Abstract
STUDY QUESTION
Does follicular flushing during assisted reproductive technologies (ART) improve the number of oocytes retrieved?
SUMMARY ANSWER
Follicular flushing during ART does not result in a greater number of oocytes in normal responders.
WHAT IS KNOWN ALREADY
Despite limited evidence supporting the use of follicular flushing, it continues to be a common procedure in many ART clinics. Prior studies have provided conflicting results regarding the routine use of flushing during oocyte retrieval.
STUDY DESIGN, SIZE, DURATION
Systematic review and meta-analysis of 518 patients who participated in 6 randomized trials over 20 years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Literature searches were conducted to retrieve randomized controlled trials on follicle or ovarian flushing in ART. Databases searched included PubMed, EMBASE, Web of Science and the Cochrane Database of Clinical Trials (CENTRAL). Six trials that included 518 subjects matched the inclusion criteria. Studies included were limited to trials that were published, randomized trials comparing oocyte retrieval with a single-lumen pick-up needle versus follicle flushing after direct aspiration with a multi-channel oocyte pick-up needle in ART patients.
MAIN RESULTS AND THE ROLE OF CHANCE
In each of the trials, measures of the oocyte yield (oocytes retrieved divided by follicles aspirated), total oocytes retrieved, fertilization or pregnancy were not different when comparing direct aspiration with follicle flushing. Four trials reported a higher operative time with follicle flushing. Results of the meta-analysis indicated no significant differences in the oocytes retrieved [weighted mean difference: 0.07, 95% confidence interval (CI): −0.13 to 0.29] or the oocyte yield (odds ratio: 1.06, 95% CI: 0.95–1.18) between the non-flushing and flushing groups.
LIMITATIONS, REASONS FOR CAUTION
All trials featured an open label design and the majority of patients in this meta-analysis were normal responders. The applications of these results to poor responders, patients undergoing natural cycle ART or minimal stimulation ART should be made with caution.
WIDER IMPLICATIONS OF THE FINDINGS
Follicle flushing does not improve ART outcomes in normal-responding patients and should not be performed. This meta-analysis should solidify this recommendation as it includes the largest trial published on the subject and is consistent with a recently published Cochrane review.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported, in part, by the Program in Reproductive and Adult Endocrinology, NICHD, NIH, Bethesda, MD. The authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: follicle flushing, oocyte retrieval, in-vitro fertilization, assisted reproductive technologies
Introduction
Over the past two decades, transvaginal ultrasound-guided follicle aspiration has become the standard procedure for oocyte retrieval during ART, both improving safety and efficacy compared with previous laparoscopic methods (Gembruch et al., 1988; Wiseman et al., 1989). This prompted refinements in the aspiration needle to maximize oocyte recovery (Eisermann et al., 1989; Miller et al., 2004). Double-lumen needles were developed to overcome potential oocyte retention, with one channel for aspiration of the oocyte and the other channel for flushing fluid into the follicle. The theoretical benefit of the double-lumen needle was that the additional flushing of fluid into the follicle would maximize the likelihood of retrieving an oocyte (Hill and Levens, 2010). This concept was initially supported by several non-randomized trials that demonstrated promising results with utilization of follicle flushing (Elhussein et al., 1992; Waterstone and Parsons, 1992; Bagtharia and Haloob, 2005). However, some subsequent randomized studies have failed to corroborate these results (Levens et al., 2009; Haydardedeoglu et al., 2011).
Despite the limited evidence supporting the routine use of follicular flushing (Knight et al., 2001; Hill and Levens, 2010), it continues to be a common procedure in many ART clinics and questions remain regarding the potential benefits of flushing on the oocyte yield and treatment cycle outcomes (Knight et al., 2001; Lozano et al., 2006; Uzelac et al., 2009). The purpose of this systematic review and meta-analysis was to summarize the available published, randomized controlled data regarding the effects of follicle flushing on the oocyte yield and other treatment cycle parameters in ART.
