potentially complete resection of residual masses who might benefit most from immediate surgery.
Adjunctive surgery in patients undergoing PC-RPLND
Additional surgical procedures of adjacent vascular or visceral structures might be necessary in up to 25% of the patients undergoing PC-RPLND (Table 3) in order to achieve complete resection of the residual masses [Nash et al. 1998; Stephenson et al. 2006; Heidenreich et al. 2004]. En bloc nephrectomy represents the most common type of adjunctive surgery for complete tumour clearance. Additional vascular procedures such as aortic replacement and resection of the IVC due to tumour infiltration will be necessary in about 1.5% and 10%, respectively [Beck and Lalka, 1998; Beck et al. 2001; Christmas et al. 1998; Heidenreich et al. 1998, 2011].
Table 3.
Indications for PC-RPLND.
| Indication | |
|---|---|
| Advanced seminoma | Positive FDG PET scan with biopsy proven vital residual cancer which can be completely resected |
| Late relapse | |
| NSGCT | Any residual mass >1 cm diameter and normalized serum tumour markers |
| Any residual mass >1 cm in diameter and plateauing serum tumour markers | |
| Residual masses <1 cm in diameter and mature teratoma in the primary orchiectomy specimen | |
| Marker negative in-field recurrence after prior RPLND | |
| Residual marker negative or plateauing markers after salvage chemotherapy | |
| Desperation RPLND in patients with chemoresistant and completely respectable masses |
PC-RPLND, postchemotherapy retroperitoneal lymph node dissection; FDG PET, fluorodeoxyglucose positron emission tomography; NSGCT, nonseminomatous germ cell tumour.
Complications after PC-RPLND
Whereas the frequency of complications is low in patients undergoing primary nsRPLND for clinical stage I NSGCT [Heidenreich et al. 2003], it increases significantly in PC-RPLND for large-volume residual disease. Although the frequency of associated complications has been decreased in recent series as compared with series of the 1990s, it still approaches 10% [Krege et al. 2008; Albers et al. 2005]. The most common complications include minor complications such as wound infections, paralytic ileus, transient hyperamylasemia, pneumonitis/atelectasis, whereas significant complications such as acute renal failure, chylous ascites, or obstructive ileus develop in less than 2% of the patients [Mosharafa et al. 2004; Leibovitch et al. 2002].
Prediction models
As outlined above, the rationale for PC-RPLND is to remove persistent retroperitoneal disease which may contain mature teratoma or vital cancer. Depending on the size of the lesion, the level of the serum tumour markers prior to chemotherapy and prior to surgery as well as the percentage shrinkage of the mass, about 50–60% of the patients might harbour necrosis/fibrosis making PC-RPLND a mere staging procedure without any therapeutic benefit for the patient.
Based on these data given any prediction model being able to identify those patients who harbour nonmalignant residual retroperitoneal lymph nodes with a high diagnostic accuracy will help to improve the current indications for surgery and it will help to prevent unnecessary treatment-associated acute and long-term toxicity. In the past, several groups have attempted to develop generally applicable prediction models based on numerous data before and after chemotherapy and preoperative parameters [Vergouwe et al. 2001, 2003, 2007; Heidenreich, 2007]. However, basically all of the models were clinically irrelevant due to a false-negative rate of about 20–30% and a diagnostic accuracy of only 70–80% making an individual decision-making process with regard to the necessity of RTR impossible. Recently, the GTCSG has evaluated prognostic risk factors to predict necrosis and fibrosis in patients with residual tumour lesions following inductive chemotherapy for advanced testis cancer [Albers et al. 2004b]. Although alpha-fetoprotein before chemotherapy less than 20 ng/ml, and tumour volume before and after chemotherapy were independently predictive of necrosis, the prediction model resulted in a diagnostic accuracy of only 75% and a false-positive rate of 20%.
