Abstract
BACKGROUND
Biological markers of ovarian reserve have the potential to advance research on fecundability, infertility and reproductive aging. Anti-Müllerian hormone (AMH) has emerged as a clinically useful measure of ovarian reserve, but the requirement for venous blood is an obstacle to application in non-clinical settings. This paper validates a new method for quantifying AMH in dried blood spot (DBS) samples—drops of whole blood collected on filter paper following a simple finger stick.
METHODS
Matched serum and DBS samples were obtained from n = 101 women of reproductive age, and AMH values were compared using regression analyses and scatter plots. The precision, reliability, linearity, recovery and lower detection limit of the DBS assay were evaluated, as well as the stability of AMH in DBS across a range of storage conditions.
RESULTS
There was a strong agreement between AMH concentrations measured in DBS and serum samples across the entire assay range. Analysis of within-assay (percent coefficient of variation, 4.7–6.5%) and between-assay (3.5–7.2%) variability indicated a high level of assay precision and reliability, respectively. The minimum detectable dose of AMH was 0.065 ng/ml. Concentrations of AMH remained stable in DBS samples stored for 2 weeks at room temperature, and for 4 weeks when refrigerated.
CONCLUSIONS
The DBS assay performs at a level that is comparable to serum-based methods, with the advantage of lower burdens and costs associated with blood collection that may be advantageous for research in clinical as well as non-clinical settings on the causes and consequences of variation in ovarian reserve.
Keywords: ovary, ovarian follicle, Müllerian inhibiting substance, infertility, immunoassay
Introduction
Anti-Müllerian hormone (AMH)—also called Müllerian inhibiting substance—has recently emerged as an important biomarker of ovarian reserve that has the potential to facilitate new research on fecundability, infertility and reproductive aging. AMH is a glycoprotein dimer produced by granulosa cells from small pre-antral and antral follicles in the ovary, and it is hypothesized to inhibit the recruitment of primordial follicles into the pool of growing follicles (Durlinger et al., 2002). Prior studies suggest that AMH represents a useful measure of ovarian reserve since it plays an important role in early-stage follicle development, can be quantified in serum or plasma and because levels fluctuate minimally during the menstrual cycle (Fauser et al., 2002; Somunkiran et al., 2007). Concentrations show a progressive decrease with age (Lee et al., 1996; Seifer et al., 2011) and predict timing of menopause in advance of clinical symptoms (e.g. menstrual irregularity) (van Rooij et al., 2004; Tehrani et al., 2009). Moreover, reduced AMH has been associated with lower fecundability in small population-based studies (Steiner et al., 2011), and AMH predicts ovarian response among women undergoing ovarian stimulation for IVF (van Rooij et al., 2002, 2004; McIlveen et al., 2007; Kwee et al., 2008; Broer et al., 2009). More recently, AMH values have been used to assess the impact of cancer treatments on ovarian reserve in female cancer survivors (Fong et al., 2008; Gracia et al., 2012).
An obstacle to the measurement of AMH in community-based studies, or studies requiring multiple blood draws, is the requirement for venous blood. Venipuncture blood draws are costly, invasive and must be performed by a trained phlebotomist in close proximity to a facility where blood samples can be centrifuged, separated and frozen. Dried blood spots (DBS)—drops of whole blood collected on filter paper following a simple finger stick—represent a minimally invasive alternative (McDade et al., 2007). The participant's finger is cleaned, pricked with a sterile, disposable lancet of the type commonly used to monitor blood glucose, and up to five drops of whole blood are applied to the paper. Samples are allowed to dry, and then stacked and stored in plastic bags prior to shipment to the laboratory. A major advantage of DBS sampling is that it is relatively painless and non-invasive, low cost and can be implemented by non-medically trained personnel in the participant's home or other non-clinical setting. The simplified logistics of DBS sampling allow investigators to collect blood from large numbers of participants in diverse research settings, and over the past 5 years, more than 35 000 DBS samples have been collected as part of major health surveys in the USA (McDade et al., 2007).
