Abstract
Cardioprotection, such as preconditioning and postconditioning, have been shown to result in a significant reduction in cell death. Many of the signaling pathways activated by cardioprotection have been elucidated, but there is still a lack of understanding of the mechanisms by which these signaling pathways reduce cell death. Mitochondria have been reported to be an important player in many types of apoptotic and necrotic cell death. If mitochondria play an important role in cell death, then it seems reasonable to consider that cardioprotective mechanisms might act, at least in part, by opposing mitochondrial cell death pathways. One of the major mechanisms of cell death in ischemia-reperfusion is suggested to be the opening of a large conductance pore in the inner mitochondrial membrane, known as the mitochondrial permeability transition pore. Inhibition of this mitochondrial pore appears to be one of the major mechanisms by which cardioprotection reduces cell death. Cardioprotection activates a number of signaling pathways that reduce the level of triggers (reactive oxygen species and calcium) or enhances inhibitors of the mitochondrial permeability transition pore at the start of reperfusion.
I) Introduction
Mitochondria are thought to have entered into a symbiotic relationship with cells many millions of years ago. Mitochondria act as the powerhouse of the cell and supply energy in the form of ATP. However, mitochondria can also play a destructive role and initiate cell death pathways or be a final effector of cell death. Mitochondria have been reported to be an important player in many types of apoptotic and necrotic cell death. If mitochondria play an important role in cell death, then it seems reasonable to consider that cardioprotective mechanisms might act, at least in part, by opposing mitochondrial cell death pathways.
II) Cardioprotection
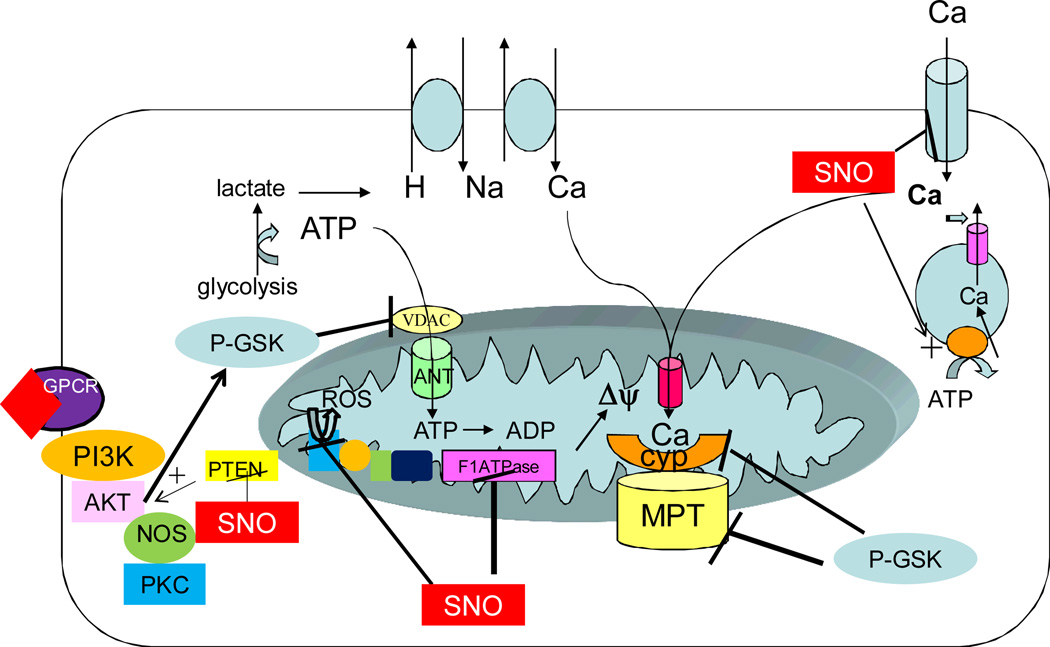
Over the years we have learned a lot about signaling pathways involved in cardioprotection (see [1–3] for recent reviews). Figure 1 summarizes current thoughts regarding cardioprotection. Cardioprotection can occur by signaling pathways initiated prior to or at the beginning of sustained ischemia (e.g.preconditioning (PC)) and cardioprotection can also occur by signaling initiated at the very start of reperfusion (postconditioning). As illustrated in figure 1, preconditioning is thought to lead to release of agonists such as adenosine, bradykinin, or opioids, which bind to G-protein coupled receptors leading to activation of a signaling cascade. A number of signaling molecules have been identified including: phosphatidylinositol 3-kinase (PI3K), protein kinase C-epsilon (PKCε, extracellular regulated kinase (ERK), glycogen synthase kinase- 3 beta (GSK3β, endothelial nitric oxide synthase (eNOS), connexin 43 (Cx43) and the mitochondrial KATP channel [1–6]. P38 MAP kinase has also been suggested to be involved in PC; however its role has been debated[7–9]. The signals generated are proposed to act on the mitochondria where they reduce cell death. Since these signaling pathways signal via phosphorylation and nitric oxide, the role of these two mediators on mitochondrial proteins and function have been extensively studied. Postconditioning has also been shown to activate some of the same signaling pathways as preconditioning [2], although there are some differences in signaling pathways between pre and postconditioning [10],[11]
Figure 1.