Vergouwe and colleagues established another prognostic model to predict necrosis/fibrosis in a large multi-institutional cohort of patients undergoing PC-RPLND [Vergouwe et al. 2001, 2003, 2007]. Benign residual masses were identified in 33% of the cases; 28% of patients with residual masses <1 cm demonstrated either mature teratoma or viable cancer in the residual lymph nodes. On multivariate analysis the absence of mature teratoma in the primary tumour (odds ratio [OR] 4.5 [2.3–8.8]) and a normal AFP serum level (OR 4.4 [2.2–8.7]) were the most predictive parameters for the presence of necrosis while the effects of normal hCG serum levels (OR 1.4 [0.68–2.9]) and standardized lactate dehydrogenase (LDH) serum levels (OR 1.9 [1.2–2.9]) were less impressive. The updated model had a somewhat higher predicted probability of benign tissue of 81% versus 79% using the old model. However, there is still a false-positive rate of about 20% with regard to the correct prediction of necrosis and fibrosis as has been shown for the previous models. The improved but still relatively high false-positive rate of 20% might be due to heterogeneity of the patient cohorts analysed and could be improved very easily. The development data used for the calculation of the updated model resulted from a treatment period between 1977 and 1993, the validation data resulted from a treatment period between 1980 and 1999. Owing to the long time frame, many individual institutional strategies and changes with regard to a risk-adapted chemotherapeutic approach, the chemotherapeutic regimen, number of cycles, close adherence to a 3-week regimen, sensitivity of CT scans, etc. might have contributed to a heterogeneous set of data resulting in a less than optimal prediction model. Therefore, recalculation of the model by recruiting only patients being treated during the last 5 years after the introduction of interdisciplinary evidence-based guidelines will help to improve the prediction model. This work is currently under way by the retrospective analysis of a multi-institutional data bank of the GTCSG.
Furthermore, the integration of molecular markers involved in response or refractoriness to cisplatin-based chemotherapy might improve the current prediction models. The presence of mature teratoma and viable cancer in the residual tumour lesions reflects the differences of intrinsic or extrinsic cisplatin resistance of the different histological subtypes of NSGCT. Recently, some groups have demonstrated that the integration of molecular markers such as bcl-2, mdr-1 and the protein product of mismatch repair genes might increase the diagnostic accuracy of new prediction models [Vergouwe et al. 2001]. In particular, mdr-1 expression in the primary testicular tumour increased the sensitivity and the positive predictive value to accurately identify patients with necrosis/fibrosis from 75% to 85% and from 78% to 86%, respectively. Although elaborative immunohistochemical studies are necessary for this approach, it might be helpful for the individual patient so that the efforts to be taken are well invested to develop prediction models for the individual patient.
Currently, the absence of mature teratoma in the primary testicular lesion, normal preoperative AFP serum levels and the size of the lesion prior to and after chemotherapy are the most useful criteria to identify patients with a potentially benign histology.
Consolidation chemotherapy after secondary surgery
After resection of necrosis or teratoma no further treatment is required. When viable undifferentiated tumour is found, the role of further consolidation chemotherapy is uncertain. A retrospective analysis demonstrated an improved progression-free survival with adjuvant chemotherapy, but failed to show an improvement in overall survival. Therefore a ‘wait-and-watch’ strategy may also be justified [Fizazi et al. 2001, 2008]. Patients in the ‘good’ prognosis group, according to the IGCCCG classification, with complete resection of residual masses and with <10% vital tumour cells in the resected specimens have a favourable outcome even without adjuvant chemotherapy. If completely resected tumour presents >10% of viable cancer, or if completeness of the resection is in doubt, consolidation chemotherapy might be justified.
Summary
The role of primary, nsRPLND in clinical stage I NSGCTs is limited according to the recent guidelines of the EAU and the EGCCCG. However, if performed properly, about 90% of the patients will remain disease free without the need for systemic chemotherapy.
PC-RPLND represents a major part of the interdisciplinary management of advanced TGCT after systemic chemotherapy. In patients with advanced seminomas, PC-RPLND is only indicated if FDG PET scan performed 6–8 weeks after completion of chemotherapy demonstrates positive findings (Table 3). In advanced NSGCT, PC-RPLND should be performed in all patients with residual masses independent of size due to the high frequency of mature teratoma and viable cancer. In patients with left-sided primaries and/or low-volume disease PC-RPLND can be performed within a modified template resection without compromising therapeutic efficacy. Complete resection of all residual masses will result in a long-term disease-free survival of 95%; in patients who undergo desperation surgery long-term cure can be achieved in about 55%. PC-RPLND requires a complex surgical approach and should be performed in experienced, tertiary referral centres only.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Axel Heidenreich, Director and Chairman, EURO Prostate Center, Department of Urology, Urologic Oncology, Pediatric Urology and Renal Transplantation, RWTH University Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany.
