Abstract
BACKGROUND
In humans, as well as in other higher primates, the infantile testis is exposed to an adult-like hormonal milieu, but spermatogenesis is not initiated at this stage of primate development. In the present study, we examined the molecular basis of this intriguing infertile state of the primate testis.
METHODS
The integrity of androgen receptor (AR) and FSH receptor (FSHR) signaling pathways in primary cultures of Sertoli cells (Scs) harvested from azoospermic infant and spermatogenic pubertal monkey testes were investigated under identical in vitro hormonal conditions. In order to synchronously harvest Scs from early pubertal testis, the activation of testicular puberty was timed experimentally by prematurely initiating gonadotrophin secretion in juvenile animals with an intermittent infusion of gonadotrophin-releasing hormone.
RESULTS
While qRT–PCR demonstrated that AR and FSHR mRNA expression in Scs from infant and pubertal testes were comparable, androgen-binding and FSH-mediated cAMP production by infant Scs was extremely low. Compromised AR and FSHR signaling in infant Scs was further supported by the finding that testosterone (T) and FSH failed to augment the expression of the T responsive gene, claudin 11, and the FSH responsive genes, inhibin-βB, stem cell factor (SCF) and glial cell line-derived neurotrophic factor (GDNF) in Scs harvested at this stage of development.
CONCLUSION
These results indicate that compromised AR and FSHR signaling pathways in Scs underlie the inability of the infant primate testis to respond to an endogenous hormonal milieu that later in development, at the time puberty, stimulates the initiation of spermatogenesis. This finding may have relevance to some forms of idiopathic infertility in men.
Keywords: androgen receptor, FSH receptor, Sertoli cells, puberty, infertility
Introduction
The quiescence of the testis in juvenile monkeys and children is guaranteed by the relative hypogonadotropism that exists between late infancy and puberty (Plant and Witchel, 2006). Infancy, in higher primates, is associated with high levels of circulating gonadotrophin concentrations and testicular testosterone (T) secretion resembling those found pubertally and post-pubertally (Plant and Witchel, 2006). Intriguingly, in spite of this adult-like hormonal milieu to which the testis of the infantile male primate is exposed, spermatogenesis is not initiated, but rather the activity of the seminiferous epithelium at this stage of development is limited to proliferation of Sertoli cells (Scs) and undifferentiated type A spermatogonia (Simorangkir et al., 2003, 2005; Plant and Witchel, 2006).
Spermatogenesis is initiated only at puberty, which typically occurs some 3–4 and 10–12 years after birth in monkeys and humans, respectively, when gonadotrophin secretion is reactivated and testicular T production rises in the adult range (Plant and Witchel, 2006). The initiation of spermatogenesis at this stage of development is the result of a combined action of the elevations in circulating FSH and testicular T levels. It is also to be noted that spermatogenesis may be initiated prematurely in the juvenile monkey by the administration of exogenous gonadotrophins or by precocious activation of endogenous LH and FSH release as a result of pulsatile GnRH treatment (Marshall and Plant, 1996; Devi et al., 2006): a finding that indicates the resistance of the seminiferous epithelium of the infantile testis to hormonal stimulation is lost sometime during subsequent juvenile development (Plant et al., 2005).
Since the action of T and FSH to initiate spermatogenesis at puberty is indirect and mediated by activation of respective signaling pathways in Scs (Plant and Marshall, 2001), it is reasonable to propose that failure of the seminiferous epithelium in the infant primate to respond to a pubertal-like gonadotrophin stimulus is related to ‘immaturity’ of the Scs at this stage of development (Sharpe et al., 2003). Furthermore, we hypothesize that the post-natal acquisition of Sc competence to support spermatogenesis is most likely correlated with the development of androgen receptor (AR) and/or FSH receptor (FSHR) signaling pathways in this somatic testicular cell. FSHR is a G protein-coupled receptor, which upon binding to FSH is activated and stimulates adenylyl cyclase-mediated production of cAMP (Simoni et al., 1997). This second messenger activates a sequence of transcriptional events that elicit the biological actions of the gonadotrophin. AR is a ligand-inducible transcription factor that binds to specific DNA sequences initiating androgen-dependant transcriptional events (Roy et al., 1999). AR expression in Scs, as determined by immunohistochemistry, has been reported to be low during early post-natal development in marmosets, rhesus monkeys and humans as compared with later maturational stages (McKinnell et al., 2001; Berensztein et al., 2006; Chemes et al., 2008; Rathi et al., 2008; Boukari et al., 2009; Rey et al., 2009). However, developmental differences in the primate Sc AR activity in terms of ligand binding and the modulation of gene expression have not yet been demonstrated.
As a first step to study the cell biology that underlies the maturation of the primate Scs during juvenile development, AR and FSHR signaling pathways were compared in primary cultures of Scs harvested from infant and ‘pubertal’ testes. The ‘pubertal’ testis was generated by prematurely activating endogenous gonadotrophin secretion in juvenile monkeys with an intermittent intravenous infusion of GnRH (Marshall and Plant, 1996; Devi et al., 2006). The induced pubertal model enabled us to (i) dictate precisely the timing of the onset of spermatogenesis and the duration of gonadotrophin exposure in order to match, in the juvenile testis, the in situ hormonal milieu of the infant testes and (ii) readily harvest Scs from ‘pubertal’ testes before the appearance of large numbers of advanced germ cells. The integrity of AR and FSHR signaling pathways were then investigated in these cells cultured under identical hormonal conditions, i.e. in the presence of both T and FSH to mimic the in vivo situation of infancy. Additionally, the action of individual hormones (T or FSH) on Scs was also investigated to fully exploit the culture system.
Materials and Methods
Animals and tissue collection
Eight juvenile (18–22 months old) and 6 infant (3–4 months old) male rhesus monkeys (Macaca mulatta) were used. Monkeys were obtained from the Primate Research Centre of the National Institute of Immunology (NII) where they were housed according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), under a 12:12h light:dark cycle with food and water provided ad libitum. The juvenile monkeys were surgically implanted with indwelling venous catheters and maintained in remote infusion withdrawal cages as previously described (Marshall and Plant, 1996; Devi et al., 2006). Blood samples from these monkeys were collected via the catheters every week for measurement of T at 5 min before, and at 20, 40, 60 and 120 min after, a GnRH pulse. In the infants, a single estimate of T was obtained from a blood sample collected by the femoral vein venepuncture ∼12 h before castration. Bilateral castration was performed as previously described (Devi et al., 2006). Experimental protocols were approved by the Institutional Animal Ethics Committee of NII and CPCSEA.