In this paper, we provide details on a new method for quantifying AMH in DBS samples in order to promote research on the causes and consequences of variation in ovarian reserve in a wider range of research settings. Following previously validated and widely used DBS assay protocols (McDade et al., 2000, 2004; McDade and Shell-Duncan 2002), the method uses commercially available supplies and is presented in sufficient detail that it can be implemented in any lab with the appropriate immunoassay equipment and technical expertise. We report on results of assay validation demonstrating levels of performance in the quantification of AMH that are comparable to gold-standard, serum-based methods.
Methods
Sample collection
For the purposes of assay validation, a matched set of finger-stick DBS samples and venipuncture serum samples were collected from 101 volunteers recruited from the University of Pennsylvania. Seventy-seven participants were recruited through local advertising, and 24 participants were infertile women over 30 years of age recruited from the university fertility clinic. Inclusion criteria were as follows: age between 18 and 45 years, the presence of both ovaries and a history of regular menstrual cycles 21–35 days in length. Exclusion criteria included pregnancy or lactation within the prior 3 months. Causes of infertility in the subsample of 24 women were unexplained, with the exception of two cases of tubal factor infertility which were removed prior to comparisons between fertile and infertile women. The subsample did not include any cases of male factor infertility. The protocol was approved by the Institutional Review Board at the University of Pennsylvania. All study participants provided informed consent before inclusion in the study.
Serum and DBS samples were collected from each participant during the same clinic visit. First, ∼4 ml blood was drawn into a serum separator tube using standard venipuncture procedures. Each tube was allowed to clot at room temperature in an upright position for 30 min and centrifuged at 1000g for 15 min. Serum was then aliquoted into cryovials and frozen at −80°C.
Immediately following venipuncture, finger-stick capillary blood samples were collected on the filter paper by delivering a controlled, uniform puncture with a sterile, disposable micro-lancet (BD Microtainer #366594, Franklin Lakes, NJ, USA). After wiping away the initial drop of blood with sterile gauze, up to five drops of whole blood were applied to the filter paper (Whatman #903, GE Healthcare, Piscataway, NJ, USA), allowed to dry at room temperature for at least 4 h, and then placed in a gas impermeable plastic bag with desiccant and stored frozen at −30°C. Serum and DBS samples were batch-shipped frozen on dry ice to the Laboratory for Human Biology Research at Northwestern University.
Serum AMH assay protocol
Concentrations of AMH were determined in serum samples using a recently validated, commercially available enzyme immunoassay kit designed for use with serum or plasma samples (AMH Gen II ELISA, Beckman Coulter #A73818, Brea, CA, USA) (Kumar et al., 2010). Samples were analyzed in duplicate using materials and procedures provided with the kit. Briefly, samples, calibrators and controls were pipetted into microtiter plate wells coated with anti-AMH capture antibody, incubated for 60 min, and then washed five times with a microplate washer (BioTek Instruments ELx50, Winooski, VT, USA). Biotinylated anti-AMH detection antibody was then added; wells were incubated for 60 min and washed, followed by the addition of streptavidin–horseradish peroxidase (HRP). Wells were incubated for 30 min, washed and tetramethylbenzidine chromogen solution was added to promote color change in proportion to the amount of bound AMH. Sulfuric acid was added to each well to stop the reaction, and absorbance was measured at 450 nm with 630 nm reference wavelength in a microplate reader (BioTek Instruments Elx808). Sample concentrations were calculated from the best-fit four-parameter logistic standard curve (KCJunior, BioTek) based on absorbance values derived from calibration materials with known concentrations of AMH (Beckman Coulter #A73819).
DBS AMH assay protocol
The protocol for analyzing AMH in DBS samples is based on modifications to the Beckman Coulter AMH enzyme immunoassay kit (Beckman Coulter #A73818). In order to minimize matrix differences and maximize comparability between calibration material and samples, DBS-based standards were manufactured by adding AMH stock of known concentration to washed erythrocytes, followed by application onto the filter paper. Washed erythrocytes were obtained as follows: (i) whole blood was collected by venipuncture in 5 ml ethylenediaminetetra-acetic acid vacutainer tubes, and centrifuged at 1500g for 15 min; (ii) plasma and buffy coat were removed and discarded; (iii) ∼3 ml normal saline (0.86 g NaCl/100 ml deionized H2O) was added; and (iv) tubes were mixed gently for 5 min on a hematology rotor and centrifuged as before. Saline and any remaining buffy coat were removed, and steps (iii) and (iv) were repeated for a total of three washes.