David Pfister, Department of Urology, Urologic Oncology, Pediatric Urology and Renal Transplantation, RWTH University Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany.
References
- Albers P., Albrecht W., Algaba F., Bokemeyer C., Cohn-Cedermark G., Horwich A., et al. (2005) Guidelines on testicular cancer. Eur Urol 48: 885–894 [DOI] [PubMed] [Google Scholar]
- Albers P., Ganz A., Hannig E., Miersch W.D., Müller S.C. (2000) Salvage surgery of chemorefractory germ cell tumors with elevated markers. J Urol 164: 381–384 [PubMed] [Google Scholar]
- Albers P., Höltl W., Heidenreich A., Aharinejad S. (2004a) Thoracoabdominal resection of retrocrural residual tumors. Akt Urol 35: 141–150 [DOI] [PubMed] [Google Scholar]
- Albers P., Melchior D., Müller S.C. (2003a) Extensive surgery in testis cancer. Eur Urol 44: 233–244 [DOI] [PubMed] [Google Scholar]
- Albers P., Siener R., Kliesch S., Weissbach L., Krege S., Sparwasser C., et al. for the German Testicular Cancer Study Group (2003a) Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol 21: 1505–1512 [DOI] [PubMed] [Google Scholar]
- Albers P., Weissbach L., Krege S., Kliesch S., Hartmann M., Heidenreich A., et al. for the German Testicular Cancer Study Group (2004b) Prediction of necrosis after chemotherapy of advanced germ cell tumors: results of a prospective multicenter trial of the German Testicular Cancer Study Group. J Urol 171: 1835–1838 [DOI] [PubMed] [Google Scholar]
- Aprikian A.G., Herr H.W., Bajorin D.F., Bosl G.J. (1994) Resection of postchemotherapy residual masses and limited retroperitoneal lymphadenectomy in patients with metastatic testicular nonseminomatous germ cell tumors. Cancer 74: 1329–1334 [DOI] [PubMed] [Google Scholar]
- Baniel J., Foster RS., Einhorn L.H., Donohue J.P. (1995) Late relapse of clinical stage I testicular cancer. J Urol 154: 1370 [PubMed] [Google Scholar]
- Barentsz J., Takahashi S., Oyen W., Mus R., De Mulder P., Reznek R. (2006) Commonly used imaging techniques for diagnosis and staging. J Clin Oncol 24: 3234–3244 [DOI] [PubMed] [Google Scholar]
- Becherer A., De Santis M., Karanikas G., Szabo M., Bokemeyer C., Dohmen B.M., et al. (2005) FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol 54: 284–288 [DOI] [PubMed] [Google Scholar]
- Beck S.D., Foster R.S., Bihrle R., Donohue J.P., Einhorn L.H. (2007) Is full bilateral retroperitoneal lymph node dissection always necessary for postchemotherapy residual tumor? Cancer 110: 1235–1240 [DOI] [PubMed] [Google Scholar]
- Beck S.D., Foster R.S., Bihrle R., Einhorn L.H., Donohue J.P. (2005) Outcome analysis for patients with elevated serum tumor markers at postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol 23: 6149–6156 [DOI] [PubMed] [Google Scholar]
- Beck S.D., Foster R.S., Bihrle R., Koch M.O., Wahle G.R., Donohue J.P. (2001) Aortic replacement during post-chemotherapy retroperitoneal lymph node dissection. J Urol 165: 1517–1520 [PubMed] [Google Scholar]
- Beck S.D., Lalka S.G. (1998) Long-term results after inferior vena cava resection during retroperitoneal lymphadenectomy for metastatic germ cell cancer. J Vasc Surg 28: 808–814 [DOI] [PubMed] [Google Scholar]
- Beck S.D.W., Foster R.S. (2006) Long-term outcome of retroperitoneal lymph node dissection in the management of testis cancer. World J Urol 24: 267–272 [DOI] [PubMed] [Google Scholar]
- Bhardwa J.M., Powles T., Berney D., Baithun S., Nargund V.H., Oliver R.T. (2005) Assessing the size and stage of testicular germ cell tumours: 1984–2003. BJU Int 96: 819–821 [DOI] [PubMed] [Google Scholar]