Cardioprotection is typically initiated by activation of G-protein coupled receptors, which result in activation of the PI3K signaling pathway. This leads to phosphorylation and activation of AKT and eNOS and activation of PKC and ERK (not shown). Activation of the PI3K pathway also leads to phosphorylation and inactivation of GSK. Inhibition of GSK has been reported to result in a reduction in ATP entry into the mitochondria thus reducing consumption of glycolytically generated ATP, which is consumed by reverse mode of the F1-F0ATPase. Inhibition of GSK has also been reported to inhibit the MPTP, the precise mechanism is unclear, but inhibition of GSK has been reported to inhibit phosphorylation of cyclophilin and to correlate with inhibition of MPTP opening. In the figure p-GSK indicates inactive GSK, which inhibits ATP entry via VDAC and cyclophilin activation of MPTP at least in part by a decrease in phosphorylation. PKC has also been reported to phopshorylated and inhibit BAD, and to phosphorylate and activate mitochondrial aldehyde dehydrogenase (not shown). Activation of NOS leads to generation of nitric oxide that in turn activates guanlyly cyclase that has been reported to activate the mitochondrial K ATP channel (not shown). Nitric oxide also leads to protein S-nitroyslation (SNO) and SNO of PTEN results in its inhibition leading to enhanced signaling via PI3K. SNO of the L-type Ca channel reduces calcium entry resulting in less calcium to enter the mitochondria and thus less calcium activation of the MPTP. SNO inhibits the F1-F0ATPase further reducing consumption of glycolytic ATP. The reduction in ATP consumption reduces lactate generation and thereby reduces calcium entry via Na-Caexchange coupled to Na-H exchange. The inhibition of the F1F0ATPase also result in a more rapid and complete decline in the mitochondrial membrane potential and since the mitochondrial membrane potential is the driving force for calcium entry into the mitochondrial, this will also reduce mitochondrial calcium. SNO of complex I has been shown to reduce generation of ROS. Thus the cardioprotective signaling pathways lead to reduction in calcium and ROS the triggers for MPTP.
III) Mitochondria and Death
It is commonly hypothesized that cardioprotective signals mediate protection by acting on the mitochondria to inhibit mitochondrially mediated cell death[12]. If this hypothesis is correct, an understanding of the mechanisms of cardioprotection is intimately linked to an understanding of the mechanisms by which mitochondria regulate cell death. So what is the role of mitochondria in cell death? The current hypothesis is that necrotic and some forms of apoptotic cell death involve prolonged opening of a large conductance pore in the mitochondria, known as the mitochondrial permeability transition pore (MPTP) [2, 13–16]. In its fully open state, the MPTP has been reported to allow unrestricted movement of solutes of <1.5 kD. The MPTP was originally described in 1970s in studies using isolated mitochondria in which it was shown that an increase in matrix calcium results in a change in mitochondrial conformation from the aggregated to orthodox state and an increase in permeability to sucrose [17–20]. The activation of MPTP in isolated mitochondria was shown to lead to mitochondrial swelling and mitochondrial swelling is thus commonly used as an assay for MPTP. These early studies also reported that the MPTP was sensitive to N-ethylmaleamide (NEM) [20, 21]. Over the ensuing 30 years, a number of activators and inhibitors of MPTP have been described (see [22, 23]).
Although initially some thought the MPTP was a laboratorycuriousity, over the past decade there has been renewed interest in the MPTP due to studies suggesting that the MPTP is involved in cell death. Table 1 summarizes the data supporting a role for the MPTP in cell death. Agents such as cyclosporin, which have been shown to inhibit the MPTP in isolated mitochondria [24–26], also reduce cell death in the setting of ischemia reperfusion injury [27–30]. Ablation of cyclophilin D (CypD), a regulator of the MPTP, reduced infarct size following ischemia reperfusion and reduced calcium activated MPTP in CypD−/− mitochondria [13, 16]. Many factors (high matrixc, reactive oxygen species (ROS), high inorganic phosphate), which have been shown to be activators of MPTP[15, 22], are present at the start of reperfusion. Low pH has also been shown to inhibit the MPTP [31]. It is proposed that the low pH during ischemia keeps the MPTP closed, but that restoration of intracellular and matrix pH at the start of reperfusion, in the presence of other activators of MPTP, results in sustained opening of MPTP. Low pH at the start of reperfusion can reduce ischemia-reperfusion mediated death[32–34].