DBS AMH standards were made as follows: (i) stock AMH (Beckman Coulter #A73819) was obtained at concentrations across the likely physiological range (22.5, 10.0, 4.0, 1.2, 0.4, 0.16 and 0.0 ng/ml); (ii) each concentration of AMH stock was added to an equal volume of washed erythrocytes (1:2 dilution); (iii) solutions were mixed gently for 5 min on a hematology rotor; (iv) standards were then applied to labeled filter paper cards in 50 µl drops using a manual pipette, dried overnight at room temperature and stored at –30°C in gas impermeable plastic bags with desiccant. Final DBS AMH standard concentrations were 11.25, 5.0, 2.0, 0.6, 0.2, 0.08 and 0.0 ng/ml. DBS-based control samples with low and high AMH levels were also manufactured using these procedures.
The day before an assay was to be performed, DBS standards, samples and controls were removed from the freezer, and discs were punched out using a 3.2 mm (1/8 in) hole punch and placed into a 96-well filter plate for overnight elution (MultiScreen HTS, Millipore #MSHVN4510, Billerica, MA, USA). For duplicate measures, six discs were punched out, with three discs placed in each of two separate wells. Assay buffer (100 μl) was added to each well, the plate was covered and then incubated overnight at 4°C. On the day of the assay, the plate was removed from refrigeration and placed on an orbital plateshaker at 250 rpm for 30 min. The filter plate was then stacked on the top of the assay plate (pre-coated with anti-AMH capture antibody) provided with the kit, and centrifuged for 2 min at 2100g. This elution protocol maximizes the recovery of sample since all material flows through the filter plate directly into the assay plate; no material is wasted due to pipetting and filter paper discs are efficiently removed from the sample.
The DBS assay was then performed as follows: (i) the assay plate was incubated with shaking (700 rpm) for 90 min and then washed five times with a microplate washer (BioTek Instruments ELx50); (ii) biotinylated anti-AMH detection antibody (100 μl) was added to each well, the plate was incubated with shaking for 90 min and washed five times; (iii) streptavidin–HRP (100 μl) was added to each well, the plate was incubated with shaking for 30 min, and washed five times; (iv) chromogen solution (100 μl) was added to each well and the plate was incubated with shaking for 15 min away from direct light exposure; (v) sulfuric acid stop solution (100 μl) was added to each well, and absorbance was measured at 450 nm (630 nm reference) in a microplate reader (BioTek Instruments Elx808). Sample concentrations were calculated from the best-fit four-parameter logistic standard curve (KCJunior, BioTek) based on absorbance values derived from DBS standards with known concentrations of AMH. Concentrations were then converted to serum equivalent values based on the Passing and Bablok fit between DBS and serum results from the sample of matched serum and DBS samples (n = 101; equation reported below). On the day the assay is performed, the protocol can be completed in ∼4.5 h.
Analysis of assay performance
The performance of the DBS assay was investigated by evaluating agreement between DBS and serum AMH concentrations in matched samples, as well as linearity, recovery, precision and reliability, and lower detection limit. In addition, we investigated the stability of DBS AMH under a range of storage conditions. Statistical analyses were performed using Analyse-it (version 2.24; Leeds, UK) and STATA (version 11.1; STATA Corp., College Station, TX, USA). The strength and linear dependence of results derived from matched DBS and serum samples was investigated with the Spearman correlation and the Passing and Bablok regression, as well as inspection of the Bland–Altman plots for evidence of bias or inconsistent variability across the range of measurement (Bland and Altman 1986, 1999).
The linearity of dilution was assessed by eluting and then serially diluting (1:2, 1:4, 1:8, 1:16) two DBS samples at the high end of the assay range. Recovery was evaluated with two control sera containing low (2.5 ng/ml) and high (8.0 ng/ml) concentrations of AMH which were diluted 1:2 with washed erythrocytes and spotted onto the filter paper. For the analysis of linearity and recovery, observed values were compared with expected values and multiplied by 100 for a measure of percent recovery. Assay precision and reliability were evaluated by calculating within-assay and between-assay coefficients of variation (CV; standard deviation/mean) from multiple determinations of two laboratory controls at the low and high end of the assay range, respectively. Precision was evaluated with 10 determinations of each control in a single assay, and reliability was evaluated with duplicate measurements of each control across 10 assays performed on different days.