- Cespedes R.D., Peretsman S.J. (1999) Retroperitoneal recurrences after retroperitoneal lymph node dissection for low-stage nonseminomatous germ cell tumors. Urology 54: 548–552 [DOI] [PubMed] [Google Scholar]
- Christmas T.J., Smith G.L., Kooner R. (1998) Vascular interventions during post-chemotherapy retroperitoneal lymph node dissection for metastatic testis cancer. Eur J Surg Oncol 292–297 [DOI] [PubMed] [Google Scholar]
- Debono D.J., Heilman D.K., Einhorn L.H., Donohue J.P. (1997) Decision analysis for avoiding postchemotherapy surgery in patients with disseminated nonseminomatous germ cell tumours. J Clin Oncol 15: 1455–1464 [DOI] [PubMed] [Google Scholar]
- De Santis M., Becherer A., Bokemeyer C., et al. (2004) 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update from the multicenter SEMPET trial. J Clin Oncol 22: 1034–1039 [DOI] [PubMed] [Google Scholar]
- de Wit M., Brenner W., Hartmann M., Kotzerke J., Hellwig D., Lehmann J., et al. (2008) [18F]-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: results of the German multicentre trial. Ann Oncol 19: 1619–1623 [DOI] [PubMed] [Google Scholar]
- Donohue J.P., Foster R.S., Rowland R.G., Bihrle R., Jones J., Geier G.(1990) Nerve-sparing retroperitoneal lymphadenectomy with preservation of ejaculation. J Urol 144:287. [DOI] [PubMed] [Google Scholar]
- Donohue J.P., Thornhill J.A., Foster R.S., Rowland R.G., Bihrle R. (1993a) Primary retroperitoneal lymph node dissection in clinical stage A nonseminomatous germ cell testis cancer. Review of the Indiana University experience 1965–1989. Br J Urol 71: 326. [DOI] [PubMed] [Google Scholar]
- Donohue J.P., Thornhill J.A., Foster R.S., Rowland R.G., Bihrle R. (1993b) Retroperitoneal lymphadenectomy for clinical stage A testis cancer (1965–1989): modifications of technique and impact on ejaculation. J Urol 149: 237–243 [DOI] [PubMed] [Google Scholar]
- Eggener S.E., Carver B.S., Loeb S., Kondagunta G.V., Bosl G.J., Sheinfeld J. (2007) Pathologic findings and clinical outcome of patients undergoing retroperitoneal lymph node dissection after multiple chemotherapy regimes for metastatic testicular germ cell tumors. Cancer 109: 528–535 [DOI] [PubMed] [Google Scholar]
- Ehrlich Y., Brames M.J., Beck S.D., Foster R.S., Einhorn L.H. (2010) Long-term follow-up of cisplatin combination chemotherapy in patients with disseminated nonseminomatous germ cell tumors: is a postchemotherapy retroperitoneal lymph node dissection needed after complete remission? J Clin Oncol 28: 531–536 [DOI] [PubMed] [Google Scholar]
- Ehrlich Y., Yossepovitch O., Kedar D., Baniel J. (2006) Distribution of nodal metastases after chemotherapy in nonseminomatous testis cancer: a possible indication for limited dissection. BJU Int 97: 1221–1224 [DOI] [PubMed] [Google Scholar]
- Fizazi K., Oldenburg J., Dunant A., Chen I., Salvioni R., Hartmann J.T., et al. (2008) Assessing prognosis and optimizing treatment in patients with postchemotherapy viable nonseminomatous germ-cell tumors (NSGCT): results of the sCR2 international study. Ann Oncol 19: 259–264 [DOI] [PubMed] [Google Scholar]
- Fizazi K., Tjulandin S., Salvioni R., Germà-Lluch J.R., Bouzy J., Ragan D. (2001) Viable malignant cells after primary chemotherapy for disseminated nonseminatous germ cell tumors: prognostic factors and role of postsurgery chemotherapy results from an international study. J Clin Oncol 19: 2647–2657 [DOI] [PubMed] [Google Scholar]
- Flechon A., Bompas E., Biron P., Droz J.P. (1979) Management of post-chemotherapy residual masses in advanced seminoma. J Urol 168: 1975–1979 [DOI] [PubMed] [Google Scholar]