Table 1.
Inhibition of MPTP Reduces I/R death
In spite of the large amount of interest in the MPTP and its apparent importance in cell death, its molecular identity is unknown. Obviously the lack of knowledge of the make-up of the MPTP is a serious limitation to our understanding of this aspect of cell death. It was proposed that the MPTP was formed by some conformational change in the association of adenine nucleotide translocator (ANT) and voltage dependent anion channel (VDAC) contact sites between the inner and outer mitochondrial membrane. Based on the observation that cyclosporin could inhibit the MPTP by its binding to cyclophilin D [24–26], cyclophilin D was usually placed as a regulator of the MPTP. In support of a role for ANT, inhibitors of ANT can both activate and inhibit MPTP [35]. Cyclophilin D was also reported to bind to ANT, although it has recently been suggested that this may be an artifact based on a non-specific antibody and that the phosphate carrier might be the target of cyclophilin D detected by this antibody[36]. However, recent studies have shown that genetic ablation of either ANT [37] or VDAC [13, 38] isoforms did not eliminate the MPTP, suggesting that neither of these proteins is an obligatory component. Ablation of cyclophilin D did reduce ischemia-reperfusion induced cell death, suggesting a role for cyclophilin in activating the MPTP [13, 16]. Recently Halestrap and coworkers have proposed that the phosphate carrier is a critical component of the MPTP and that the interaction between ANT and the phosphate carrier can modulate MPTP [36, 39]. They show that cyclophilin D can bind to the phosphate carrier and that several inhibitors of the MPTP (NEM and ubiquinone analogues) have a similar concentration dependent inhibition of phosphate transport. An alternative proposed by He et al. suggests that oxidized proteins may misfold in the inner membrane leading to an unregulated channel such as the MPTP [40]. It is suggested that this might explain why ablation of one protein does not lead to loss of MPTP, other oxidized proteins can take their place. A composite hypothesis would be that there is a preferred molecular composition of the MPTP, but if this preferred composition is absent due to genetic deletion of one of the components, other proteins can misfold, leading to a delay in MPTP opening but not permanent elimination of the channel.
The MPTP is a channel in the inner mitochondrial membrane so it seems likely that it involves some inner mitochondrial protein that can span the membrane. This group would include all the inner membrane channels and transporters, such as the ANT, the Pi carrier, the glutamate-aspartate carrier, the calcium uniporter, Carnitine palmitoyltransferase 1 (CPT-1), the Na-Calcium exchanger, Cx 43, etc. It would also include many of the components of the electron transport chain such as the F1-F0ATPase. Recently Bernardi and coworkers have reported that cyclophilin can bind to components of the stalk of the F0ATPase in a phosphate dependent manner [41]. Thus there are a large number of potential candidates. However, Brookes et al have recently reported that uncoupling protein 2 (UCP2) does not appear to serve as MPTP [42]. One challenge is that many mitochondrial channels and transporters have yet to be identified at the molecular level. Based on the inhibitor/activator data the MPTP (or its regulator) is likely to be sensitive to cyclosporin, calcium, NEM, ROS, Pi, pH, and NADH. However it is unclear which effects are attributable to the MPTP itself, and which are due to regulators of the pore.
IV) How might Cardioprotection Reduce Cell Death?
If we assume that activation of the MPTP is the cause of cell death, then how do cardioprotective signals reduce MPTP opening? There are two general mechanisms by which PC might reduce MPTP. PC could act directly on the MPTP to inhibit its opening or PC could reduce the level of triggers of MPTP such as calcium or ROS. These two possibilities are not mutually exclusive and both may contribute.
A) PC reduces levels of triggers of MPTP
MPTP has been shown to open at the start of reperfusion [2, 43–45] and its opening is generally attributed to an increase in matrix calcium and/or ROS. It is therefore possible that cardioprotective signaling works by reducing matrix calcium and/or ROS, rather than by direct modulation of MPTP components. What are the main sources of the rise in matrix calcium and ROS generation at the start of reperfusion? We will discuss these separately.