The lower detection limit (minimum detectable dose) was evaluated based on 10 determinations of the zero standard (assay buffer and washed erythrocytes) measured on a single assay plate. The mean absorbance of the zero standard was calculated, and the point 2 SD above zero was plotted on the assay standard curve to determine the lowest DBS AMH concentration that could be differentiated from zero with confidence.
The stability of AMH in blood spots was determined over a 4-week period in which DBS samples from three individuals were exposed to one of three steady temperature conditions [4°C, room temperature (21–23°C), 37°C], and one oscillating condition (12 h at 32°C and 12 h at 21°C to represent ambient conditions in tropical environments). Samples were considered to be stable so long as values remained within a 2 SD range of initial baseline values (based on the mean and SD of 10 determinations of samples placed in the freezer after drying overnight). Samples were exposed for 1, 2, 3, 4, 5, 6, 7, 14, 21 or 28 days in gas-impermeable bags with desiccant, stored at −30°C after the period of exposure, and then analyzed together along with baseline samples. In addition, the stability of AMH in DBS to repeated cycles of freezing and thawing was also evaluated to consider the potential effects of removing samples from the freezer during assay set-up. Three DBS samples were removed from their plastic bags and placed on the benchtop at room temperature for 1 h, and then returned to the freezer. The procedure was repeated over 5 different days.
Results
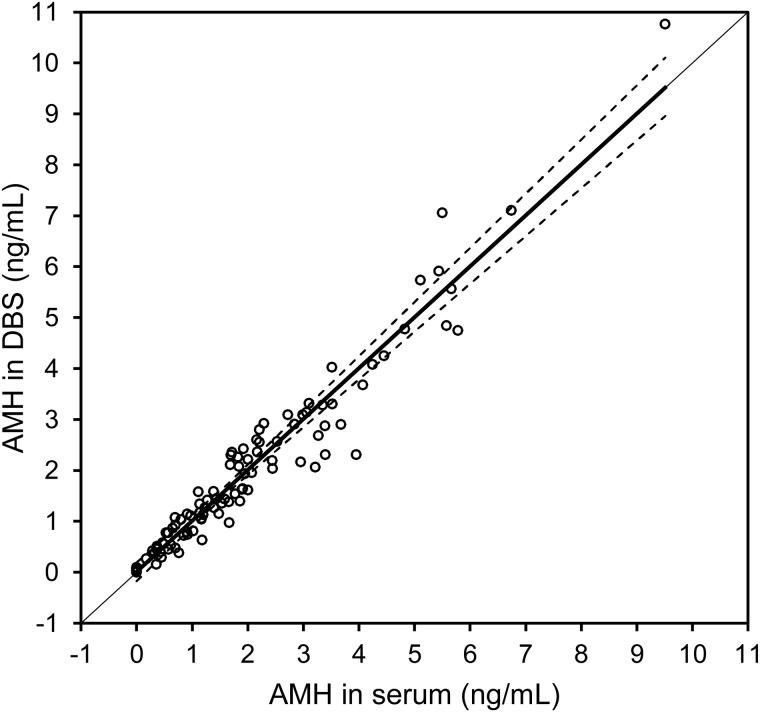
The mean age of women providing validation samples was 31.4 (SD 7.7) years, and the mean BMI was 25.8 (SD 6.5). The mean age of women in the infertility subsample (36.2 years) was significantly higher than those in the sample without diagnosed infertility (30.0 years, P < 0.01). Analysis of matched DBS and serum values indicated that the association between AMH values was strong and linear across the entire assay range, with the Spearman r = 0.97 (P < 0.0001). Based on this association, we calculated serum equivalent values according to the following equation: serum (ng/ml) = 1.26(DBS) (ng/ml). The level of agreement between serum and DBS-derived serum equivalent AMH values was high (Fig. 1). The mean serum equivalent AMH concentration in DBS samples for the entire sample was 1.96 ng/ml (SD 1.79), and the mean serum concentration was 1.98 ng/ml (SD 1.73). The mean AMH in DBS for the infertility subsample was 1.29 ng/ml (SD 1.20), compared with 1.23 ng/ml (SD 1.19) in serum.