Reagents
The recombinant monkey (rm) FSH (AFP6940), GnRH and an anti-cAMP antibody (Lot CV-27) were obtained from the National Hormone and Peptide Program (NHPP), NIH (Torrance, CA, USA). A polyclonal antibody to T (T3-125) was purchased from Endocrine Sciences Laboratories (Calabasas, CA, USA) and used for the radioimmunoassay of this steroid. All other reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA) unless stated otherwise.
Iodination of 5 µg of rm FSH (iodination grade) was carried out with 50 µCi of 125I-Na by the iodogen method (Dahia and Rao, 2006).
Induction of precocious puberty
Puberty was triggered precociously in the juvenile monkeys by eliciting premature endogenous gonadotrophin secretion with an intermittent i.v. infusion of GnRH (0.15 µg/min for 2 min every 3 h) administered for 4–5 weeks as previously described (Marshall and Plant, 1996; Devi et al., 2006). Plasma T concentrations tracked in weekly samples from these monkeys reached peak levels by 4 weeks of GnRH treatment that were comparable to those observed in the infants (Supplementary Fig. S1 and Supplementary Table SI), and similar to those previously reported for pubertal male rhesus monkeys (Plant, 1985). In the monkey, circulating concentrations of LH and T are tightly correlated (Plant, 1981) and serum T therefore provides a reliable and sensitive bioassay for LH secretion under the experimental conditions employed here. In previous studies of infants and of GnRH-stimulated juvenile monkeys, increases in circulating FSH levels have paralleled those in LH (Plant, 1985; Majumdar et al., 1995; Majumdar et al., 1997). For the foregoing reasons, it is reasonable to propose that gonadotrophin levels in the infant and GnRH-stimulated (pubertal) monkeys were comparable. Scs from the testes of the GnRH-stimulated juveniles are subsequently referred to as ‘pubertal’ Scs. This model of puberty was employed because it enabled the testes from different monkeys to be harvested at a synchronized stage of development before the appearance of a large number of advanced germ cells, which are known to interfere with the isolation of Scs (Majumdar et al., 1998; Devi et al., 2006). During spontaneous puberty in the monkey, the precise timing of the initial activation of testicular function is neither predictable nor reliably identifiable.
Isolation and culture of Scs
Scs from infant and pubertal monkeys were isolated by sequential enzyme digestion with collagenase and pancreatin, as described by us previously (Devi et al., 2006). The cells were then suspended in the Dulbecco modified eagle medium/nutrient mixture F-12 HAM containing 1% fetal calf serum. Equal numbers of cell clusters (0.5 × 104 clusters/well) were seeded in each well of a 24-well culture plate (Day 1) and cultures were kept at 34°c in a 5% CO2 incubator. On Day 2, cells were washed and maintained in the media containing serum replacement factors (sodium selenite, 5 µg/ml; insulin, 10 µg/ml; transferrin, 5 µg/ml and epidermal growth factor, 2.5 ng/ml). Media were replaced every 24 h. The testes of each infant generated a sufficient number of cells to seed one 24-well plate, in contrast to 5–6 such plates seeded from the testes of a pubertal monkey. We have previously reported that the cultured monkey Scs maintain hormone responsiveness for 5–6 days with serum replacement factors (Majumdar et al., 1998; Devi et al., 2006).
Treatments
Since germ cells in the culture influence the behavior of primate Scs (Foucault et al., 1994), residual germ cells were removed by hypotonic shock on Day 4 of culture (Galdieri et al., 1983). At 24 h post-hypotonic shock, one set of cultured Scs were treated with TRIZOL and stored at −80°C for extraction of RNA to determine the basal levels of gene expression (0 h). For clarity, the experimental flow chart is presented in Supplementary Table SII. Cells in the remaining wells were exposed for 24 h to T (100 nM) and rmFSH (5 ng/ml), either alone, or in combination, or to media alone, all in the presence of isobutyl-methyl-xanthine (IBMX, 0.1 mM). The dose of rmFSH was that found in preliminary studies to be bioactive in cultures of monkey Scs (Devi et al., 2006). At the end of culture, Scs were washed with calcium-free buffer and then dissociated using trypsin-EDTA. After washing, cells were lyzed in TRIZOL and stored at −80°C before the extraction of RNA. The extracted RNA was treated with DNase I to remove trace DNA contamination, and DNase I was removed using a DNase I inactivation solution (Ambion Inc., Austin, TX, USA). In a separate experiment, cultured Scs were treated for 0.5 h with either rmFSH (5 ng/ml) or Cholera toxin (1 IU/ml) or media alone in the presence of IBMX (0.1 mM). At the end of culture, media were collected for estimating cAMP levels.
Cytochemical evaluation of cultured cells
On Day 5 of culture, cell viability was determined by trypan blue exclusion. Leydig cell contamination was evaluated by cytochemical staining for 3β-hydroxysteroid dehydrogenase (3β-HSD) activity (Klinefelter et al., 1987). A crude preparation of monkey Leydig cells, which was obtained while isolating Scs from testis of pubertal monkeys, was used as a positive control to detect 3β-HSD activity. Peritubular cells and Scs were identified by staining for alkaline phosphatase activity and Oil Red-O, respectively (Chapin et al., 1987).
Ligand-binding ability of AR
Androgen binding to Scs was assayed as previously described (Fix et al., 2004). Briefly, cells were incubated for 4 h with either 100 nM radioactive T ([3H] specific activity 115 Ci/mmol; Perkin Elmer, Inc., MA, USA) in the presence or absence of cold T (10000 nM) or with 5 nM radioactive R1881 ([3H], specific activity 82 Ci/mmol; Perkin Elmer, Inc.) in the presence or absence of cold R1881 (5000 nM). R1881 is non-aromatizable androgen.
After incubation, cells were washed five times with ice cold PBS, and the bound ligand was extracted by treating cells with 1 ml of ethanol for 30 min at room temperature. Radioactivity in an aliquot of ethanol (0.9 ml) was measured in a liquid scintillation counter. The remaining ethanol was evaporated and the cells were treated with cell lysis buffer for protein estimation.
Ligand-binding ability of FSHR
FSH binding to Scs was assayed as described previously (Dahia and Rao, 2006). On Day 5 of culture, Scs were dislodged and washed, then 1 million Scs from each age group were suspended in PBS containing 100 000 cpm of 125I-rm-FSH in the presence or absence of 1000-fold excess of cold rm FSH for 2 h at 34°C. Scs were then washed and specifically bound FSH (in cpm/million Scs) was calculated.
Cyclic AMP and T assay
cAMP concentrations in the culture medium were determined by radio-immuno assay (RIA) using 125I-cAMP-TME (2-0′ monosuccinyl cAMP tyrosine methyl ester) and anti-cAMP antibody in accordance with instructions provided by NHPP, USA and circulating levels of T were measured by RIA as described previously (Devi et al., 2006).