1) Calcium: It has been shown that PC and other models of cardioprotection reduce the rise in cytosolic calcium during ischemia ([46–48]). There are several mechanisms (reviewed in figure 1) by which PC is proposed to reduce the rise in cytosolic calciumduring ischemia and early reperfusion. As reviewed elsewhere, during ischemia, there is a rise in cytosolic sodium due to a combination of Na entry via persistent sodium channels and Na-H exchange[49]. This rise in sodium results in a rise in calcium via the plasma membrane Na-c exchanger. Calcium can also enter the cell via the L-type Calcium channel [50]. The rise in mitochondrial matrix calcium at the start of reperfusion can be attributed to a rise in Cai that occurs during ischemia as well as at the start of reperfusion. Re-oxygenation at the start of reperfusion allows re-energization of the mitochondria, and restoration of membrane potential, which is the driving force for calcium uptake into the matrix. Thus mitochondria can be a major sink for the rise in calciumat the start of reperfusion. In the next section we will discuss how PC might alter mechanisms responsible for the rise in matrix calcium.
a) PC can reduce the rise in calciumvia the sodium-calcium exchanger (NCX): PC has been shown by a number of investigators to slightly reduce ischemic acidification[47, 51]. This could conceivably make the opening of the MPTP more likely during ischemia, but the reduction in ischemic pH with PC is small (~0.3–4 pH units) and so the pH is still in the range to inhibit the MPTP. However the reduction in hydrogen ion during ischemia would reduce the I/R induced rise in sodium via sodium-proton exchanger (NHE). It has been suggested that a rise in sodium occurs during ischemia and early reperfusion as a result of a combination of Na-H exchange and Na entry via persistent Na channels (see[49]). This increase in sodium will serve as the driving force for reverse mode of Na-Ca exchange resulting in calcium entry into the cytosol. Normally calcium is extruded from myocytes by NCX operating in the forward mode (calciumextrusion mode) using the Na gradient as a driving force. As mentioned, at the start of ischemia, the sodium gradient is reduced and NCX now functions to allow an increase in intracellular calcium. The plasma membrane Ca ATPase is a low capacity transporter and it is likely to be inhibited due to low ATP during ischemia, so it only slowly reduces this rise in cytosolic calcium. Inhibitors of the rise in sodiumduring ischemia and reperfusion have been shown to reduce ischemia-reperfusion injury (see [49]for discussion). PC has been shown not only to reduce ischemic acidosis and/or lactate production, but it has also been shown to reduce the rate of decline in ATP[47, 52]. The combination of reduced lactate production coupled with a reduced rate of fall in ATP suggest that PC reduces the rate of ATP breakdown[52]; the mechanisms responsible are unclear, but one hypothesis is that PC by several possible mechanisms might slow the rate of the reverse mode of the F1-F0ATPase. Normally the protons that are extruded from the matrix during electron transport re-enter the matrix via the F1-F0ATPase and the energy of this reaction is used to drive the synthesis of ATP. However, during ischemia when electron transport is blocked due to lack of oxygen, the mitochondrial membrane potential declines and the F1-F0ATPase can run in reverse, becoming a consumer of ATP[53, 54]. The energy from ATP consumption is used to generate a mitochondrial membrane potential [55]. It had been proposed that PC might directly inhibit the reverse mode of the F1-F0ATPase by more rapid binding of the the inhibitory factor (IF); however measurements of F1-F0ATPase activity in PC hearts showed no difference in activity [56, 57]. However, Sun et al have reported that PC results in S-nitrosylation (SNO) of the F1-F0ATPase, which in turn inhibits its activity [58]. SNO is a labile modification and it is lost during isolation of mitochondria unless care is taken to retain it (e.g. samples are kept in the dark and metals are chelated). Furthermore, SNO is shown to be rapidly reversed during reperfusion making it an ideal modification for temporary inhibition of the F1-F0ATPase. Das et al have also reported that PC mediated inhibition of GSK results in a decrease in phosphorylation of the VDAC which results decrease ATP entry into the matrix via VDAC[59]. During ischemia, ATP is primarily generated in the cytosol via anaerobic glycolysis. This glycolytic ATP can then enter the mitochondria via VDAC and the ANT where is can be consumed by the reverse mode of the F1-F0ATPase. Thus inhibition of F1-F0ATPase and/or ATP entry via VDAC would result in a decrease in the rate of ATP breakdown during ischemia and would reduce generation of protons during ischemia. It has also been suggested that PC can lead to a metabolic slowdown with a slow wake-up [60]. The exact mechanism for this is still somewhat unclear, but it is interesting that many components of the electron transport chain are modified by PC and other cardioprotective mechanisms.
b) PC can reduce Calcium entry via the L-type Ca channel: We have found that PC and other forms of cardioprotection can lead to S-nitrosylation of the L-type Ca channel, which in turn reduces the activity of the L-type Ca channel and thereby reduces calcium entry during ischemia [58, 61]. Consistent with this hypothesis, activation of the L-type Ca channel has also been shown to increase necrotic cell death [50])
c) PC can activate sarcoplasmic-endoplasmic reticulum CaATPase (SERCA): The calcium uptake and release mechanisms of the sarcoplasmic reticulum (SR) are also frequently disrupted during ischemia-reperfusion. Given the close localization of the mitochondria and the SR, altered SR calciumhandling can lead to alterations in mitochondrial calcium (see [62]). Consistent with a role for SR calciumhandling, increasing the expression of the SERCA2a has been shown to reduce infarct size [63]. SERCA is also activated by S-nitrosylation [58].