- Fosså S.D., Borge L., Aass N., Johannessen N.B., Stenwig A.E., Kaalhus O. (1987) The treatment of advanced metastatic seminoma: experience in 55 cases. J Clin Oncol 5: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Fossa S.D., Ous S., Lien H.H., Stenwig A.E. (1989) Post-chemotherapy lymph node histology in radiologically normal patients with metastatic nonseminomatous testicular cancer. J Urol 141: 557–559 [DOI] [PubMed] [Google Scholar]
- Friedman E.L., Garnick M.B., Stomper P.C., Mauch P.M., Harrington D.P., Richie J.P. (1985) Therapeutic guidelines and results in advanced seminoma. J Clin Oncol 3: 1325–1332 [DOI] [PubMed] [Google Scholar]
- Fujioka T., Nomura K., Okamoto T., Aoki H., Ohhori T., Kubo T. (1993) Retroperitoneal lymph node dissection for testicular tumors using the thoracoabdominal approach. Int Surg 78: 154–158 [PubMed] [Google Scholar]
- Gerl A., Clemm C., Schmeller N., Hentrich M., Lamerz R., Williams W. (1997) Late relapse of germ cell tumors after cisplatin-based chemotherapy. Ann Oncol 8: 41–47 [DOI] [PubMed] [Google Scholar]
- Hartmann J.T., Candelaria M., Kuczyk M.A., Schmoll H.J., Bokemeyer C. (1997) Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumours. Eur J Cancer 33: 843–847 [DOI] [PubMed] [Google Scholar]
- Heidenreich A. (2007) Residual tumor resection following inductive chemotherapy in advanced testicular cancer. Eur Urol 51: 299–301 [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Albers P., Hartmann M., Kliesch S., Kohrmann K.U., Krege S., et al. for the German Testicular Cancer Study Group (2003) Complications of primary nerve sparing retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell tumors of the testis: experience of the German Testicular Cancer Study Group. J Urol 169: 1710–1714 [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Derakhshani P., Krug B., Engelmann U.H. (1998a) Evaluation of the inferior vena cava by magnetic resonance imaging in advanced testicular germ cell tumors. Eur Urol 49: 196 [Google Scholar]
- Heidenreich A., Dieckmann K.P., Schmelz H., Winter C., Pfister D. (2011) Prognostic clinical parameters to predict the necessity of reconstructive vascular surgery for patients who undergo postchemotherapy retroperitoneal lymph node dissection for advanced nonseminomatous germ cell tumours. Results of the German Testicular Cancer Study Group and the Association of Urologic Oncology. J Urol, AUA abstract # 832 [Google Scholar]
- Heidenreich A., Moul J.W., McLeod D.G., Mostofi F.K., Engelmann U.H. (1997) The role of retroperitoneal lymphadenectomy in mature teratoma of the testis. J Urol 157: 160–163 [PubMed] [Google Scholar]
- Heidenreich A., Ohlmann C., Hegele A., Beyer J. (2005) Repeat retroperitoneal lymphadenectomy in advanced testicular cancer. Eur Urol 47: 64–71 [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Pfister D., Witthuhn R., Thüer D., Albers P. (2009) Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol 55: 217–224 [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Seger M., Schrader A.J., Hofmann R., Engelmann U.H. (2004) Surgical considerations in residual tumor resection following inductive chemotherapy for advanced testicular cancer. Eur Urol 162(Suppl. 3): 632 [Google Scholar]
- Heidenreich A., Sesterhenn I.A., Mostofi F.K., Moul J.W. (1998b) Prognostic factors that identify patients with clinical stage I nonseminomatous germ cell tumors at low risk and high risk for metastasis. Cancer 83: 1002–1011 [PubMed] [Google Scholar]