Quantitative real-time-PCR analyses
Quantitative real-time-PCR (q-PCR) amplifications were performed in a 96-well plate in a RealplexS PCR machine (Eppendorf, Germany) in a total volume of 10 μl [1 or 2 μl of cDNA (depending upon the abundance of the transcripts), 0.5 µM of each primer and 5 μl of Power SYBR Green Master Mix (Applied Biosystems)]. Primers for each gene (Supplementary Table SIII) were independently validated by a standard curve calculated from the serial dilutions of the cDNAs. The Ct values for each sample were plotted against the log of the mRNA concentration present in each cDNA dilution. The efficiency of reaction was derived from the slope [efficiency = 10(1/−slope)]. Primers with an efficiency of 1 ± 0.2 were considered. The q-PCR reaction was initiated with melting of cDNA at 95°C for 15 min, followed by 40 amplification cycles (15 s at 95°C, 45 s at 60°C and 45 s at 65°C). Melting curve analyses for each gene were performed immediately after the amplification to detect the specific amplification peak for each gene. Basal expression (0 h, i.e. before initiation of treatment) of mRNA of the target genes [FSH-R, AR, inhibin-βB, claudin 11, stem cell factor (SCF) and glial cell line-derived neurotrophic factor (GDNF)] was determined by the efficiency-corrected ΔCt method [quantity = (efficiency + 1)−Ct] as described before (Wood and Walker, 2009). For each sample, the relative quantity of each target gene was calculated by normalization with the relative quantity of the endogenous control 60S ribosomal protein L32 (RPL32): a housekeeping gene, reported in monkeys to be superior to that of other popular housekeeping genes, in part, because its expression remains uniform throughout different developmental stages (Ahn et al., 2008). Hormone-mediated augmentation of FSHR, AR, inhibin-βB, claudin 11, SCF and GDNF mRNAs were calculated by the traditional 2(−ΔΔCt) method as described before (Viswanathan et al., 2009). Briefly, ΔCt was calculated for each sample (ΔCt = treated − control). The ΔΔCt was calculated by comparing each sample with the RPL32 according to the following equation: ΔΔCt = ΔCttarget gene − ΔCtRPL32. The fold change was then calculated relative to the reference according to the following formula: fold change = 2(−ΔΔCt). For each gene, the mean ± SEMs of at least three individual experiments (i.e. three animals in each of the two age groups) were determined for each treatment group.
Presentation of data and data analysis
Mean values of the relative mRNA levels were derived from at least three independent cultures (from each of three monkeys) for each age group. For determination of androgen-binding activity and cAMP production, each treatment for a given monkey was examined in at least triplicate wells. The average values for replicate wells were calculated and used to determine the overall mean for that infant or pubertal monkey (n= 3 or more monkeys for each age group). Means of triplicates for a given treatment (for each monkey) were used to compare the differences from other treatments in that particular monkey. Such means from at least three different infant or pubertal monkeys were used for determining the standard error within a particular developmental age group. For gene expression studies, a paired t-test was used for determining significant changes. P < 0.05 was considered as significant. Multiple measures of one-way ANOVA followed by Dunnett's post-test using InStat version 3.0 statistical program (Graphpad Software, Inc. San Diego, CA, USA) was employed for the evaluation of cAMP production as reported by us earlier (Devi et al., 2006) to determine the significance of differences between means.
Results
Preparation of pubertal monkeys
As was to be expected, plasma T levels in GnRH-treated juvenile monkeys increased significantly after 4 weeks of GnRH stimulation and were similar to those found during infancy and puberty (see section Materials and Methods for detail). Testicular weight increased ∼4-fold at the end of GnRH treatment. A histological analysis of such pubertal testes revealed the enlargement of seminiferous tubules and initiation of Gc differentiation as reflected by the presence of abundant type B spermatogonia and occasionally primary spermatocytes (Supplementary Fig. S2A–C).
Viability and purity of Sc cultures
As previously described (Majumdar et al., 1998; Devi et al., 2006), infant and pubertal Scs in culture formed confluent monolayers with purities >95% as indicated by oil Red O staining. Viability of cells on Day 5 of culture was >98%. Although peritubular cell-specific alkaline phosphatase activity was present occasionally, contamination of peritubular cells was <2%. Leydig cells were absent, as indicated by the lack of 3β-HSD activity in the cultured cells (Supplementary Fig. S3A–E).
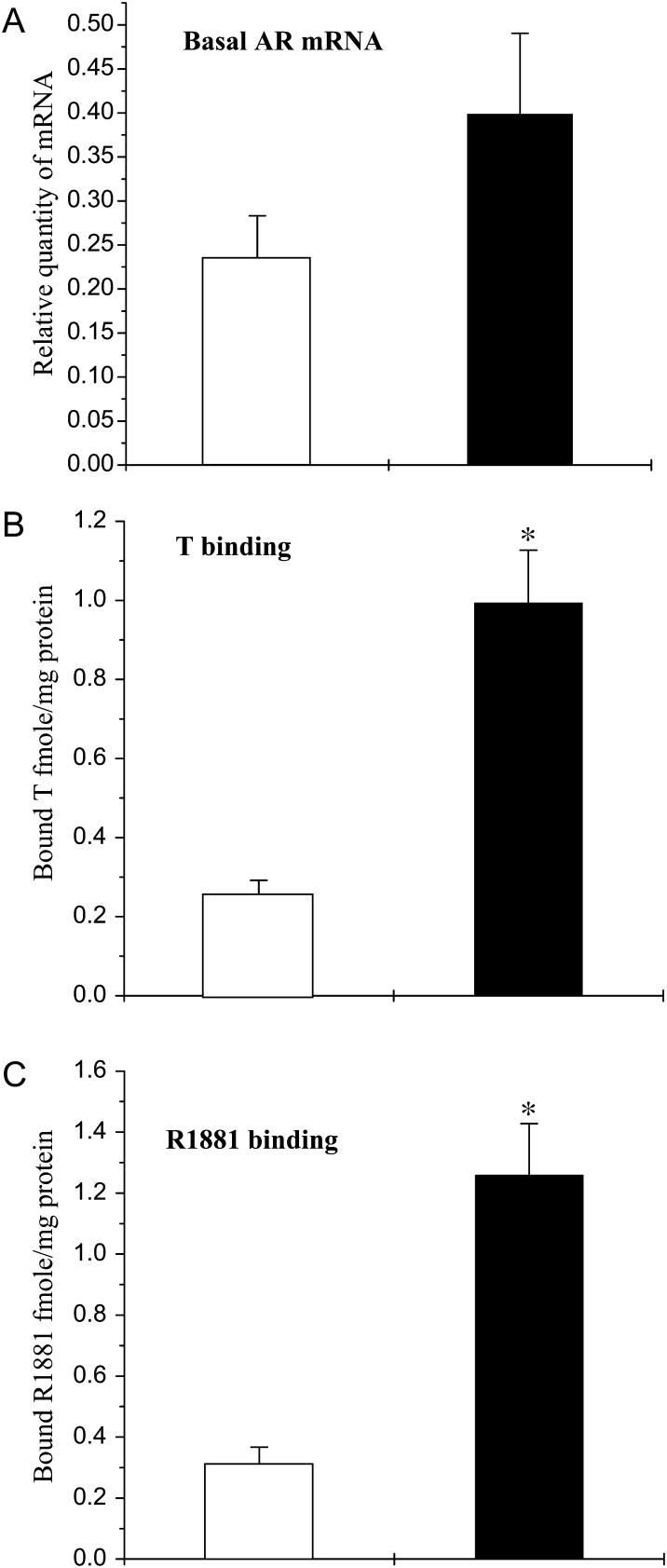
Basal AR mRNA levels and androgen binding activity
Basal AR mRNA levels determined at time 0 h (i.e. before initiation of treatment) were not significantly different in Scs isolated from infant or pubertal monkeys (Fig. 1A). In contrast, androgen-binding ability as reflected by both aromatizable and non-aromatizable ligands (T and R1881, respectively) was 4- or 5-fold higher (P < 0.05) in pubertal Scs as than those in infant Scs (Fig. 1B and C).