Figure 1.
Scatterplot and Passing and Bablok regression analysis of the association between AMH concentrations obtained from matched serum and DBS samples for n = 101 reproductive age women. Regression results were as follows: y = 1.000(x); r = 0.97, P < 0.0001.
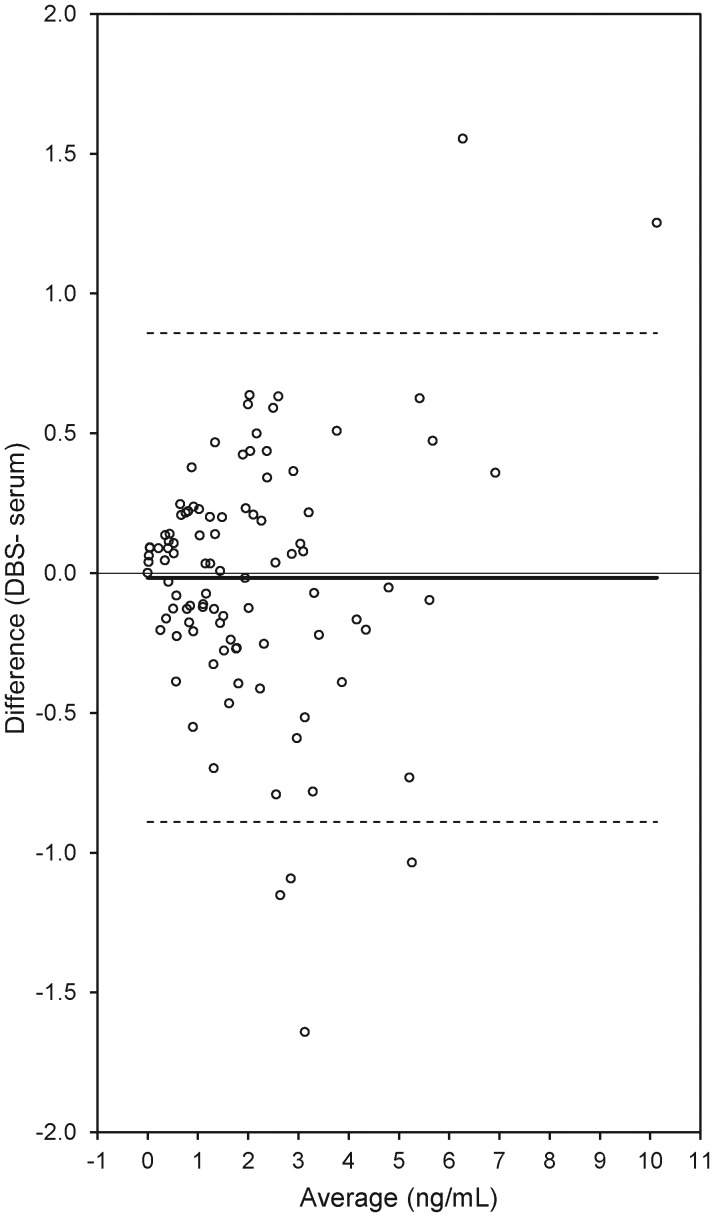
Serum and DBS results were also compared by calculating the difference between AMH concentrations in DBS and serum, and visually inspecting for evidence of bias or inconsistent variability across the range of measurement (Bland and Altman, 1986, 1999). The mean difference was −0.016, with six values lying outside the 95% limits of agreement (−0.890, 0.858) (Fig. 2). There was no evidence of systematic variation in the difference between serum and DBS results across the assay range.
Figure 2.
Scatterplot of the difference in AMH concentrations between DBS and serum samples as a function of the average AMH concentration across the two sample types (n = 101). Mean difference = −0.016; limits of agreement (Bland and Altman, 1999) upper 95% = 0.858: lower 95% = −0.890.