- Hendry W.F., Norman A.R., Dearnaley D.P., Fisher C., Nicholls J., Huddart R.A., et al. (2002) Metastatic nonseminomatous germ cell tumors of the testis: results of elective and salvage surgery for patients with residual retroperitoneal masses. Cancer 94: 1668–1676 [DOI] [PubMed] [Google Scholar]
- Herr H.W. (1997) Does necrosis on frozen-section analysis of a mass after chemotherapy justify a limited retroperitoneal resection in patients with advanced testis cancer? Br J Urol 80: 653–657 [DOI] [PubMed] [Google Scholar]
- Herr H.W., Sheinfeld J., Puc H.S., Heelan R., Bajorin D.F., Mencel P., et al. (1997) Surgery for a post-chemotherapy residual mass in seminoma. J Urol 157: 860–862 [PubMed] [Google Scholar]
- Hofmockel G., Gruss A., Theiss M. (1996) Chemotherapy in advanced seminoma and the role of postcytostatic retroperitoneal lymph node dissection. Urol Int 57: 38–42 [DOI] [PubMed] [Google Scholar]
- Hogeboom W.R., Hoekstra H.J., Mooyart E.L., Sleijfer D.T., Schraffordt Koops H. (1993) Magnetic resonance imaging of retroperitoneal lymph node metastases of non-seminomatous germ cell tumors of the testis. Eur J Surg Oncol 19: 429–437 [PubMed] [Google Scholar]
- Huddart R.A., O’Doherty M.J., Padhani A., Rustin G.J., Mead G.M., Joffe J.K., et al. For the NCRI Testis Tumour Clinical Study Group (2007) 18fluorodeoxyglucose positron emission tomography in the prediction of relapse in patients with high-risk, clinical stage I nonseminomatous germ cell tumors: preliminary report of MRC Trial TE22–the NCRI Testis Tumour Clinical Study Group. J Clin Oncol 25: 3090–3095 [DOI] [PubMed] [Google Scholar]
- Jewett M.A., Kong Y.S., Goldberg S.D., Sturgeon J.F., Thomas G.M., Alison R.E. (1988) Retroperitoneal lymphadenectomy for testis tumor with nerve sparing for ejaculation. J Urol 139: 1220 [DOI] [PubMed] [Google Scholar]
- Kamat M.R., Kulkarni J.N., Tongoankar H.B., Ravi R. (1992) Value of retroperitoneal lymph node dissection in advanced testicular seminoma. J Surg Oncol 51: 65–67 [DOI] [PubMed] [Google Scholar]
- Kollmannsberger C., Daneshmand S., So A., Chi K.N., Murray N., Moore C., et al. (2010) Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J Clin Oncol 28: 537–542 [DOI] [PubMed] [Google Scholar]
- Krege S., Beyer J., Souchon R., Albers P., Albrecht W., Algaba F., et al. (2008) European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): part I. Eur Urol 53: 478–496 [DOI] [PubMed] [Google Scholar]
- Krug B., Heidenreich A., Dietlein M., Lackner K. (1999) Lymphknotenstaging maligner testikulärer Keimzelltumoren. Fortschr Röntgenstr 171: 87–94 [PubMed] [Google Scholar]
- Leibovitch I., Foster R.S., Kopecky K.K., Donohue J.P. (1995a) Improved accuracy of computerized tomography based clinical staging in low stage nonseminomatous germ cell tumor using size criteria of retroperitoneal lymph nodes. J Urol 154: 1759–1763 [PubMed] [Google Scholar]
- Leibovitch I., Foster R.S., Kopecky K.K., Albers P., Ulbright T.M., Donohue J.P. (1998) Identification of clinical stage A nonseminomatous testis cancer patients at extremely low risk for metastatic disease: a combined approach using quantitative immunohistochemical, histopathologic, and radiologic assessment. J Clin Oncol 16: 261–268 [DOI] [PubMed] [Google Scholar]
- Leibovitch I., Foster R.S., Ulbright T.M., Donohue J.P. (1995b) Adult primary pure teratoma of the testis. The Indiana experience. Cancer 75: 2244–2250 [DOI] [PubMed] [Google Scholar]
- Leibovitch I., Mor Y., Golomb J., Ramon J. (2002) The diagnosis and management of postoperative chylous ascites. J Urol 167: 449–457 [DOI] [PubMed] [Google Scholar]