Figure 1.
Basal AR mRNA levels and androgen-binding activity in Scs isolated from testes of infant (open histograms) and pubertal (closed histograms) monkeys. (A) Basal AR mRNA evaluated by q-RT–PCR using RPL32 as an internal control. (B) Specifically bound T and (C) specifically bound R1881. For all three parameters, the means ± SEM of at least three independent experiments for each age group were analyzed and statistical significance (P < 0.05) is indicated by asterisks.
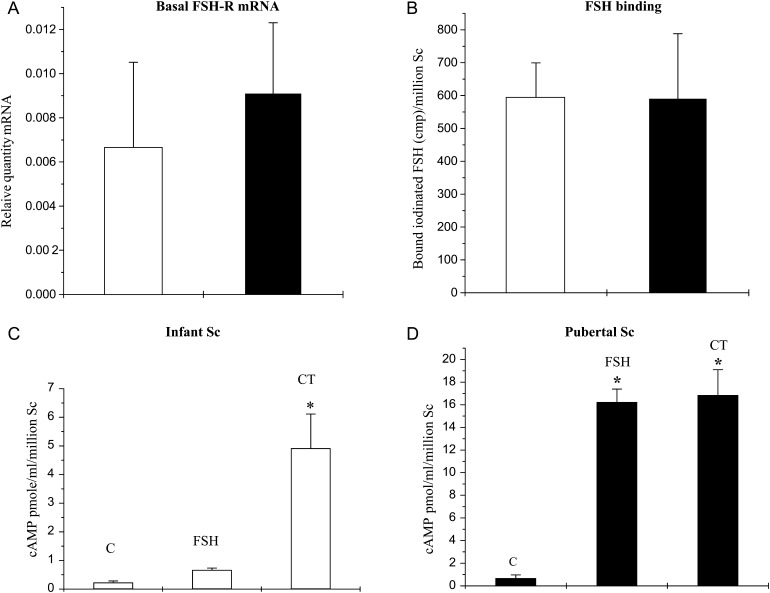
Basal FSHR mRNA levels, FSH binding ability and cAMP production
Basal FSHR mRNA levels (at time 0 h i.e. before initiation of treatment) in infant and pubertal Scs were indistinguishable (Fig. 2A), as was FSH binding (Fig. 2B). FSH stimulation for 30 min, however, resulted in about 10-fold increase in cAMP production by pubertal Scs, while in infant Scs this treatment was only marginally effective in augmenting cAMP production (Fig. 2C and D). Treatment with cholera toxin (CT), which is known to augment cAMP production by an action on GαS distal to FSHR, elicited a robust response in both infant and pubertal Scs (Fig. 2C and D).
Figure 2.
FSHR mRNA expression, FSH-binding activity and cAMP generation in Scs isolated from testes of infant (open histograms) and pubertal (closed histograms) monkeys. (A) Basal levels of FSHR mRNA were evaluated by q-RT–PCR using RPL32 as an internal control. (B) Iodinated rm-FSH-binding activity in cultured Scs obtained from infant and pubertal monkeys. (C) and (D) Production of cAMP by Scs cultured from infant and pubertal Scs, respectively. C, control; FSH, rm-FSH (5 ng/ml); CT, cholera toxin (1 IU/ml). In all cases, the mean ± SEM of at least three independent experiments for each age group was analyzed and statistical significance (P < 0.05) is indicated by an asterisk.
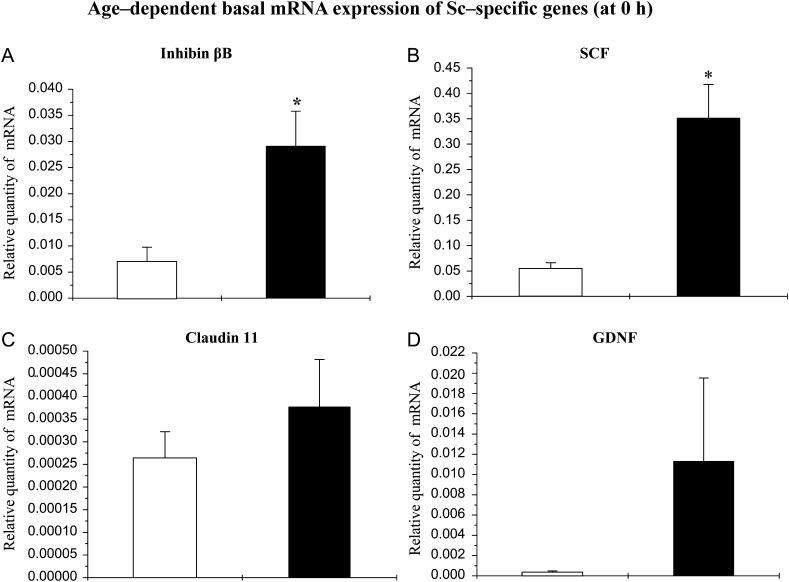
Basal levels of mRNAs coding for inhibin-βB, SCF, claudin11 and GDNF
Basal levels (at time 0 h i.e. before initiation of treatment) of the mRNA coding for inhibin-βB and SCF were elevated significantly in pubertal Scs compared with infant Scs (Fig. 3A and B). However, the basal levels of claudin11 mRNA in infant and pubertal monkey Scs were comparable (Fig 3C). Although basal GDNF mRNA levels were higher in pubertal Scs (Fig 3D), there was considerable animal-to-animal variation and hence this developmental difference was not significant.