Taken together, the PC mediated reduction in calcium entry via the plasma membrane NCX and the L-type Ca channel and the increase in calcium uptake into the SR result in less calcium available for transport into the matrix where it can activate the MPTP. Interestingly, Griffiths et al [64] have reported a rise in matrix calcium during ischemia that is suggested to occur due to calcium influx into the matrix on the mitochondrial NCX. Under normal conditions the electrogenic mitochondrial NCX primarily extrudes calcium from the matrix. However, during ischemia when the mitochondrial membrane potential is lost, NCX can lead to an increase in matrix calcium as indicated by the lower matrix calcium during ischemia in the presence of CGP37157, an inhibitor of mitochondrial NCX.
2) Reactive Oxygen Species (ROS): Along with calcium, an increase in ROS is thought to be a primary activator of the MPTP at the start of reperfusion[65, 66]. One protective effect of PC is to reduce ROS production at the start of reperfusion [67]. What are some of the targets of PC that might reduce ROS generation? It is generally thought that ROS generation at the start of reperfusion is primarily mediated by enhanced electron leak in the electron transport chain due to oxidative damage that occurs during ischemia. It has also been suggested, and we have obtained some data to support the concept, that PC might lead to increased SNO of proteins that might protect them from oxidation [58]. We have identified [68]several proteins that show increased SNO and decreased oxidation during ischemia and reperfusion. It is interesting to speculate that PC mediated SNO of these proteins protects them from oxidation and thereby reduces ROS generation at the start of reperfusion. Complex I and Complex III are thought to be important sources for ROS production. Brookes and coworkers have reported that PC results in SNO of complex I which reduces ROS generation during ischemia [69]. ROS generation by monoamine oxidase and shc66 have also been shown to play a role in ROS generation during ischemia and reperfusion [66, 70]. In addition to the electron transport chain, it is becoming clear that lipomamide containing dehydrogenases such as pyruvate dehydrogenase (PDH) and alpha-ketoglutarate dehydrogenase (αKGDH) can be an important contributor to ROS production [71]. SNO and phosphorylation of PDH and αKGDH have been reported to occur with PC[58]; it will be important to establish whether these post translational modifications (PTM) protect these enzymes from oxidation and if they contribute to reduced ROS generation in PC heart.
B) PC acts directly on the MPT to inhibit its opening
If cardioprotective signaling pathways protect by direct inhibition of MPTP, then one would expect that these signaling pathways would modify or alter components or modulators of the MPTP. If this is the case one might expect cardioprotective signaling to result in post translational modifications in mitochondrial proteins that are part of the MPTP. Unfortunately the components of the MPTP have not been identified so there are no clear targets to evaluate for post translational modifications. Thus there are two approaches that one can take to begin to examine whether cardioprotection might alter putative MPTP components. One can take a candidate approach and examine whether cardioprotection results in alterations in proteins that are suggested to be part of the MPTP. Alternatively one can employ unbiased approaches to see if they provide common changes in proteins and might thus provide insight into possible candidates for the MPTP. In either approach, it is useful to consider what criteria one might use in selecting candidates. Genetic ablation of cyclophilin D significantly reduced MPTP[13, 16], and addition of cyclosporin acutely inhibits the MPTP [24, 26, 27]. Thus it is generally assumed that cyclophilin D is likely to bind to at least some of the components of the MPTP. The MPTP has also been shown to be sensitive to NEM and other cysteine modifying agents, so it is likely that the MPTP contains reactive cysteine residues [21, 36]. The MPTP is an inner membrane permeability, so it is also likely that the candidates will span the inner membrane.
1) Candidate approach
The problem with the candidate approach is that the putative components of MPTP are unknown. PC mediated modifications in VDAC and ANT were examined based on the rationale that they were components of the MPTP [72]; however genetic studies have suggested that they are not required MPTP components [37, 38, 73]. Until the components of the MPTP are identified it may be difficult to address whether cardioprotection results in alteration in MPTP components. However, cyclophilin D has been shown to be a regulator of the MPTP, and recent data suggest that GSK can phosphorylate cyclophilin D leading to activation of MPTP [74]. These data are consistent with the observations that cardioprotection is associated with inhibition of GSK [75,77].