Methods
Literature search
Literature searches were conducted to retrieve randomized controlled trials (RCTs) on follicle or ovarian flushing in ARTs.
Databases searched included PubMed, EMBASE, Web of Science and the Cochrane Database of Clinical Trials. The searches were not limited by language or by date and were executed from 15 to 30 September 2011. References were exported into an endnote bibliographic management database and duplicates were removed. Searches utilized keywords and specific database indexing terminology when available.
The following search strategy was performed: (ovarian follicle[mh] OR ovary OR ovarian OR follicle OR follicular) AND (washing OR flushing) AND (reproductive techniques, assisted[mh] OR ‘embryo transfer’ OR fertilization, in vitro[mh] OR ‘in vitro fertilization’ OR IVF OR ‘intracytoplasmic sperm injection*’ OR ICSI OR ‘artificial insemination’ OR ‘gamete intrafallopian transfer’ OR ‘oocyte donation’ OR ‘oocyte retrieval’ OR ‘ovulation induction’ OR ‘zygote intrafallopian transfer’ OR ‘assisted reproduction’ OR ‘assisted reproductive’) AND (random* OR randomized controlled trial[pt]).
Study selection
Criteria for inclusion in the study were established prior to the literature search. Inclusion was limited to studies that were published RCTs comparing oocyte retrieval with a single-lumen pickup needle (non-flushing group) versus follicle flushing after direct aspiration with a multi-channel oocyte pick-up needle (flushing group) in ART patients. There were no additional inclusion or exclusion criteria pertaining to patient population. Eligible trials were included regardless of the number of follicle flushes performed or the volume of flushing media utilized. Non-randomized trials, trials without a non-flushing control group, studies published as abstracts only and review articles were excluded.
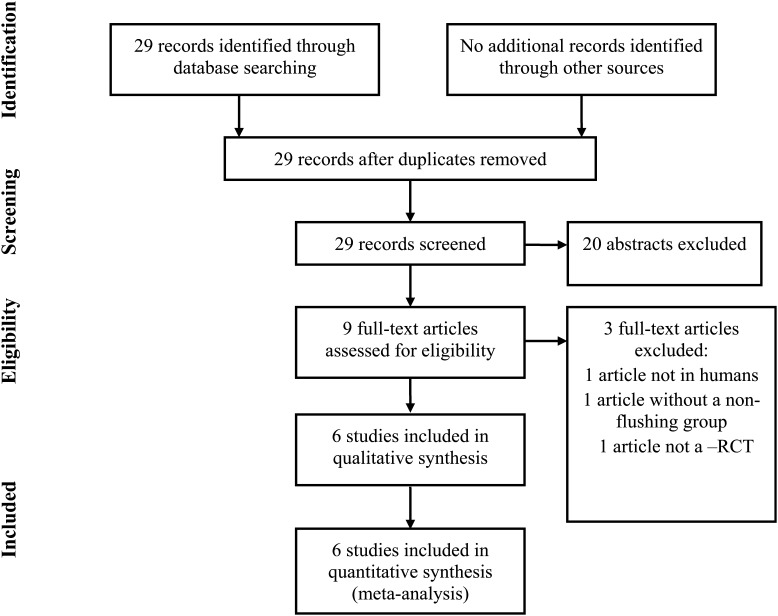
Studies retrieved by the literature search were independently screened for inclusion by two authors (G.L. and M.J.H.), and there was consensus in all studies identified for inclusion. The search strategy yielded 29 records. Searches executed in other databases yielded no additional studies (Fig. 1). All 29 abstracts were reviewed and 20 abstracts were excluded during this review based on clear failure to meet the inclusion criteria. Nine full text papers were evaluated further for inclusion and exclusion criteria. Of these, six papers met the full inclusion criteria. One article was excluded as the study was conducted in cattle, one article was excluded for not having a non-flush group and one article was excluded for not being an RCT. Two authors (G.L. and M.J.H.) were involved in all aspects of abstract and manuscript review and final study selection. Risks of bias within each study was also ascertained by the same two authors, specifically evaluating the randomization method, group allocation, concealment and the flow of patients through the study following the methodology established by Jadad et al. (1996).