- McKiernan J.M., Motzer R.J., Bajorin D.F., Bacik J., Bosl G.J., Sheinfeld J. (2003) Reoperative retroperitoneal surgery for nonseminomatous germ cell tumor: clinical presentation, patterns of recurrence and outcome. Urology 62: 732–736 [DOI] [PubMed] [Google Scholar]
- Meyer C.A., Conces D.J. (2002) Imaging of intrathoracic metastases of nonseminomatous germ cell tumors. Chest Surg Clin N Am 12: 717–738 . [DOI] [PubMed] [Google Scholar]
- Mosharafa A.A., Foster R.S., Koch M.O., Bihrle R., Donohue J.P. (2004) Complications of post-chemotherapy retroperitoneal lymph node dissection for testis cancer. J Urol 171: 1839–1841 [DOI] [PubMed] [Google Scholar]
- Mosharafa A.A., Foster R.S., Leibovich C.C., Bihrle R., Johnson C., Donohue J.P. (2003) Is post-chemotherapy resection of seminomatous elements associated with higher acute morbidity? J Urol 169: 2126–2128 [DOI] [PubMed] [Google Scholar]
- Nash P.A., Leibovitch I., Foster R.S., Bihrle R., Rowland R.G., Donohue J.P. (1998) En bloc nephrectomy in patients undergoing post-chemotherapy retroperitoneal lymph node dissection for nonseminomatous testis cancer: indications, implications and outcomes. J Urol 159: 707–710 [PubMed] [Google Scholar]
- Nicolai N., Miceli R., Necchi A., Biasoni D., Catanzaro M., Milani A., et al. (2010) Retroperitoneal lymph node dissection with no adjuvant chemotherapy in clinical stage I nonseminomatous germ cell tumours: long-term outcome and analysis of risk factors of recurrence. Eur Urol [ePub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Oldenburg J., Alfsen G.C., Lien H.H., Aas N., Waehre H., Fossa S.D. (2003) Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol 21: 3310–3317 [DOI] [PubMed] [Google Scholar]
- Perrotti M., Ankem M., Bancilla A., deCarvalho V., Amenta P., Weiss R. (2004) Prospective metastatic risk assignment in clinical stage I nonseminomatous germ cell testis cancer: a single institution pilot study. Urol Oncol 22: 174–177 [DOI] [PubMed] [Google Scholar]
- Pfister D., Busch J., Winter C., Albers P., Schrader M., Dieckmann K.P., et al. (2011) Pathohistological findings in patients with nonseminomatous germ cell tumours who undergo postchemotherapy retroperitoneal lymph node dissection for small tumours. J Urol, AUA abstract # 830 [Google Scholar]
- Pont J., Höltl W., Kosak D., Machacek E., Kienzer H., Julcher H. (1990) Risk-adapted treatment choice in stage I nonseminomatous testicular germ cell cancer by regarding vascular invasion in the primary tumor: a prospective trial. J Clin Oncol 8: 16–19 [DOI] [PubMed] [Google Scholar]
- Puc H.S., Heelan R., Mazumdar M., Herr H., Sheinfeld J.E., Vlasmis V., et al. (1996) Management of residual mass in advanced seminoma: results and recommendations from the Memorial Sloan Kettering Cancer Center. J Clin Oncol 14: 454–460 [DOI] [PubMed] [Google Scholar]
- Qvist H.L., Fossa S.D., Ous S., Hoie J., Stenwig A.E., Giercksky K.E. (1991) Post-chemotherapy tumor residuals in patients with advanced nonseminomatous testicular cancer. Is it necessary to resect all residual masses? J Urol 145: 300–302 [DOI] [PubMed] [Google Scholar]
- Rabbani F., Goldenberg S.L., Gleave M.E., Paterson R.F., Murray N., Sullivan L.D. (1998) Retroperitoneal lymphadenectomy for post-chemotherapy residual masses: is a modified dissection and resection of the residual mass sufficient? Br J Urol 81: 295–300 [DOI] [PubMed] [Google Scholar]
- Rassweiler J.J., Scheitlin W., Heidenreich A., Laguna M.P., Janetschek G. (2008) Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol 54: 1004–1015 [DOI] [PubMed] [Google Scholar]
- Ravi R., Rao R.R., Shanta V. (1994) Integrated approach to the management of patients with advanced germ cell tumors of the testis. J Surg Oncol 55: 47–51 [DOI] [PubMed] [Google Scholar]