Figure 3.
Basal levels of mRNAs coding for inhibin-βB, claudin 11, SCF and GDNF in Scs isolated from testes of infant (open histograms) and pubertal monkeys (closed histograms). On Day 5 of culture, the basal level of (A) inhibin-βB, (B) SCF, (C) claudin 11 and (D) GDNF mRNA were evaluated by q-RT–PCR with RPL32 as an internal control. Means of at least three individual monkeys for each age group were used for statistics. Statistical significance (P < 0.05) is indicated by an asterisk.
Effects of hormone treatment on AR mRNA levels
The relative fold changes in the expression of AR mRNA in Scs from either infant or pubertal monkeys during stimulation for 24 h with FSH and T, either alone or in combination, were similar (data not shown).
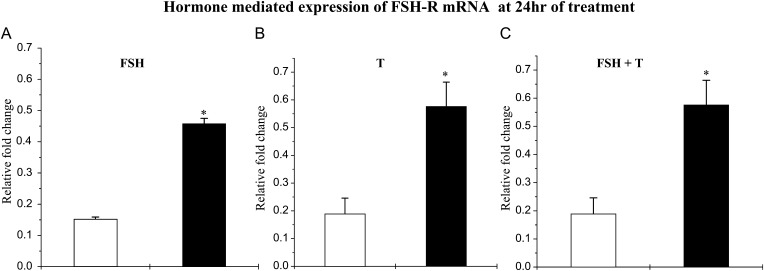
Effects of hormone treatment on FSHR mRNA levels
After 24 h of stimulation with either FSH (Fig. 4A) or T (Fig. 4B), the expression of FSHR mRNA was significantly higher in Scs from pubertal animals as compared with those from infants. Combined stimulation for 24 h with FSH and T also resulted in a significantly greater increase in FSHR mRNA in the pubertal Scs (Fig. 4C).
Figure 4.
Hormonal regulation of FSH-R mRNA in infant (open histograms) and pubertal (closed histograms) monkey Scs. Hormone-mediated expression of FSHR mRNA was evaluated by q-RT–PCR. The augmentation of FSH-R mRNA by (A) FSH (5 ng/ml), (B) T (100 nM) and (C) FSH and T in combination was evaluated at 24 h. Each bar represents the mean ± SEM from at least three individual monkeys. Statistical significance (P < 0.05) is indicated by an asterisk.
Effects of hormone treatment on inhibin-βB, claudin11, SCF and GDNF mRNA level
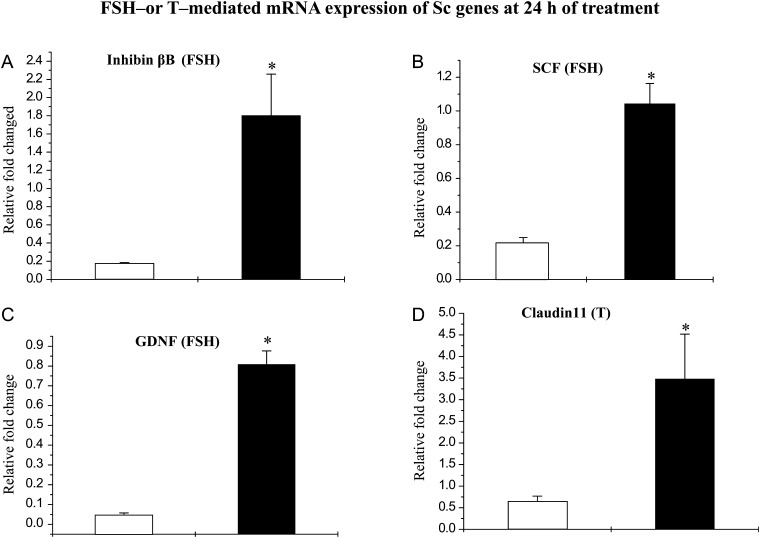
Treatment with FSH for 24 h resulted in the expression levels of inhibin-βB (Fig. 5A), SCF (Fig. 5B) and GDNF (Fig. 5C) mRNAs that were 5- to 10-fold higher (P < 0.05%) in Scs isolated from pubertal monkeys than those from infant monkeys. However, FSH stimulation failed to augment claudin 11 mRNA expression at 24 h in either developmental groups (data not shown).
Figure 5.
FSH-mediated augmentation of levels of mRNAs coding for inhibin-βB, SCF, GDNF and T-mediated augmentation of claudin 11 mRNA in Scs isolated from testes of infant (open histograms) and pubertal (closed histograms) monkeys. The effect of rm-FSH (5 ng/ml) on Sc genes was evaluated by q-RT–PCR. FSH-mediated augmentation of (A) inhibin-βB mRNA, (B) SCF mRNA and (C) GDNF mRNA was evaluated at 24 h. T (100 nM) mediated augmentation of (D) claudin 11 mRNA was evaluated by q-RT–PCR. Each bar represents the mean ± SEM from at least three individual monkeys. Statistical significance (P < 0.05) is indicated by an asterisk.
Treatment with T for 24 h resulted in an expression of claudin 11 mRNAs that was 7-fold higher (P < 0.05%) in Scs isolated from pubertal monkeys than those from infant monkeys (Fig 5D). However, T treatment did not augment inhibin-βB, SCF and GDNF mRNA expression at 24 h in either developmental groups (data not shown).
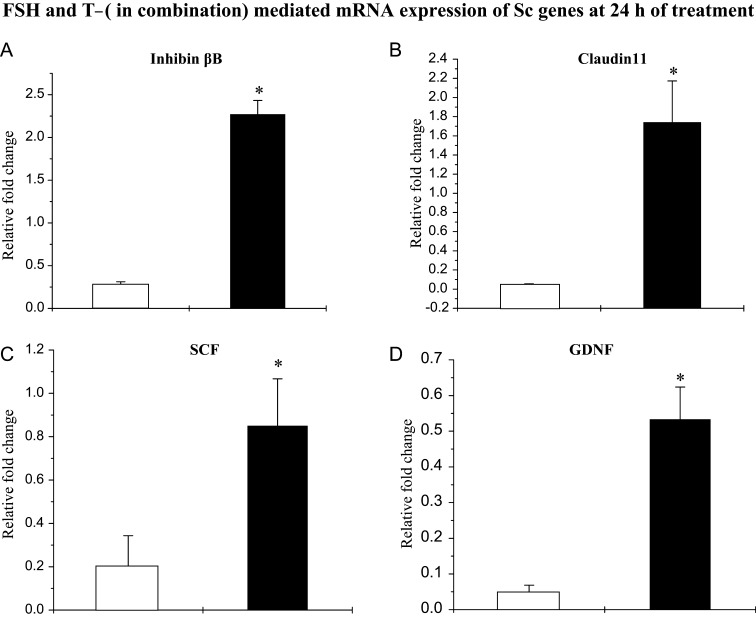
Combined FSH and T treatment for 24 h augmented inhibin-βB (Fig. 6A), claudin 11 (Fig. 6B), SCF (Fig. 6C) and GDNF (Fig. 6D) to significantly higher mRNA levels in pubertal Scs compared with those in infants Scs.