Figure 1.
PRISMA four-phase flow diagram of search yield, screening and inclusion steps.
Data collection
Data were abstracted in parallel by two authors (G.L. and M.J.H.). Dichotomous outcomes data (live birth per retrieval and oocyte yield) were extracted from the source papers in the form of 2 by 2 tables. Continuous data (oocytes retrieved and procedure time) were extracted in the form of mean, standard deviation and population size. Additional extracted data included: author, year of publication, journal, randomization method, group concealment and allocation methods, the number of patients randomized to non-flush or follicle flushing intervention, volume of flushing media utilized per follicle, number of flushes allowed per follicle and fertilization. The primary outcome was the oocyte yield. Secondary outcomes included oocytes retrieved, oocyte retrieval procedure time, fertilization, clinical pregnancy, ongoing pregnancy and live birth. Outcomes were measured on a per patient basis.
Data synthesis (Moher et al., 1999)
Heterogeneity was evaluated using the Q test and I2 index values and reported for each outcome as P-value and percentage, respectively (Higgins et al., 2009). If these tests indicated the presence of heterogeneity, a random-effects model was used for analysis. If these tests indicated a lack of heterogeneity, a fixed-effects model was used. Bias was assessed at the study level using a qualitative review assessing randomization, concealment, blinding and patient flow. Publication bias was assessed at the outcome level by visual inspection of funnel plots. Dichotomous outcome data were reported as odds ratios (ORs) with 95% confidence intervals (CIs) and continuous data were synthesized using weighted means with 95% CIs. An a priori subgroup analysis of normal-responding and poor-responding ART patients was performed. As the systematic review included two studies published from 2009 to 2012 and four studies published from 1988 to 1992, a post hoc subgroup analysis of studies published recently and studies published remotely was also performed.
Data collection was performed in Excel (Microsoft Office, 2007) and statistical analysis was performed using Mix 2.0 Pro (Bax L: MIX 2.0; professional software for meta-analysis in Excel, version 0.0.1.4. BiostatXL, 2011: http://www.meta-analysis-made-easy.com). Data were analyzed per patient randomized. This study was exempted by the Institutional Review Board (IRB) given the nature of the work.
Results
Selection of studies for meta-analysis
A total of 29 abstracts were identified, 9 full text articles were reviewed and 6 trials met the inclusion criteria (Fig. 1; Haines et al., 1989; Scott et al., 1989; Kingsland et al., 1991; Tan et al., 1992; Levens et al., 2009; Haydardedeoglu et al., 2011). These studies represented a total of 518 ART cycles in 518 patients with randomization to direct aspiration with a single-lumen oocyte pick-up needle (non-flushing group) versus follicle flushing after direct aspiration with a double-lumen oocyte retrieval needle (flushing group). One study was performed in poor responders only, defined as fewer than 8 follicles >12 mm on the day of hCG administration (Levens et al., 2009). Another trial excluded poor responders, defined as patients with fewer than 6 follicles <12 mm on the day of hCG administration (Haydardedeoglu et al., 2011). Of the 518 ART cycles included in the meta-analysis, 488 were performed in a general, normal-responding ART population.
Assessment of bias within each individual trial was performed by qualitative evaluation of randomization, concealment, allocation and patient flow. Three of the trials failed to report on the method of randomization, concealment, allocation or patient flow (Haines et al., 1989; Scott et al., 1989; Kingsland et al., 1991), but were included in the meta-analysis as the methods sections clearly stated that the trials were prospective and randomized. Tan et al. (1992) adequately described their method of randomization, but failed to describe concealment, allocation or patient flow. Both Levens et al. (2009) and Haydardedeoglu et al. (2011) adequately described randomization, concealment and allocation, and the studies had no patient drop-out. Five of the trials were non-blinded study designs with neither patients nor providers blinded to group allocation (Haines et al., 1989; Scott et al., 1989; Kingsland et al., 1991; Tan et al., 1992; Haydardedeoglu et al., 2011). Levens et al. (2009) was the only study that employed a blinding scheme. The physicians performing the oocyte retrieval were blinded to the number of oocytes retrieved until the completion of the surgery and the embryologist evaluating the oocytes was blinded to patient group assignment (Levens et al., 2009). Visual inspection of funnel plots did not suggest publication bias.