2) Unbiased Proteomic Approach
One might consider an alternative approach, that is, using unbiased proteomic approaches to see if alterations in mitochondrial proteins that occur with cardioprotection might provide some insight into components of the MPTP. Indeed, several groups have taken the approach of identifying mitochondrial proteins that are modified by cardioprotection. Arrell et alexamined the effects of the cardioprotective drugs adenosine and diazoxide on the proteome using 2D gel electrophoresis[78]. Arrell et al found consistent differences in 28 protein spots that represented 19 non-redundant proteins in hearts treated with cardioprotective drugs[78]. Adenosine and diazoxide both resulted in an increase in amounts of isocitrate dehydrogenase, NADH ubiquinone oxidoreductase subunit 23 and subunit 24, ATP synthase gamma, and DJ-1 antioxidant protein. Adenosine and diazoxide both resulted in a decrease in metaxin 2. Adenosine and/or diazoxide also resulted in post translational modification (PTM) of four proteins, but only ADP ribosyl hydrolase showed PTM with both adenosine and diazoxide.
Wong et al [79] also studied changes in mitochondrial proteins in the setting of cardioprotection. They used proteomics to examine mitochondrial protein levels/post-translational modifications that were common between two models of cardioprotection, PC and treatment with GSK inhibitors. These studies were done in a Langendorff perfused heart model. Levels of cytochrome c oxidase subunits Va and VIb, ATP synthase-coupling factor 6, and cytochrome b-c1 complex subunit 6 were increased while cytochrome c was decreased in PC and GSK inhibition. Furthermore, with PC and GSK inhibitor treatment the amount of cytochrome c oxidase subunit VIb was found to be increased in mitochondrial supercomplexes, which are comprised of complexes I, III, and IV. This result would suggest that changes in complex subunits associated with cardioprotection may affect supercomplex composition.
Feng et al [80] reported that isoflurane mediated cardioprotection was associated with phosphorylation of the adenine nucleotide translocator on Tyr 194. Mayr et al [81]examined the proteomic difference in mice with constitutively active PKCε. Hearts from mice with constitutively active PKCε exhibit smaller infarcts than WT littermates. Compared to control and hearts with dominant negative overexpression of PKCε (which did not show cardioprotection) Mayr et al found differences in only 2 mitochondrial proteins (mitofilin and manganeses SOD) in hearts from mice with constitutively active PKCε.
In contrast to these studies Clarke et al [82] reported that PC resulted in no significant changes in phosphorylation of the mitochondrial proteome, although they did find changes in carbonylation. It should be noted that in the study by Clarke et al the mitochondria were highly purified. Such purification is important if one wants to establish the mitochondrial location of a protein. Phosphorylation and other post translational modifications could be lost during the purification. This difference might account for some of the differences observed in post translational modification of proteins associated with cardioprotection. However, they did observed phosphorylated mitochondrial proteins, just no change with PC. It is possible the PC mediated phosphorylation is dynamic and quickly lost during the isolation and purification. Additional studies will be needed to resolve this issue.
SNO of several mitochondrial proteins has also been shown with cardioprotection[83, 84]. SNO is the addition of a nitric oxide group to cysteine groups in proteins (see [84] for a review). An increase in nitric oxide, as has been shown to occur during ischemia [85–87], would lead to an increase in SNO. The increase in nitric oxide during ischemia appears to be due to both an increase in NOS activity as well an increase in non-enzymatic production likely via breakdown of nitrite. Interestingly MPTP appears to have several important cysteine residues and is blocked by low levels of NEM [21]. Depending on the nitric oxide level, SNO might react with these cysteines and regulate MPTP activity. Furthermore, SNO is rapidly reversible so that changes would be rapidly reversed on reperfusion. Sun et al reported that cardioprotection by PC or GSNO treatment resulted in an increase in SNO of several mitochondrial proteins including complex I (75KDa subunit), F1-F0ATPase alpha 1 subunit, creatine kinases, acyl CoA dehydrogenase, alpha ketoglutarate dehydrogenase and malate dehydrogenase [58]. Brookes and coworkers showed that PC results in SNO of complex I which they suggest results in less ROS generation in the setting of ischemia and reperfusion[69].
So a number of proteomic changes associated with cardioprotection have been described. Do these changes provide any insight into mechanisms by which cardioprotection might reduce MPTP opening or provide any insight into the identity of the MPTP? A number of changes in mitochondrial proteins have been associated with cardioprotection; however there are no clear consistent changes that suggest an obvious target. It should be noted that due to dynamic range issues, proteomic methods tend to select for high abundance proteins. Thus if the MPTP is a low abundance protein, or if reduced MPTP in cardioprotection is due to alteration in a low abundance regulatory protein, changes in this protein might not be consistently observed in these proteomic studies. Also with the 2D gel methods used in the studies by Arnell et al and Wong et al, membrane proteins are frequently not well resolved. It appears that although general proteomic methods provide information on changes associated with cardioprotection, at present these methods provide no clear consensus regarding the identity of the MPTP. However, newer quantitative, direct mass spectrometry approaches are identifying more low abundance proteins and these newer proteomic approaches may provide insight into the elusive MPTP.