Figure 6.
FSH- and T-mediated augmentation of levels of mRNAs coding for inhibin-βB, claudin 11, SCF and GDNF in Scs isolated from testes of infant (open histograms) and pubertal (closed histograms) monkeys. Effects of rm-FSH (5 ng/ml) and T (100 nM) on Sc genes were evaluated by q-RT–PCR. FSH- and T-mediated augmentation of (A) inhibin-βB mRNA, (B) claudin 11 mRNA, (C) SCF mRNA and (D) GDNF mRNA was evaluated at 24 h. Each bar represents the mean ± SEM from at least three individual monkeys. Statistical significance (P < 0.05) is indicated by an asterisk.
Discussion
The present study examined the hypothesis that, in primates, restricted AR and/or FSHR signaling by the infant Scs underlies the azoospermia of the testis at this stage of development in the face of adult-like levels of T and FSH in the circulation. For this purpose, androgen and FSH signaling pathways in Scs from infant or pubertal monkey testis were interrogated in vitro and the results then compared to identify any deficits in signaling in infant Scs that might underlie the immaturity of the seminiferous epithelium that is reflected by the failure of the testis to support germ cell differentiation at this stage of development. For the preparation of pubertal Scs cultures, we harvested these cells from animals in which puberty had been prematurely induced by the administration of an intermittent i.v. infusion of GnRH that leads to elevated secretion of endogenous LH and FSH. The reason for using an experimentally induced puberty rather than spontaneous puberty was that gonadotrophin-induced initiation of testicular activation in the juvenile monkey may be synchronized and precisely timed, whereas the timing of spontaneous puberty is quite variable and difficult to pinpoint. As anticipated, plasma T concentrations increased and testicular growth accelerated over the 4–5-week period of GnRH stimulation indicating that precocious pubertal activation of both the interstitial and seminiferous compartments of the testis had been achieved (Devi et al., 2006). It is recognized at the outset of this discussion that in spite of employing Scs that had been harvested from monkeys in which the initiation of puberty had been experimentally synchronized, considerable variability between individual animals was observed. In this regard it should be remembered that, in contrast to studies reported for rats where each culture typically consisted of Scs pooled from several animals (Devi et al., 2006; Wood and Walker, 2009), in the present series of experiments each Scs culture was derived from a single monkey: an experimental design that probably accounts for the greater variability in the present monkey data.
The critical importance of AR expression in Scs for the regulation of spermatogenesis has been elegantly shown in transgenic mice with a Sc-specific AR knock-out (Chang et al., 2004; De Gendt et al., 2004; Holdcraft and Braun, 2004). These mice display normal urogenital tract development and testicular descent, but spermatogenesis is arrested during meiosis at the diplotene premeiotic stage. AR signaling is also generally recognized to be obligatory for the initiation and maintenance of spermatogenesis in primates including man (Plant and Marshall, 2001; McLachlan et al., 2002). Moreover, defective AR is speculated to be one of the potential causes of infertility in 20–30% of infertile patients who are not hypoandrogenic but do not respond to T or gonadotrophin treatment (Lombardo et al., 2005).
For the foregoing reasons, the finding of restricted androgen binding and an associated unresponsiveness of infant Scs to androgen, in spite of basal expression of AR mRNA during this stage of development that is comparable to that at puberty, is of particular interest. Together, the observations suggest that in Scs of the infant testis, either translation of AR mRNA is compromised or appropriate conformation of the translated receptor required for ligand binding (Keller et al., 1996) does not occur. That translation or appropriate post-translational conformation is limited in infant Scs is suggested by previous immunohistochemical studies that have shown low levels of AR in Scs from infant (4–6 weeks of age) marmosets (McKinnell et al., 2001), infant (3 and 6 months old) rhesus monkeys (Rathi et al., 2008) and infant boys (Berensztein et al., 2006; Chemes et al., 2008; Boukari et al., 2009).
Claudin 11 is an AR-regulated Scs gene (Florin et al., 2005; Kaitu'u-Lino et al., 2007) and a major component of the blood testes barrier (Morrow et al., 2010). Although the basal expression of claudin 11 mRNA was found to be similar in infant and pubertal Scs, the expression of this gene was significantly augmented by 24 h of T treatment (either alone or in combination with FSH) only at the later stage of development. This finding is consistent with the androgen-binding data and provides compelling evidence for the view that AR bioactivity is extremely low in infant Scs. Since the blood–testis barrier is a necessary prerequisite for initiation of spermatogenesis at puberty (Wong and Cheng, 2005), it may also be inferred from these data that establishment of the blood–testis barrier at puberty is likely activated due to up-regulation of claudin 11 in response to androgen at this stage of development. We also recognize that the deficits in AR signaling in the infant Scs revealed by the present study does not exclude the potential for deficiencies in AR signaling in other somatic cells of the testis at this stage of development.
The finding that cAMP production, an early event in the FSHR signaling pathway (Walker and Cheng, 2005), was markedly lower following FSH treatment in infant Scs than that in pubertal Scs is consistent with an earlier observation suggesting that FSH signaling may not be fully operational in the infant monkey Scs (Lee et al., 1983), as is also the case in 5-day old rat Scs (Crepieux et al., 2001). It is unlikely, however, that FSHR signaling is completely absent in infant monkey Scs, as cAMP production by Scs at this stage of development was augmented, albeit minimally, in response to FSH. It is likely that such FSH stimulation is necessary at this stage of primate development for the optimal proliferation of Scs (Simorangkir et al., 2003; Plant et al., 2005). That FSH driven Scs proliferation during infancy is mediated through the activation of the MAP kinase pathway, instead of the cAMP-mediated pathway as shown in rats (Crepieux et al., 2001), is also possible and this notion merits investigation in primates.
Although FSHR activity was greater in pubertal Scs, basal levels of FSHR mRNA and ligand binding of FSHR at the two developmental stages were indistinguishable. Our observation that FSHR is expressed during infancy is consistent with the earlier demonstration of specific high affinity binding of radiolabeled FSH in homogenates of near term fetal rhesus monkey testes (Huhtaniemi et al., 1987). In addition, a recent study demonstrated that FSHR mRNA is expressed from 28 weeks of gestational age in human and levels of expression of this mRNA remain unaltered up to adulthood (Boukari et al., 2009). In the present study, CT significantly augmented cAMP production by infant Scs suggesting that the GαS subunit of FSHR is operational in infant Scs, and that defective transduction of the signal through the transmembrane domain of FSHR underlies the limited cAMP response during infancy. Since, we used IBMX in our cultures, we eliminated the possibility of interference by endogenous phosphodiesterases, the expression of which in rat Scs is developmentally regulated (Levallet et al., 2007).