Comparison of oocyte yield
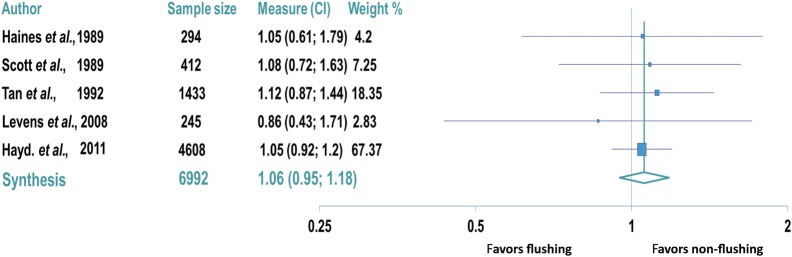
Five trials reported on the oocyte yield (oocytes retrieved divided by follicles aspirated) for a total of 6992 follicles aspirated (Haines et al., 1989; Scott et al., 1989; Tan et al., 1992; Levens et al., 2009; Haydardedeoglu et al., 2011). In all five trials, no differences were observed between the oocyte yields in the flushing and non-flushing groups (Table I). No difference in the oocyte yield was also observed in the trial evaluating poor responders (non-flushing group: 83% versus flushing group: 85%, P= 0.70; Levens et al., 2009). No significant heterogeneity was suggested by results of the Q test (P= 0.97) and the I2 index (I2 value= 0%). Therefore, a fixed effects model was used for meta-analysis of these studies to evaluate the oocyte yield. No difference was observed in the oocyte yield between the non-flushing and flushing groups (OR: 1.06, 95% CI: 0.95–1.18; Fig. 2).
Table I.
Description and results of the six trials included in the meta-analysis.
| Study |
Patients (n) |
Flushing |
Oocyte yield (%) |
Mean oocytes retrieved (n) |
Fertilization (%) |
Procedure time (min) |
Pregnancy (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Flush | No flush | Volume of flush (ml) | Number of flushes | Flush | No flush | Flush | No flush | Flush | No flush | Flush | No flush | Flush | No flush |
| Haines et al. | 1989 | 18 | 18 | NR | Up to 5 | 75 | 76 | 7.1 | 8.8 | 64 | 70 | NR | NR | NR | NR |
| Scott et al. | 1989 | 22 | 22 | 1-3 | No limit | 63 | 65 | 5.9 | 6.3 | NR | NR | NR | NR | NR | NR |
| Kinglsand et al. | 1991 | 18 | 16 | 2 | Up to 5 | NR | NR | 7 | 8.5 | 64 | 60 | 35 | 20a | 3b | 3b |
| Tan et al. | 1992 | 50 | 50 | 1.5 | Up to 6 | 77 | 77 | 9 | 11 | 60 | 56 | 30 | 15a | 13c | 12c |
| Levens et al | 2009 | 15 | 15 | 2 | 1 | 85 | 83 | 7.2 | 6.5 | 57 | 67 | 6 | 3a | 40b | 20b |
| Haydardedeoglu et al. | 2011 | 149 | 125 | 2 | 1 | 75 | 75 | 12.2 | 13.0 | 69 | 67 | 12 | 8a | 39d | 38d |
NS, not statistically significant. NR, not reported.
aP-value <0.05.
bOngoing pregnancy (%).
cClinical pregnancy (%).
dLive birth (%).
Figure 2.
Forest plot comparing oocyte yield (oocytes retrieved/follicles aspirated) in the flushing and non-flushing groups. Data reported per oocyte aspirated.