V) Summary and Future Perspective
Cardioprotection activates a number of signaling pathways that reduce the level of triggers or enhances inhibitors of the MPTP at the start of reperfusion. This appears to be one of the major mechanisms by which cardioprotection such as PC reduces cell death. PC has been shown to reduce ROS, calcium and Pi (activators of MPTP) at the start of reperfusion. This hypothesis suggests that PC protects by acting on multiple targets rather than by acting via a linear pathway that leads to one final target. This raises the question of why many inhibitors block the protection. Many of the inhibitors such as inhibitors of PKCε or PI3K appear to act early in the signaling cascade and it appears that PI3K and PKCε activation result in changes in many of the pathways. It is also possible that PC might alter the activity of the MPTP (either directly or via some regulatory protein). However, given the paucity of information about the identity of the MPTP, it is not possible at the present time to determine whether cardioprotection can more directly alter the activity of the MPTP. Thus a major area for future research needs to be directed to identifying the MPTP.
References
- 1.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R. Postconditioning and Protection from Reperfusion Injury : Where Do We Stand? Cardiovasc Res. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 3.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 4.Ardehali H, O'Rourke B. Mitochondrial K(ATP) channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G, Hoppe UC. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest. 2010;120:1441–1453. doi: 10.1172/JCI40927. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Schulz R, Belosjorow S, Gres P, Jansen J, Michel MC, Heusch G. p38 MAP kinase is a mediator of ischemic preconditioning in pigs. Cardiovasc Res. 2002;55:690–700. doi: 10.1016/s0008-6363(02)00319-x. [DOI] [PubMed] [Google Scholar]
- 8.Schneider S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK alpha/beta reduces ischemic injury and does not block protective effects of preconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 9.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 10.Heusch G, Buchert A, Feldhaus S, Schulz R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res Cardiol. 2006;101:354–356. doi: 10.1007/s00395-006-0589-0. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- 13.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 14.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 17.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- 21.Costantini P, Colonna R, Bernardi P. Induction of the mitochondrial permeability transition by N-ethylmaleimide depends on secondary oxidation of critical thiol groups. Potentiation by copper-ortho-phenanthroline without dimerization of the adenine nucleotide translocase. Biochim Biophys Acta. 1998;1365:385–392. doi: 10.1016/s0005-2728(98)00090-5. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 23.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 25.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19:297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 28.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 29.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 30.Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 24:85–87. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halestrap AP. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at pH values below 7. Implications for the protective effect of low pH against chemical and hypoxic cell damage. Biochem J. 1991;278(Pt 3):715–719. doi: 10.1042/bj2780715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 33.Inserte J, Barba I, Hernando V, Abellan A, Ruiz-Meana M, Rodriguez-Sinovas A, Garcia-Dorado D. Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage. Cardiovasc Res. 2008;77:782–790. doi: 10.1093/cvr/cvm082. [DOI] [PubMed] [Google Scholar]
- 34.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 35.Le Quoc K, Le Quoc D. Involvement of the ADP/ATP carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: importance of the orientation of the nucleotide binding site. Arch Biochem Biophys. 1988;265:249–257. doi: 10.1016/0003-9861(88)90125-7. [DOI] [PubMed] [Google Scholar]
- 36.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 40.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 41.Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem. 2009;284:33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brookes PS, Parker N, Buckingham JA, Vidal-Puig A, Halestrap AP, Gunter TE, Nicholls DG, Bernardi P, Lemasters JJ, Brand MD. UCPs--unlikely calcium porters. Nat Cell Biol. 2008;10:1235–1237. doi: 10.1038/ncb1108-1235. author reply 1237-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 44.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res. 1991;68:1250–1258. doi: 10.1161/01.res.68.5.1250. [DOI] [PubMed] [Google Scholar]
- 47.Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- 48.Ylitalo KV, Ala-Rami A, Liimatta EV, Peuhkurinen KJ, Hassinen IE. Intracellular free calcium and mitochondrial membrane potential in ischemia/reperfusion and preconditioning. J Mol Cell Cardiol. 2000;32:1223–1238. doi: 10.1006/jmcc.2000.1157. [DOI] [PubMed] [Google Scholar]