In rat Scs, FSH-induced cAMP production triggers a sequence of signal transduction events, which leads to increased expression of FSH-inducible genes (Walker and Cheng, 2005), such as inhibin-βB (Najmabadi et al., 1993), SCF (Yan et al., 1999), GDNF (Tadokoro et al., 2002), FSHR (Viswanathan et al., 2009) and claudin 11 (Kaitu'u-Lino et al., 2007). Inhibin-βB, SCF, GDNF and FSHR mRNA levels were all significantly (P < 0.05) augmented in the presence of FSH (alone or with T) in pubertal Scs, but not in infant Scs. This finding is consistent with the cAMP data and further demonstrates an enhanced FSH activity in pubertal Scs compared with that in infant Scs. However, FSH alone failed to up-regulate claudin 11 expression in pubertal Scs as compared with those of infants. Although the expression of claudin 11 has been shown to be up-regulated by FSH in prepubertal (20-days-old) rat Scs (Kaitu'u-Lino et al., 2007), such may not be the case in primates.
While the present study demonstrates that FSH responsiveness in the monkey Scs is spontaneously acquired somewhere between 3–4 and 18–22 months of age, the precise point in this developmental continuum at which this switch occurs remains to be determined. Studies by Dobrinski et al., however, shed some light on this problem, as this group found that when gonadotrophin stimulation of the infant monkey testis was sustained beyond 3 months of age by xenografting infant monkey testis into recipient adult mice, Sc maturation in the grafted testis was initiated before 10 months of age (Rathi et al., 2008). Interestingly, in two infant boys with congenital hypogonadotropic hypogonadism, FSH responsiveness of the testes, as reflected by robust increases in inhibin B and anti-Mullerian hormone secretion in response to 6 months of treatment with recombinant human FSH and LH, was evident when the boys were 7 and 10 months of age (Bougneres et al., 2008).
Estrogens via their receptors on Scs and germ cells are also considered to play a role in spermatogenesis (Carreau and Hess, 2010). Moreover, studies with human Scs at various stages of development (Foucault et al., 1992; Pereyra-Martinez et al., 2001) and with pubertal monkey Scs (Devi et al., 2006) have demonstrated high endogenous aromatase activity in these cells, which is independent of FSH or cAMP (Foucault et al., 1992; Devi et al., 2006). Thus, the possibility that deficits in intratesticular estrogen receptor signaling in infant Scs may contribute to the azoospermia of the testis at this stage of development cannot be excluded.
In summary, the present study provides substantial evidence for the view that limited AR activity in the Scs is a major factor underlying the azoospermia of the infant primate testis, which at this stage of development is exposed to an adult-like hormonal (T and FSH) milieu. FSH-induced augmentation of cAMP production was also found to be limited in infant Scs. The ability of the primate Scs to respond fully to androgen and FSH is not acquired until later in prepubertal development, by which time circulating gonadotrophin levels are low thereby guaranteeing that spermatogenesis is not initiated until puberty, when there is a resurgence in the secretion of LH and FSH (Plant and Witchel, 2006). The mechanisms underlying the switch from hormonal resistance of the Scs during infancy to hormone responsiveness later during prepubertal development are of considerable interest because it is reasonable to predict that if this switch fails to occur in Scs before puberty, infertility will prevail in spite of hormonal support (Fisher et al., 2003). Further studies for identifying the molecular bases of this switch might therefore be of interest and pave the way for novel treatment modalities for certain forms of male idiopathic infertility.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
S.S.M., K.S. and I.B. participated in designing various parts of this study, execution, analysis and manuscript drafting. T.M.P. contributed in designing this study, analysis of the data, critical discussion and preparation of the manuscript.
Funding
This work was supported by funding from the Department of Biotechnology, India, and the National Institutes of Health, USA, through an Indo-US Program on contraceptive and Reproductive Health Research and NIH HD08610 (TMP).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgements
We thank Dr W.H. Walker (University of Pittsburgh School of Medicine) for his advice during the early stages of this project. We are thankful to the staff of Primate Research Centre of NII, including Dr P. Nagrajan and Dr Venkatesan for providing facilities and expertise during the course of the study. We thank present and past Directors of NII for constant support for the primate work. We also thank Mukkesh Gautam, Bhola Shankar Pradhan and Abul Usmani for their help during the course of study.
References
- Ahn K, Huh JW, Park SJ, Kim DS, Ha HS, Kim YJ, Lee JR, Chang KT, Kim HS. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol Biol. 2008;9:78. doi: 10.1186/1471-2199-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, Rivarola MA, Belgorosky A. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr Res. 2006;60:740–744. doi: 10.1203/01.pdr.0000246072.04663.bb. [DOI] [PubMed] [Google Scholar]
- Bougneres P, Francois M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, Roger D, Lahlou N. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J Clin Endocrinol Metab. 2008;93:2202–2205. doi: 10.1210/jc.2008-0121. [DOI] [PubMed] [Google Scholar]
- Boukari K, Meduri G, Brailly-Tabard S, Guibourdenche J, Ciampi ML, Massin N, Martinerie L, Picard JY, Rey R, Lombes M. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J Clin Endocrinol Metab. 2009;94:1818–1825. doi: 10.1210/jc.2008-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Phelps JL, Miller BE, Gray TJ. Alkaline phosphatase histochemistry discriminates peritubular cells in primary rat testicular cell culture. J Androl. 1987;8:155–161. doi: 10.1002/j.1939-4640.1987.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, Gonzalez-Peramato P, Serrano A. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab. 2008;93:4408–4412. doi: 10.1210/jc.2008-0915. [DOI] [PubMed] [Google Scholar]
- Crepieux P, Marion S, Martinat N, Fafeur V, Vern YL, Kerboeuf D, Guillou F, Reiter E. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene. 2001;20:4696–4709. doi: 10.1038/sj.onc.1204632. [DOI] [PubMed] [Google Scholar]