Comparison of oocytes retrieved
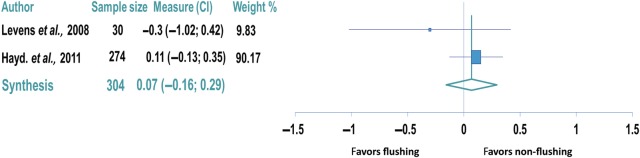
There were no significant differences in the number of oocytes retrieved between the two groups in any of the individual studies (Table I). There was also no difference in the number of oocytes retrieved in the flushing group in the trial evaluating poor responders (non-flushing group: 6.5 oocytes versus flushing group: 7.2 oocytes, P= 0.38; Levens et al., 2009). Only two studies adequately reported data on oocytes retrieved to allow for statistical synthesis comprising 304 ART cycles (Levens et al., 2009; Haydardedeoglu et al., 2011). The other studies did not report standard deviations, which did not allow statistical inclusion of the data into the meta-analysis. Meta-analysis of oocytes retrieved showed no difference between the non-flushing and flushing groups (weighted mean difference: 0.07, 95% CI: −0.13 to 0.29; Fig. 3).
Figure 3.
Forest plot comparing the number of oocytes retrieved in the flushing and non-flushing groups. Data reported per patient.
Comparison of procedure time
Four of the trials reported on the time required for oocyte retrieval (Kingsland et al., 1991; Tan et al., 1992; Levens et al., 2009; Haydardedeoglu et al., 2011). In all four studies, procedural time for oocyte retrieval was significantly longer in the follicle flushing group than in the non-flushing group (Kingsland et al., 1991; Tan et al., 1992; Levens et al., 2009; Haydardedeoglu et al., 2011). In trials performed using older ART techniques, follicle flushing was associated with a 15-min longer procedure time as compared with non-flushing (Table I; Kingsland et al., 1991; Tan et al., 1992). Tan et al. (1992) and an increased amount of anesthetic needed for the procedure in the follicle flushing group when compared with the non-flushing group (100 versus 50 mg of pethidine, P < 0.0001). In two more recent trials, follicle flushing was associated with a 3- and 4-min longer procedure time when compared with non-flushing (Table I; Levens et al., 2009; Haydardedeoglu et al., 2011). Procedural times for oocyte retrieval shortened from the older to the newer studies; however, follicle flushing was associated with a longer procedure time for oocyte retrieval during all time periods in this study. Both of the two most recent trials demonstrated a substantially longer duration of oocyte retrieval in the follicle flushing group of 3- and 4–min, respectfully (Levens et al., 2009; Haydardedeoglu et al., 2011). A busy ART clinic that performs a large number of oocyte retrievals daily may find the additional time clinically significant.
Secondary outcomes
Five trials reported no differences in the fertilization of MII oocytes (oocytes that have completed meiosis I) between the flushing and non-flushing groups (Haines et al., 1989; Kingsland et al., 1991; Tan et al., 1992; Levens et al., 2009; Haydardedeoglu et al., 2011). Kingsland et al. (1991 and Tan et al. (1992) reported similar clinical pregnancy rates between the non-flushing and flushing groups. Levens et al. (2009) reported ongoing pregnancies in 40% of the non-flushing group and 20% of the flushing group (P= 0.19). Haydardedeoglu et al. (2011) reported live birth in 39.4% of the non-flushing group and 38.1% of the flushing group (P= 0.68). Statistical synthesis of pregnancy outcomes was not performed given the heterogeneity of the outcomes reported in the primary studies.
Subgroup analysis
An a priori subgroup analysis was performed to evaluate the effect of follicle flushing in poor-responding and normal-responding ART patients. Only the Levens et al. (2009) trial evaluated the use of follicle flushing in poor responders. There was no difference in the oocytes retrieved or oocyte yield in this study between the two groups, but the study was limited by a small sample size of 30 ART cycles (Table I). The only significant difference reported in this trial was an increased mean retrieval time with follicle flushing (non-flushing group: 186s versus flush group: 366 s, P < 0.001; Levens et al., 2009). Five trials were performed in general, normal-responding ART populations (Haines et al., 1989; Scott et al., 1989; Kingsland et al., 1991; Tan et al., 1992; Haydardedeoglu et al., 2011). Subgroup analysis of normal responders showed no increase in the oocyte yield between the flushing and non-flushing groups (OR: 1.06, 95% CI: 0.95–1.19).