Analysis of two serially diluted samples indicated a high degree of assay linearity, with observed values ranging from 83.3 to 102.6% of expected for the first sample (9.72 ng/ml starting concentration), with a mean of 94.1%. Observed values ranged from 92.7 to 98.6% of expected for the second sample (6.99 ng/ml starting concentration), with a mean of 95.4%. Analysis of recovery produced similar results: for low and high control samples, the observed values were 107.7 and 97.1% of expected, respectively.
Repeat analysis of two control samples within and across assay plates indicated a high degree of precision and reliability. Within-assay %CV for the low control (mean = 1.50 ng/ml) was 6.5%, and between-assay %CV as 7.2%. For the high control (mean = 4.68 ng/ml), within-assay %CV was 4.7%, and between-assay %CV was 3.5%.
The lower detection limit of the assay was determined to be acceptably low. Based on a criterion of 2 SD above the zero standard, the minimum detectable dose of AMH was 0.065 ng/ml. A more conservative 3 SD criterion resulted in a minimum detectable dose of 0.095 ng/ml. It is worth noting that in our analysis of paired DBS/serum samples, the DBS assay was able to detect AMH for all women who also had detectable levels of AMH as determined with the serum assay. The lower detection limit of the DBS assay therefore approximates that of the serum assay (Kumar et al., 2010).
Analysis of AMH stability in DBS indicates that samples can be stored refrigerated at 4°C for at least 4 weeks, and for 2 weeks at room temperature, without significant decreases in AMH concentration compared with baseline. Concentrations of AMH declined after 3 weeks of storage at room temperature, with values across the samples averaging 89.8% of baseline. Samples remained stable for 7 days when exposed to the oscillating condition, and declined to 91.4% of baseline by 14 days. Concentrations of AMH declined rapidly in samples stored at 37°C. By day 3, concentrations were reduced on average to 85.7% of baseline. There was no consistent pattern of degradation in AMH concentrations across five cycles of freezing and thawing.
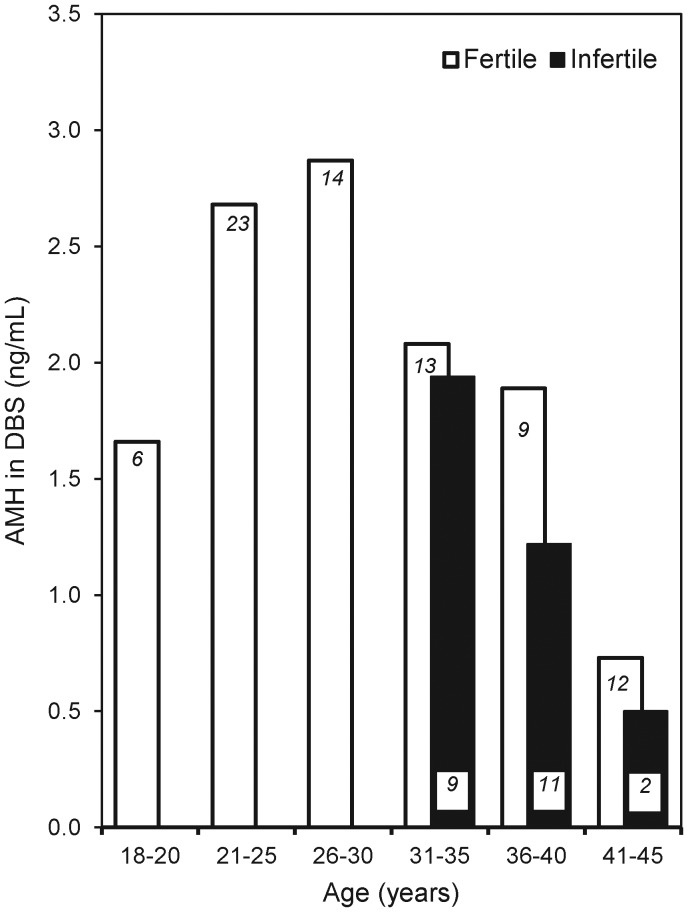
The age distribution of DBS AMH concentrations in women in our validation sample is presented in Fig. 3. AMH concentrations are highest between the ages of 21 and 30 years, and decline with age. Women presenting with infertility have substantially lower AMH concentrations than age-matched women without diagnosed infertility.
