Abstract
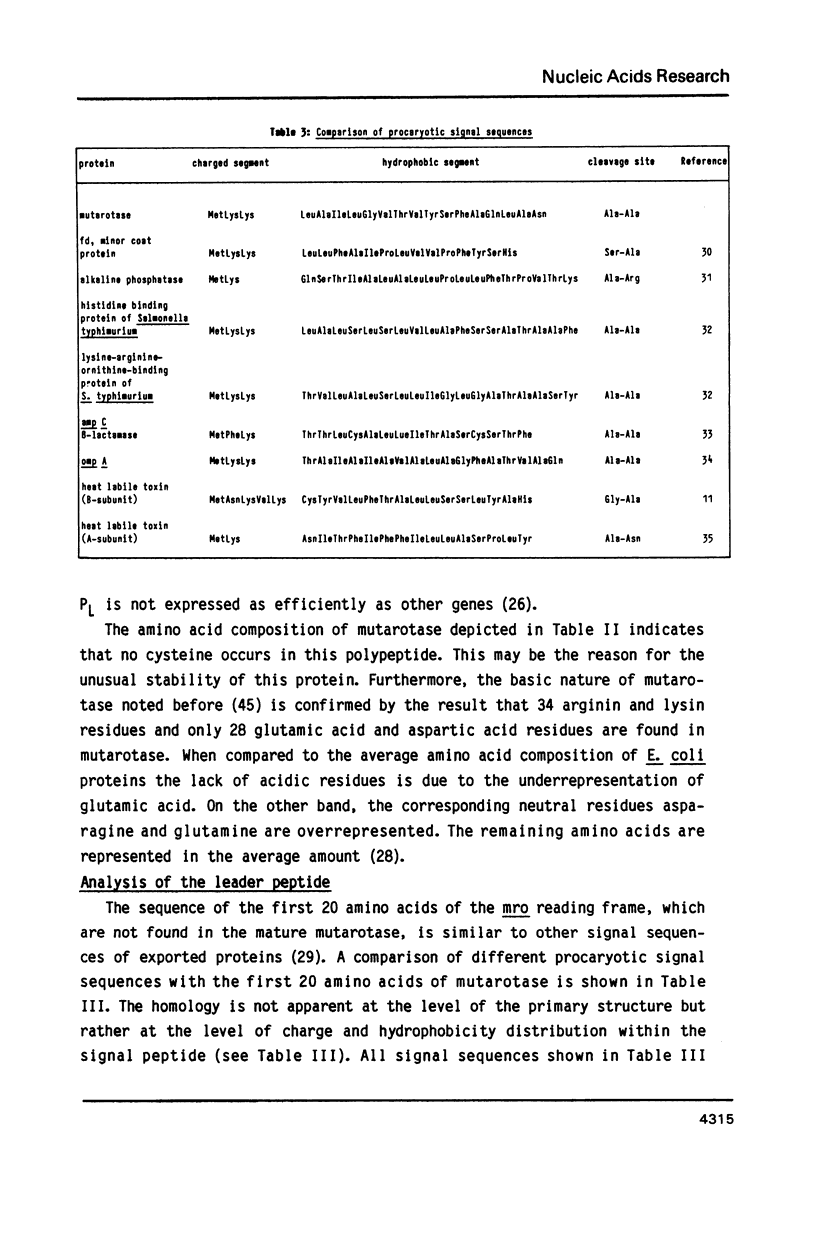
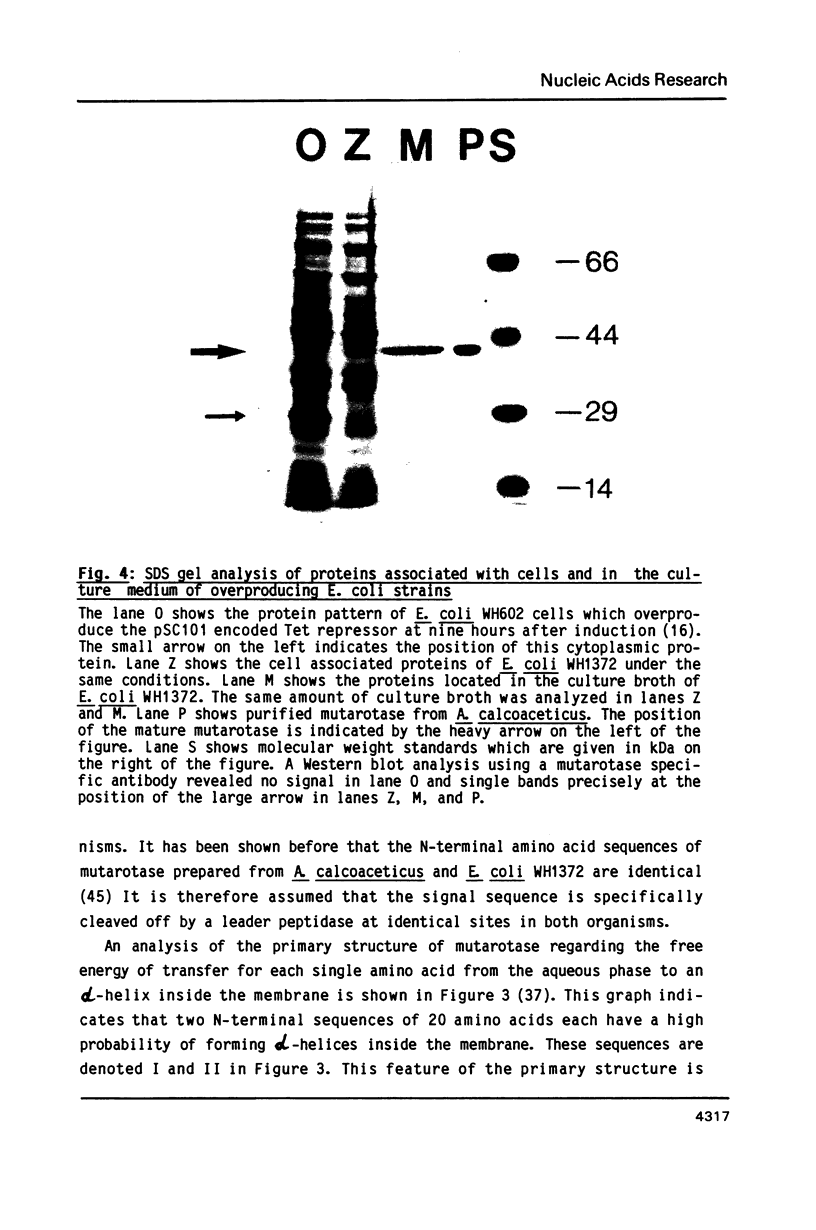
The nucleotide sequence of the mutarotase gene from Acinetobacter calcoaceticus has been determined. It reveals an open reading frame of 381 amino acids. The codon usage of A. calcoaceticus for this gene is similar to E. coli except for the amino acids Leu, Ala, Glu, and Arg where major differences exist. This did not interfere drastically with high level expression in E. coli. The regulatory sequences for the initiation of translation are similar to the ones described for E. coli. The N-terminal 20 amino acids, which are not found in the mature enzyme, show homology to signal sequences of exported proteins. In A. calcoaceticus and E. coli mutarotase is specifically secreted into the periplasmic space. Processing of the signal sequence occurs at identical sites in both organisms. The mature mutarotase consists of 361 amino acids and has a calculated molecular weight of 38457 Da. Expression of mutarotase at a high level in a recombinant E. coli destabilizes the outer membrane. This results in coordinated leakage of mutarotase and beta-lactamase into the culture broth.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banauch D., Brümmer W., Ebeling W., Metz H., Rindfrey H., Lang H., Leybold K., Rick W., Staudinger H. J. Eine Glucose-Dehydrogenase für die Glucose-Bestimmung in Körperflüssigkeiten. Z Klin Chem Klin Biochem. 1975 Mar;13(3):101–107. [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M. Codon frequencies in 119 individual genes confirm consistent choices of degenerate bases according to genome type. Nucleic Acids Res. 1980 May 10;8(9):1893–1912. doi: 10.1093/nar/8.9.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6038–6042. doi: 10.1073/pnas.78.10.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Barnes W., Beckwith J. Signal sequence of alkaline phosphatase of Escherichia coli. J Bacteriol. 1982 Feb;149(2):434–439. doi: 10.1128/jb.149.2.434-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Jakes K. S., Model P. Mechanism of export of colicin E1 and colicin E3. J Bacteriol. 1979 Jun;138(3):770–778. doi: 10.1128/jb.138.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Juni E. Genetics and physiology of Acinetobacter. Annu Rev Microbiol. 