A post hoc subgroup analysis was performed to evaluate the primary outcomes of the meta-analysis in the older RCTs (1989–1992) and the newer RCTs (2009–2011) to account for changes in the follicle flushing equipment and oocyte retrieval technique. In the four RCTs published between 1989 and 1992, there was no difference in the oocyte yield between the non-flushing and flushing groups utilizing a fixed effects model (OR: 1.10, 95% CI: 0.90–1.34; Haines et al., 1989; Scott et al., 1989; Kingsland et al., 1991; Tan et al., 1992). In the two trials performed between 2009 and 2011, there was no difference in the oocyte yield between the non-flushing and flushing groups utilizing a fixed effects model (OR: 1.03, 95% CI: 0.91–1.09; Levens et al., 2009; Haydardedeoglu et al., 2011).
Discussion
In this meta-analysis, there was no demonstrable benefit to follicle flushing over aspiration alone. Both techniques resulted in a similar total number of oocytes retrieved and oocyte yield. These findings were consistent across all six RCT included in the analysis. Follicle flushing was also associated with a longer procedure time, which could theoretically lead to a small increase in the cost and risk of the procedure, although data are lacking to adequately address this. Our findings are consistent with those of a 2010 Cochrane review demonstrating no benefit in follicle flushing (Wongtra-ngan et al., 2010). That review was performed prior to the largest publication by Haydardedeoglu et al. (2011) which allowed this meta-analyis to consider data from 518 ART cycles for analysis compared with 164 in the prior review (Wongtra-ngan et al., 2010). Despite the substantially larger data set and additional power of this current meta-analysis, ART outcomes were not different between the flushing and non-flushing groups.
These data differ from several non-randomized studies that have suggested that follicle flushing increases oocyte yield (Elhussein et al., 1992; Waterstone and Parsons, 1992; Bagtharia and Haloob, 2005). However, these studies have methodologic issues in their design that may have influenced the results. In all three trials, every patient underwent flushing and there was no control group. Each follicle was punctured a single time and aspiration occurred followed by flushing. Oocytes retrieved early were attributed to direct aspiration and oocytes retrieved later were attributed to flushing. It is possible that oocytes attributed to retrieval from follicle flushing were trapped in the aspiration tubing and therefore actually retrieved from aspiration alone, although credited to follicle flushing. Some of these trials attempted to address this issue by counting oocytes retrieved in the first flush as resulting from aspiration alone. Randomizing patients to flushing versus non-flushing is the best way to compare these approaches, and to address the potential for inaccurate attribution of oocytes retrieved to aspiration or flushing, and therefore this meta-analysis only included RCTs.
In theory, increasing oocyte yield should lead to an increase in the number of embryos available to select from for embryo transfer, potentially increasing the odds of live birth. In an analysis of over 400 000 ART cycles, Sunkara et al. (2011) demonstrated that the odds of live birth from ART increased markedly as the total number of oocytes retrieved increased from 1 to 10 oocytes. Similar increases were not observed for higher oocytes retrievals, and with retrieval of between 15 and 30 oocytes the odds of live birth essentially plateaued. Based on this association, little benefit might be expected from follicle flushing in normal-responding patients, where the additional benefit of one to two more oocytes may be negligible. However, patient groups where a small number of oocytes are available for retrieval may represent patients most likely to benefit from follicle flushing. Such patient groups may include poor responders, natural cycle ART and minimal stimulation ART (Lozano et al., 2006; Lozano et al., 2008; Levens et al., 2009). The only randomized trial that evaluated poor responders observed no significant differences between groups, demonstrating a non-significant increase in the oocyte yield and total number of oocytes retrieved with follicle flushing (Levens et al., 2009). However, this study was limited by a small sample size of 30 patients. The systematic review did not find any randomized controlled data evaluating follicle flushing in natural cycle or minimal stimulation ART.