Figure 3.
Association between age and mean AMH concentration in DBS samples from n = 77 reproductive age women without a diagnosis of infertility (open bars), and for n = 22 women with a diagnosis of infertility (black bars). The number of observations for each group is indicated in italics.
Discussion
We have developed and validated a minimally invasive method for quantifying AMH in DBS samples in order to facilitate research on ovarian reserve. Analysis of assay performance indicates that the DBS method returns AMH results that are accurate, precise, reliable and in strong agreement with the current gold standard serum-based assay method. In addition, since our protocol uses commercially available supplies and commonly available enzyme immunoassay equipment, barriers to implementation are relatively low.
The age distribution of DBS AMH concentrations in women in our validation sample is generally consistent with previously published studies using serum samples (Lee et al., 1996; Seifer et al., 2011). Our sample was recruited for the purposes of assay validation, and the small, non-representative nature of the sample may account for the lower AMH concentrations among 18–20 year-olds, although this pattern is in agreement with a recent study in which AMH concentrations were shown to peak at age 25 and subsequently decline (Kelsey et al., 2011). The pattern of association between AMH and age in our sample is identical whether we use serum- or DBS-based results; any deviations from prior research therefore likely derive from sample composition rather than assay methodology.
Previously, validated methods for quantifying gonadotrophins and steroid hormones in DBS have promoted comparative, community-based research on human reproductive function for almost two decades (Worthman and Stallings, 1994, 1997). Our AMH method adds an important biomarker to this methodological toolkit, and takes advantage of the low costs and simplified logistics associated with collecting DBS samples. Recent applications have yielded high rates of participant compliance in multiple community- and population-based studies (Borders et al., 2007; Williams and McDade, 2009; McDade, 2011). For example, in a large, nationally representative study of young adults, 94% of participants consented to provide a DBS sample (Harris, 2010).
Requirements for storage and transportation are simplified by the fact that DBS samples can be stacked and stored in air-tight containers and kept at ambient temperatures. Results of our stability analysis indicate that samples can be stored for 2 weeks at normal room temperature without loss of AMH, and that this period can be extended to 4 weeks or longer with refrigeration. However, the AMH in DBS is sensitive to elevated temperatures, with more rapid degradation with exposure to temperatures common in tropical regions, and during summer months in other areas. Efforts should therefore be made to protect samples from prolonged exposure to high temperatures during storage and shipping.
While there are many advantages to DBS sampling, disadvantages associated with quantifying AMH in DBS should also be considered. First, proper placement of whole blood on the filter paper is essential, since the dispersion of analytes within the sample will be inconsistent if blood is blotted or smeared onto the paper, or if a drop of blood is placed on the top of a previously collected drop. In addition, the AMH assay requires a relatively large quantity of whole blood: six 3.2 mm discs are required for duplicate analyses, which is the volume that can be obtained from one large drop of blood (∼50 μl). The filter papers we used include pre-printed circles as guides for blood placement, and by collecting at least one large drop of blood that fills the border of this circle one can be assured of having enough sample. While the process of collecting DBS samples is relatively straightforward, implementing procedures that ensure sufficient sample volume and that avoid blotting and smearing are important for successful quantification of AMH.
Lastly, measuring AMH in DBS complicates efforts to compare results with studies using serum-based methods. Current AMH reference values apply to plasma and serum (Almog et al., 2011), and DBS values will differ substantially due to the presence of lysed erythrocytes and associated matrix effects. This issue is not of particular concern for relative comparisons within studies using DBS samples, but it poses challenges for comparisons with clinical or epidemiological data sets assaying AMH in serum, and for the application of clinically relevant cut-off values. This limitation can be overcome by comparing serum and DBS AMH concentrations for a large set of matched samples to derive a conversion formula that produces serum equivalent values based on DBS results. Our serum-DBS comparison indicates a high level of correlation in AMH values, but a much larger, and more diverse sample is required to establish a reliable and generalizable conversion factor. Alternative approaches include the development of AMH reference values specific to the DBS assay, or for investigators to develop study-specific conversion factors based on matched serum and DBS samples collected from a subset of participants.