1978;32:349–371. doi: 10.1146/annurev.mi.32.100178.002025. [DOI] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Biological catalysis of mutarotation of glucose. Biochem J. 1952 Jan;50(3):341–348. doi: 10.1042/bj0500341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T., Kato C., Horikoshi K. Excretion of the penicillinase of an alkalophilic Bacillus sp. through the Escherichia coli outer membrane. J Bacteriol. 1983 Nov;156(2):949–951. doi: 10.1128/jb.156.2.949-951.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miwa I. Rapid polarographic mutarotase assay with -D-glucose oxidase. Anal Biochem. 1972 Feb;45(2):441–447. doi: 10.1016/0003-2697(72)90205-9. [DOI] [PubMed] [Google Scholar]
- Miwa I., Toyoda Y., Okuda J. Purification and properties of hog kidney mutarotase. Chem Pharm Bull (Tokyo) 1981 Jun;29(6):1702–1707. doi: 10.1248/cpb.29.1702. [DOI] [PubMed] [Google Scholar]
- Mulhern S. A., Fishman P. H., Kusiak J. W., Bailey J. M. Physical characteristics and chemi-osmotic transformations of mutarotases from various species. J Biol Chem. 1973 Jun 25;248(12):4163–4173. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmichen R., Klock G., Altschmied L., Hillen W. Construction of an E. coli strain overproducing the Tn10-encoded TET repressor and its use for large scale purification. EMBO J. 1984 Mar;3(3):539–543. doi: 10.1002/j.1460-2075.1984.tb01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J., Miwa I. Mutarotase effect on micro determinations of D-glucose and its anomers with -D-glucose oxidase. Anal Biochem. 1971 Sep;43(1):312–315. doi: 10.1016/0003-2697(71)90140-0. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U. pUR 250 allows rapid chemical sequencing of both DNA strands of its inserts. Nucleic Acids Res. 1982 Oct 11;10(19):5765–5772. doi: 10.1093/nar/10.19.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- So M., McCarthy B. J. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Kavanaugh W. M., Dallas W. S., Falkow S., Konigsberg W. H., Schafer D. E. Sequence homologies between A subunits of Escherichia coli and Vibrio cholerae enterotoxins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):50–54. doi: 10.1073/pnas.78.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W., Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. doi: 10.1128/jb.144.1.53-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger B., Becker J., Hillen W. Nucleotide sequence of the gene, protein purification and characterization of the pSC101-encoded tetracycline resistance-gene-repressor. Gene. 1984 Nov;31(1-3):103–108. doi: 10.1016/0378-1119(84)90199-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]