- 49.Murphy E, Allen DG. Why did the NHE inhibitor clinical trials fail? J Mol Cell Cardiol. 2009;46:137–141. doi: 10.1016/j.yjmcc.2008.09.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kida M, Fujiwara H, Ishida M, Kawai C, Ohura M, Miura I, Yabuuchi Y. Ischemic preconditioning preserves creatine phosphate and intracellular pH. Circulation. 1991;84:2495–2503. doi: 10.1161/01.cir.84.6.2495. [DOI] [PubMed] [Google Scholar]
- 52.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 53.Jennings RB, Reimer KA, Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol. 1991;23:1383–1395. doi: 10.1016/0022-2828(91)90185-o. [DOI] [PubMed] [Google Scholar]
- 54.Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol. 2004;287:H1747–H1755. doi: 10.1152/ajpheart.01019.2003. [DOI] [PubMed] [Google Scholar]
- 55.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vander Heide RS, Hill ML, Reimer KA, Jennings RB. Effect of reversible ischemia on the activity of the mitochondrial ATPase: relationship to ischemic preconditioning. J Mol Cell Cardiol. 1996;28:103–112. doi: 10.1006/jmcc.1996.0010. [DOI] [PubMed] [Google Scholar]
- 57.Green DW, Murray HN, Sleph PG, Wang FL, Baird AJ, Rogers WL, Grover GJ. Preconditioning in rat hearts is independent of mitochondrial F1F0 ATPase inhibition. Am J Physiol. 1998;274:H90–H97. doi: 10.1152/ajpheart.1998.274.1.H90. [DOI] [PubMed] [Google Scholar]
- 58.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 59.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 62.Ruiz-Meana M, Abellan A, Miro-Casas E, Agullo E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am J Physiol Heart Circ Physiol. 2009;297:H1281–H1289. doi: 10.1152/ajpheart.00435.2009. [DOI] [PubMed] [Google Scholar]
- 63.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffiths EJ, Ocampo CJ, Savage JS, Stern MD, Silverman HS. Protective effects of low and high doses of cyclosporin A against reoxygenation injury in isolated rat cardiomyocytes are associated with differential effects on mitochondrial calcium levels. Cell Calcium. 2000;27:87–95. doi: 10.1054/ceca.1999.0094. [DOI] [PubMed] [Google Scholar]
- 65.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 67.Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 68.Kohr M, Sun J, Murphy E, Steenbergen C. S-nitrosylation exerts cardioprotection during ischemia-reperfusion (IR) injury by reducing cysteine oxidation. Journal of Molecular and Cellular Cardiology. 2010;48:S100. [Google Scholar]
- 69.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasola A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci U S A. 2010;107:726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 76.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 78.Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res. 2006;99:706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 79.Wong R, Aponte AM, Steenbergen C, Murphy E. Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol. 2010;298:H75–H91. doi: 10.1152/ajpheart.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res. 2008;80:20–29. doi: 10.1093/cvr/cvn161. [DOI] [PubMed] [Google Scholar]
- 81.Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI, Young G, Vondriska TM, Ladroue C, Madhu B, Griffiths JR, Gomes A, Xu Q, Ping P. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J Mol Cell Cardiol. 2009;46:268–277. doi: 10.1016/j.yjmcc.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 84.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 86.Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- 87.Martin C, Schulz R, Post H, Boengler K, Kelm M, Kleinbongard P, Gres P, Skyschally A, Konietzka I, Heusch G. Microdialysis-based analysis of interstitial NO in situ: NO synthase-independent NO formation during myocardial ischemia. Cardiovasc Res. 2007;74:46–55. doi: 10.1016/j.cardiores.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 88.Weinbrenner C, Liu GS, Downey JM, Cohen MV. Cyclosporine A limits myocardial infarct size even when administered after onset of ischemia. Cardiovasc Res. 1998;38:678–684. doi: 10.1016/s0008-6363(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 89.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol. 2009;46:902–909. doi: 10.1016/j.yjmcc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 90.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 91.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 92.West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, Keith RJ, Schrader J, Guo Y, Conklin DJ, Prabhu SD, Zweier JL, Bolli R, Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation. 2008;118:1970–1978. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ladilov Y, Haffner S, Balser-Schafer C, Maxeiner H, Piper HM. Cardioprotective effects of KB-R7943: a novel inhibitor of the reverse mode of Na+/Ca2+ exchanger. Am J Physiol. 1999;276:H1868–H1876. doi: 10.1152/ajpheart.1999.276.6.H1868. [DOI] [PubMed] [Google Scholar]
- 94.Przyklenk K, Hata K, Kloner RA. Is calcium a mediator of infarct size reduction with preconditioning in canine myocardium? Circulation. 1997;96:1305–1312. doi: 10.1161/01.cir.96.4.1305. [DOI] [PubMed] [Google Scholar]
- 95.Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res. 2005;97:916–921. doi: 10.1161/01.RES.0000187456.06162.cb. [DOI] [PubMed] [Google Scholar]
- 96.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]