A potential weakness of this meta-analysis is that all trials evaluated featured open-label designs that potentially introduce physician bias into patient management. Only Levens et al. (2009) attempted to address this potential confounding factor by blinding the treating physicians to the number of oocytes retrieved while the procedure was ongoing. However, open-label designs are thought to more commonly favor bias towards overestimating the positive effect of the intervention (Wood et al., 2008), a phenomenon not seen in this meta-analysis. Three of the studies failed to adequately report on randomization and allocation, which allows for potential additional bias within the data. Another weakness is the heterogeneity of the studies with respect to which aspiration needle was used, the volume of the flushing media, and the number of flushes performed per follicle. Additionally, this heterogeneity was unlikely to affect the results of this meta-analysis as no individual study showed the evidence of benefit from its specific flushing technique.
The data in this meta-analysis did not show any benefit in total oocytes retrieved or oocyte yield with follicle flushing during oocyte retrieval. Follicle flushing was associated with a significant increase in the procedure time for oocyte retrieval. RCTs are needed to assess the utility of follicle flushing in poor responders, natural cycle ART and minimal stimulation ART.
Authors’ roles
G.L. and M.J.H. were involved in the substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data, in addition to drafting the article. C.I.R. and L.C. were involved in drafting the article and revising the content. M.E.R. was involved in acquisition, analysis and interpretation of the data. A.H.D. and E.D.L. were involved in revising the article critically for content and final manuscript approval. B.W.W. was involved in revising the article critically for content, data interpretation and final manuscript approval.
Funding
This work was supported, in part, by the Program in Reproductive and Adult Endocrinology, NICHD, NIH, Bethesda, MD.
Conflict of interest
None declared.
References
- Bagtharia S, Haloob ARK. Is there a benefit from routine follicular flushing for oocyte retrieval? J Obstet Gynaecol. 2005;25:374–376. doi: 10.1080/01443610500118970. [DOI] [PubMed] [Google Scholar]
- Eisermann J, Yang V, Register K, Swank N, Strickler RC. Ovarian stimulation with pure follicle-stimulating hormone/human menopausal gonadotropin and improved laparoscopic aspiration needles influence the success of an in vitro fertilization program. Fertil Steril. 1989;51:112–116. doi: 10.1016/s0015-0282(16)60438-7. [DOI] [PubMed] [Google Scholar]
- Elhussein E, Balen AH, Tan SL. A prospective-study comparing the outcome of oocytes retrieved in the aspirate with those retrieved in the flush during transvaginal ultrasound directed oocyte recovery for in vitro fertilization. Br J Obstet Gynaecol. 1992;99:841–844. doi: 10.1111/j.1471-0528.1992.tb14417.x. [DOI] [PubMed] [Google Scholar]
- Gembruch U, Diedrich K, Welker B, Wahode J, van der Ven H, Al-Hasani S, Krebs D. Transvaginal sonographically guided oocyte retrieval for in-vitro fertilization. Hum Reprod. 1988;3(Suppl. 2):59–63. doi: 10.1093/humrep/3.suppl_2.59. [DOI] [PubMed] [Google Scholar]
- Haines CJ, Emes AL, Oshea RT, Weiss TJ. Choice of needle for ovum pickup. J In Vitro Fert Embryo Transf. 1989;6:111–112. doi: 10.1007/BF01130737. [DOI] [PubMed] [Google Scholar]
- Haydardedeoglu B, Cok T, Kilicdag EB, Parlakgumus AH, Simsek E, Bagis T. In vitro fertilization-intracytoplasmic sperm injection outcomes in single- versus double-lumen oocyte retrieval needles in normally responding patients: a randomized trial. Fertil Steril. 2011;95:812–814. doi: 10.1016/j.fertnstert.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC. Higgins JPT, Green S, editors. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Version 5.1.0 (updated March 2011) The Cochrane Collaboration www.cochrane-handbook.org . [Google Scholar]