- Dahia CL, Rao AJ. Demonstration of follicle-stimulating hormone receptor in cauda epididymis of rat. Biol Reprod. 2006;75:98–106. doi: 10.1095/biolreprod.105.047704. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi YS, Sarda K, Stephen B, Nagarajan P, Majumdar SS. Follicle-stimulating hormone-independent functions of primate Sertoli cells: potential implications in the diagnosis and management of male infertility. J Clin Endocrinol Metab. 2006;91:1062–1068. doi: 10.1210/jc.2005-2072. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin A, Maire M, Bozec A, Hellani A, Chater S, Bars R, Chuzel F, Benahmed M. Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology. 2005;146:1532–1540. doi: 10.1210/en.2004-0834. [DOI] [PubMed] [Google Scholar]
- Foucault P, Carreau S, Kuczynski W, Guillaumin JM, Bardos P, Drosdowsky MA. Human Sertoli cells in vitro. Lactate, estradiol-17 beta and transferrin production. J Androl. 1992;13:361–367. [PubMed] [Google Scholar]
- Foucault P, Drosdowsky MA, Carreau S. Germ cell and Sertoli cell interactions in human testis: evidence for stimulatory and inhibitory effects. Hum Reprod. 1994;9:2062–2068. doi: 10.1093/oxfordjournals.humrep.a138394. [DOI] [PubMed] [Google Scholar]
- Galdieri M, Zani BM, Monaco L, Ziparo E, Stefanini M. Changes of Sertoli cell glycoproteins induced by removal of the associated germ cells. Exp Cell Res. 1983;145:191–198. doi: 10.1016/s0014-4827(83)80020-2. [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Yamamoto M, Ranta T, Jalkanen J, Jaffe RB. Follicle-stimulating hormone receptors appear earlier in the primate fetal testis than in the ovary. J Clin Endocrinol Metab. 1987;65:1210–1214. doi: 10.1210/jcem-65-6-1210. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- Keller ET, Ershler WB, Chang C. The androgen receptor: a mediator of diverse responses. Front Biosci. 1996;1:d59–d71. doi: 10.2741/a116. [DOI] [PubMed] [Google Scholar]
- Klinefelter GR, Hall PF, Ewing LL. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod. 1987;36:769–783. doi: 10.1095/biolreprod36.3.769. [DOI] [PubMed] [Google Scholar]
- Lee BC, Pineda JL, Spiliotis BE, Brown TJ, Bercu BB. Male sexual development in the nonhuman primate. III. Sertoli cell culture and age-related differences. Biol Reprod. 1983;28:1207–1215. doi: 10.1095/biolreprod28.5.1207. [DOI] [PubMed] [Google Scholar]
- Levallet G, Levallet J, Bouraima-Lelong H, Bonnamy PJ. Expression of the cAMP-phosphodiesterase PDE4D isoforms and age-related changes in follicle-stimulating hormone-stimulated PDE4 activities in immature rat sertoli cells. Biol Reprod. 2007;76:794–803. doi: 10.1095/biolreprod.106.055343. [DOI] [PubMed] [Google Scholar]
- Lombardo F, Sgro P, Salacone P, Gilio B, Gandini L, Dondero F, Jannini EA, Lenzi A. Androgens and fertility. J Endocrinol Invest. 2005;28:51–55. [PubMed] [Google Scholar]
- Majumdar SS, Mikuma N, Ishwad PC, Winters SJ, Attardi BJ, Perera AD, Plant TM. Replacement with recombinant human inhibin immediately after orchidectomy in the hypophysiotropically clamped male rhesus monkey (Macaca mulatta) maintains follicle-stimulating hormone (FSH) secretion and FSH beta messenger ribonucleic acid levels at precastration values. Endocrinology. 1995;136:1969–1977. doi: 10.1210/endo.136.5.7720645. [DOI] [PubMed] [Google Scholar]
- Majumdar SS, Winters SJ, Plant TM. A study of the relative roles of follicle-stimulating hormone and luteinizing hormone in the regulation of testicular inhibin secretion in the rhesus monkey (Macaca mulatta) Endocrinology. 1997;138:1363–1373. doi: 10.1210/endo.138.4.5058. [DOI] [PubMed] [Google Scholar]
- Majumdar SS, Winters SJ, Plant TM. Procedures for the isolation and culture of Sertoli cells from the testes of infant, juvenile, and adult rhesus monkeys (Macaca mulatta) Biol Reprod. 1998;58:633–640. doi: 10.1095/biolreprod58.3.633. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction. 2001;122:419–429. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- Morrow CM, Mruk D, Cheng CY, Hess RA. Claudin and occludin expression and function in the seminiferous epithelium. Philos Trans R Soc Lond B Biol Sci. 2010;365:1679–1696. doi: 10.1098/rstb.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmabadi H, Rosenberg LA, Yuan QX, Reyaz G, Bhasin S. Transcriptional regulation of inhibin beta B messenger ribonucleic acid levels in TM.4 or primary rat Sertoli cells by 8-bromo-cyclic adenosine monophosphate. Mol Endocrinol. 1993;7:561–569. doi: 10.1210/mend.7.4.8502238. [DOI] [PubMed] [Google Scholar]
- Pereyra-Martinez AC, Roselli CE, Stadelman HL, Resko JA. Cytochrome P450 aromatase in testis and epididymis of male rhesus monkeys. Endocrine. 2001;16:15–19. doi: 10.1385/ENDO:16:1:15. [DOI] [PubMed] [Google Scholar]
- Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light-dark cycle. Biol Reprod. 1981;25:244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Strauss JD, Plant TM, Pfaff DW, Challis JRG, deKretser DM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. San Diego, USA: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- Plant TM, Ramaswamy S, Simorangkir D, Marshall GR. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann N Y Acad Sci. 2005;1061:149–162. doi: 10.1196/annals.1336.016. [DOI] [PubMed] [Google Scholar]
- Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149:5288–5296. doi: 10.1210/en.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey RA, Musse M, Venara M, Chemes HE. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech. 2009;72:787–795. doi: 10.1002/jemt.20754. [DOI] [PubMed] [Google Scholar]
- Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Plant TM. Sertoli cell proliferation during prepubertal development in the rhesus monkey (Macaca mulatta) is maximal during infancy when gonadotropin secretion is robust. J Clin Endocrinol Metab. 2003;88:4984–4989. doi: 10.1210/jc.2002-021858. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Ehmcke J, Schlatt S, Plant TM. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Wood MA, Walker WH. Follicle-stimulating hormone (FSH) transiently blocks FSH receptor transcription by increasing inhibitor of deoxyribonucleic acid binding/differentiation-2 and decreasing upstream stimulatory factor expression in rat Sertoli cells. Endocrinology. 2009;150:3783–3791. doi: 10.1210/en.2008-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- Wong CH, Cheng CY. The blood–testis barrier: its biology, regulation, physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- Wood MA, Walker WH. USF1/2 transcription factor DNA-binding activity is induced during rat Sertoli cell differentiation. Biol Reprod. 2009;80:24–33. doi: 10.1095/biolreprod.108.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Linderborg J, Suominen J, Toppari J. Stage-specific regulation of stem cell factor gene expression in the rat seminiferous epithelium. Endocrinology. 1999;140:1499–1504. doi: 10.1210/endo.140.3.6590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.