- Read G., Stenning S.P., Cullen M.H., Parkinson M.C., Horwich A., Kaye S.B. (1992) Medical Research Council prospective study of surveillance for stage I testicular teratoma. Medical Research Council Testicular Tumors Working Party. J Clin Oncol 10: 1762–1768 [DOI] [PubMed] [Google Scholar]
- Schultz S.M., Einhorn L.H., Conces D.J., Williams S.D., Loehrer P.J. (1989) Management of postchemotherapy residual mass in patients with advanced seminoma: Indiana University experience. J Clin Oncol 7: 1497–1503 [DOI] [PubMed] [Google Scholar]
- Sexton W.J., Wood C.G., Kim R., Pisters L.L. (2003) Repeat retroperitoneal lymph node dissection for metastatic testis cancer. J Urol 169: 1353–1356 [DOI] [PubMed] [Google Scholar]
- Skinner D.G., Melamud A., Lieskovsky G. (1982) Complications of thoracoabdominal retroperitoneal lymph node dissection. J Urol 127: 1107–1110 [DOI] [PubMed] [Google Scholar]
- Sonneveld D.J., Sleijfer D.T., Koops H.S., Keemers-Gels M.E., Molenaar W.M., Hoekstra H.J. (1998) Mature teratoma identified after postchemotherapy surgery in patients with disseminated nonseminomatous testicular germ cell tumors: a plea for an aggressive surgical approach. Cancer 82: 1343–1351 [DOI] [PubMed] [Google Scholar]
- Stephenson A.J., Bosl G.J., Motzer R.J., Kattan M.W., Stasi J., Bajorin D.F., et al. (2005) Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol 23: 2781–2788 [DOI] [PubMed] [Google Scholar]
- Stephenson A.J., Tal R., Sheinfeld J. (2006) Adjunctive nephrectomy at post-chemotherapy retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer. J Urol 176: 1996–1999 [DOI] [PubMed] [Google Scholar]
- Toner G.C., Panicek D.M., Heelan R.T., Geller S.L., Lin S.Y., Bajorin D., et al. (1990) Adjunctive surgery after chemotherapy for nonseminomatous germ cell tumors: recommendations for patient selection. J Clin Oncol 8: 1683–1694 [DOI] [PubMed] [Google Scholar]
- Vergouwe Y., Steyerberg E.W., de Wit R., Roberts J.T., Keizer H.J., Collette L. (2003) External validation of a prediction rule for residual mass histology in testicular cancer: an evaluation for good prognosis patients. Br J Cancer 88: 843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouwe Y., Steyerberg E.W., Foster R.S., Habbema J.D.F., Donohue J.P. (2001) Validation of a prediction model and its predictors for the histology of residual masses in nonseminonatous testicular cancer. J Urol 165: 84–88 [DOI] [PubMed] [Google Scholar]
- Vergouwe Y., Steyerberg E.W., Foster R.S., Sleijfer D.T., Fossa S.D., Gerl A., et al. (2007) Predicting retroperitoneal histology in postchemotherapy testicular germ cell cancer: a model update and multicenter validation with more than 1000 patients. Eur Urol 51: 424–432 [DOI] [PubMed] [Google Scholar]
- Waples M.J., Messing E.M. (1993) Redo retroperitoneal lymphadenectomy for germ cell tumor. Urology 42: 1–4 [DOI] [PubMed] [Google Scholar]
- White P.M., Adamson D.J.A., Howard G.C.W., Wright A.R. (1999) Imaging of the thorax in the management of germ cell testicular tumours. Clin Radiol 54: 207–211 [DOI] [PubMed] [Google Scholar]
- Wood D.P., Herr H.W., Heller G., Vlamis V., Sogani P.C., Motzer R.J., et al. (1992) Distribution of retroperitoneal metastases after chemotherapy in patients with nonseminomatous germ cell tumors. J Urol 148: 1812–1816 [DOI] [PubMed] [Google Scholar]
- Yoon G.H., Stein J.P., Skinner D.G. (2005) Retroperitoneal lymph node dissection in the treatment of low-stage nonseminomatous germ cell tumors of the testicle: an update. Urol Oncol 23: 168. [DOI] [PubMed] [Google Scholar]