AMH is a promising measure of ovarian reserve, with potential utility for clinical practice related to reproductive function, aging and the reproductive capacity of ovaries compromised by iatrogenic treatments. Methodological tools that facilitate research on AMH in non-clinical settings, with larger, more diverse and representative samples, provide opportunities for clarifying the physiological significance of AMH, and for broadening our understanding of the determinants and consequences of variation in female reproductive function, aging and disease.
Authors' roles
The individual contributions of each author are as follows: T.W.M. conceived and designed the study, analyzed and interpreted the data and drafted the manuscript; T.K.W. contributed substantially to study design and manuscript drafting and revision; Y.-y.H. contributed substantially to data acquisition and analysis, as well as manuscript revision; W.E.F. contributed substantially to data acquisition, analysis and interpretation, as well as manuscript revision; M.P. contributed substantially to data acquisition and manuscript revision; L.K. contributed substantially to data acquisition and manuscript revision; C.R.G. contributed substantially to study design, data acquisition and analysis, and manuscript drafting and revision. All authors approved the final version of the manuscript.
Funding
Support provided by the Oncofertility Consortium (NIH UL1 RRDE019587, 5RL1-HD058294 and 1PL1-EB008542) as part of the NIH Roadmap Interdisciplinary Research Consortia.
Conflict of interest
None declared.
References
- Almog B, Shehata F, Suissa S, Holzer H, Shalom-Paz E, La Marca A, Muttukrishna S, Blazar A, Hackett R, Nelson SM, et al. Age-related normograms of serum antimullerian hormone levels in a population of infertile women: a multicenter study. Fertil Steril. 2011;95:2359–2363. doi: 10.1016/j.fertnstert.2011.02.057. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Borders AEB, Grobman WA, Amsden LB, Collins ET, Holl JL. Factors that influence the acceptability of collecting in-home finger stick blood samples in an urban, low-income population. J Health Care Poor Underserved. 2007;18:100–115. doi: 10.1353/hpu.2007.0004. [DOI] [PubMed] [Google Scholar]
- Broer SL, Mol BWJ, Hendriks D, Broekmans FJM. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–714. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Durlinger ALL, Gruijters MJG, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JTJ, Grootegoed JA, Themmen APN. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Fauser BCJM, de Vet A, Laven JSE, de Jong FH, Themmen APN. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- Fong SL, Lugtenburg PJ, Schipper I, Themmen APN, de Jong FH, Sonneveld P, Laven JSE. Anti-mullerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod. 2008;23:674–678. doi: 10.1093/humrep/dem392. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–140. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. An integrative approach to health. Demography. 2010;47:1–22. doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A validated model of serum anti-Mullerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, Lambalk C. Evaluation of anti-Mullerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008;90:737–743. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, Hasegawa Y, Noto RA, Schoenfeld D, MacLaughlin DT. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocr Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- McDade TW. The state and future of blood-based biomarkers in the Health and Retirement Study. Forum Health Econ Policy. 2011;14 article 5. [Google Scholar]
- McDade TW, Shell-Duncan B. Whole blood collected on filter paper provides a minimally invasive method for assessing human transferrin receptor level. J Nutr. 2002;132:3760–3763. doi: 10.1093/jn/132.12.3760. [DOI] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Angold A, Costello EJ, Burleson M, Cacioppo JT, Glaser R, Worthman CM. Epstein–Barr virus antibodies in whole blood spots: a minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000;62:560–567. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- McIlveen M, Skull JD, Ledger WL. Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod. 2007;22:778–785. doi: 10.1093/humrep/del435. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–750. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- van Rooij IAJ, Broekmans FJM, te Velde ER, Fauser BCJM, Bancsi LFJMM, de Jong FH, Themmen APN. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, Themmen AP, te Velde ER. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- Williams SR, McDade TW. The use of dried blood spot sampling in the National Social Life, Health, and Aging Project. J Gerontol B Psychol. 2009;64:I131–I136. doi: 10.1093/geronb/gbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Measurement of gonadotropins in dried blood spots. Clin Chem. 1994;40:448–453. [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997;104:1–22. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]