- Hill MJ, Levens ED. Is there a benefit in follicular flushing in assisted reproductive technology? Curr Opin Obstet Gynecol. 2010;22:208–212. doi: 10.1097/GCO.0b013e3283373bfe. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Kingsland CR, Taylor CT, Aziz N, Bickerton N. Is follicular flushing necessary for oocyte retrieval—a randomized trial. Hum Reprod. 1991;6:382–383. doi: 10.1093/oxfordjournals.humrep.a137344. [DOI] [PubMed] [Google Scholar]
- Knight DC, Tyler JP, Driscoll GL. Follicular flushing at oocyte retrieval: a reappraisal. Aust N Z J Obstet Gynaecol. 2001;41:210–213. doi: 10.1111/j.1479-828x.2001.tb01212.x. [DOI] [PubMed] [Google Scholar]
- Levens ED, Whitcomb BW, Payson MD, Larsen FW. Ovarian follicular flushing among low-responding patients undergoing assisted reproductive technology. Fertil Steril. 2009;91:1381–1384. doi: 10.1016/j.fertnstert.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano DH, Fanchin R, Chevalier N, Feyereisen E, Hesters L, Frydman N, Frydman R. Optimising the semi natural cycle IVF: the importance of follicular flushing. J Indian Med Assoc. 2006;104:423–427. [PubMed] [Google Scholar]
- Lozano DHM, Scheffer JB, Frydman N, Fay S, Fanchin R, Frydman R. Optimal reproductive competence of oocytes retrieved through follicular flushing in minimal stimulation IVF. Reprod Biomed Online. 2008;16:119–123. doi: 10.1016/s1472-6483(10)60564-0. [DOI] [PubMed] [Google Scholar]
- Miller KA, Elkind-Hirsch K, Benson M, Bergh P, Drews M, Scott RT. A new follicle aspiration needle set is equally effective and as well tolerated as the standard needle when used in a prospective randomized trial in a large in vitro fertilization program. Fertil Steril. 2004;81:191–193. doi: 10.1016/j.fertnstert.2003.06.010. [DOI] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- Scott RT, Hofmann GE, Muasher SJ, Acosta AA, Kreiner DK, Rosenwaks Z. A prospective randomized comparison of single-lumen and double-lumen needles for trans-vaginal follicular aspiration. J In Vitro Fert Embryo Transf. 1989;6:98–100. doi: 10.1007/BF01130734. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- Tan SL, Waterstone J, Wren M, Parsons J. A prospective randomized study comparing aspiration only with aspiration and flushing for transvaginal ultrasound-directed oocyte recovery. Fertil Steril. 1992;58:356–360. doi: 10.1016/s0015-0282(16)55230-3. [DOI] [PubMed] [Google Scholar]
- Uzelac PS, Christensen GL, Nakajima ST. Follicular flushing avoids multiple vaginal punctures and may aid in oocyte recovery in in vitro maturation (IVM) Fertil Steril. 2009;91 S5. [Google Scholar]
- Waterstone JJ, Parsons JH. A prospective-study to investigate the value of flushing follicles during transvaginal ultrasound-directed follicle aspiration. Fertil Steril. 1992;57:221–223. doi: 10.1016/s0015-0282(16)54806-7. [DOI] [PubMed] [Google Scholar]
- Wiseman DA, Short WB, Pattinson HA, Taylor PJ, Nicholson SF, Elliott PD, Fleetham JA, Mortimer ST. Oocyte retrieval in an in vitro fertilization-embryo transfer program: comparison of four methods. Radiology. 1989;173:99–102. doi: 10.1148/radiology.173.1.2528782. [DOI] [PubMed] [Google Scholar]
- Wongtra-ngan S, Vutyavanich T, Brown J. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD004634.pub2. CD004634. [DOI] [PubMed] [Google Scholar]
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, Gluud C, Martin RM, Wood AJG, Sterne JAC. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. Br Med J. 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]