Abstract
Studies of bear cognition are notably missing from the comparative record despite bears’ large relative brain size and interesting status as generalist carnivores facing complex foraging challenges, but lacking complex social structures. We investigated the numerical abilities of three American black bears (Ursus Americanus) by presenting discrimination tasks on a touch-screen computer. One bear chose the larger of two arrays of dot stimuli, while two bears chose the smaller array of dots. On some trials the relative number of dots was congruent with the relative total area of the two arrays. On other trials number of dots was incongruent with area. All of the bears were above chance on trials of both types with static dots. Despite encountering greater difficulty with dots that moved within the arrays, one bear was able to discriminate numerically larger arrays of moving dots, and a subset of moving dots from within the larger array, even when area and number were incongruent. Thus, although the bears used area as a cue to guide responding, they were also able to use number as a cue. The pattern of performance was similar to that found previously with monkeys, and suggests that bears may also show other forms of sophisticated quantitative abilities.
Keywords: bears, quantity estimation, number, area, ratio
Given that bears have the largest relative brain size of any carnivore (Gittleman, 1986) even in comparison to other social species such as canines, about which there is a recent explosion of research (Hare, 2007; Kubinyi, Virányi, & Miklósi, 2007; Miklói, & Topál, 2004), it is surprising that there are few published reports of their cognitive abilities. Other than reports on visual and spatial abilities (Bacon & Burghardt, 1976; Dungl, Schratter & Huber, 2008; Kelling, et. al, 2006; Perdue, Snyder, Zhihe, Marr & Maple, 2011; Tarou, 2004) and tool use (Bentley-Condit, & Smith, 2010; Deecke, 2012) nothing is known of their cognitive traits in comparison to social species such as corvids (Emery, & Clayton; 2004; Seed, Emery & Clayton, 2009), other large-brained mammals, such as primates (Rosati, Santos & Hare, 2010; Tomasello & Call, 1997), and other carnivores, such as canines (Hare, 2007; Kubinyi, et al., 2007; Miklósi, & Topál, 2004). This is a serious shortcoming in comparative psychology, and remedying this shortcoming could allow for better tests of the Social Intelligence (Humphrey, 1976; Jolly, 1966) and Foraging hypotheses (Milton, 1981, 1988). For instance, demonstrating that a non-social mammal that faces significant foraging challenges exhibits the same sorts of cognitive abilities as more social species within the same order may indicate that adaptive problems faced in the physical environment, such as with foraging, is a better predictor of these kinds of cognitive traits, than is social-living.
There are many examples of non-social animals that face significant foraging problems and demonstrate impressive cognitive skills, such as tool use and observational learning. For example, octopi and cuttlefish have the capacity to make conditional discriminations (Hvorecny et al., 2007; Ikeda, 2009). However, although these species also exhibit problem-solving behaviour similar to that of several vertebrate species, their strategies sometimes demonstrate fixed behavioural patterns, rather than significant behavioural flexibility (Fiorito, Biederman, Davey & Gherardi, 1998; but also see Mather, 2006). Interestingly, Mather (2006) assumes behavioural flexibility in part based on flexible prey choice, and this idea suggest that bears make an interesting test case for assessing such flexibility because bears show flexibility in their diet (Gittleman, 1986). Comparisons of closely related species, such as bears, to other carnivores that vary in their sociality and feeding regime, would be vastly informative with regard to hypotheses about the relative importance of sociality versus foraging demands. Unfortunately, the data with regard to cognitive abilities in carnivores, particularly in ursines and felines, is still too scarce to allow for many direct comparisons.
Clearly, however, it is useful, not only to make comparisons between species that are more closely related, as in the order Carnivore, but also to those species that are more distantly related, as with primates. Researchers can make inferences about when in a species’ evolutionary history a trait may have been most likely to emerge by examining the presence or absence of such traits in species both closely and distantly related. Of course, such inferences must be made cautiously with consideration to the possibility of convergent evolutionary processes. One can look for convergence by examining differences in species’ behavioural ecologies, such as arboreal versus terrestrial lifestyles, different mating strategies, home range size (Perdue et al., 2011) and distribution of food resources (Milton, 1981). By doing so we can best determine which selective pressures are most likely to have given rise to different cognitive abilities, such as spatial memory (Perdue et al., 2011; Tarou, 2005: Vonk & Zamisch, in press), concept formation (Vonk et al., in review), and social cognition (Hare et al., 2002; Miklósi, & Topál, 2004).
One very well studied area in comparative cognition is quantity estimation by non-humans. Numerous species are capable of relative numerousness judgments (fish, Agrillo, Dadda, Serena & Bisazza, 2009; Gomez-Laplaza & Gerlai, 2011; gorillas, Anderson, Stoinski, Bloomsmith, Marr, Smith & Maple, 2005; chimpanzees, Beran, 2001; Boysen & Berntson, 1995; Boysen, Mukobi & Berntson; 1999; rhesus macaques, Beran, 2007, 2008; Brannon, Cantlon & Terrace, 2006; Brannon & Terrace, 2000; Cantlon & Brannon, 2006; pigeons, Emmerton, 1998; Emmerton, Lohmann, & Niemann, 1997; dolphins, Jaakkola, Fellner, Erb, Rodriguez & Guarino, 2005; Kilian, Yaman, von Fersen & Gunturkun, 2003; capuchins, Beran, 2008; Judge, Evans & Vyas, 2005;; African grey parrots, Pepperberg, 2006; Roberts, & Mitchell, 1994; lemurs, Santos, Barnes & Mahagan, 2005, squirrel monkeys, Thomas & Chase, 1980; salamanders, Uller, Jaeger, Guidry & Martin, 2003), including a number of non-primate species such as birds (Emmerton, 1998; Emmerton et al., 1997; Pepperberg, 2006), amphibians (Uller et al., 2003), elephants (Perdue, Talbot, Stone, & Beran, under review) and fish (Agrillo et al., 2009; Gomez-Laplaza & Gerlai, 2011). That is, they are able to choose among sets of items on the basis of the quantities or even numbers of items in those sets.
In some cases, food items are the stimuli to be discriminated, and here it is natural for animals to “go for more” if they can. For example, chimpanzees will select the greater number of food items (e.g., Beran & Beran, 2004), and salamanders will move toward larger numbers of prey items (Uller et al., 2003). In other cases, however, non-edible items are relevant. For example, fish (Agrillo et al., 2009; Gomez-Laplaza & Gerlai, 2011) have been tested for their approach to a larger group of conspecifics. Sometimes, totally arbitrary stimuli are used, presumably because those stimuli release subjects from prepotent responding as would occur to food items or other naturalistic stimuli. For example, primate and bird species are presented with two arrays of dots on a touch-screen computer with one array containing a greater number of dots (Beran, 2007, 2008; Emmerton, 1998) and are required to choose the larger array. Alternatively, they may be required to order dot stimuli in ascending or descending order (Brannon, et al., 2006; Brannon & Terrace, 2000; Cantlon & Brannon, 2006). The use of arbitrary stimuli such as dots allows the researcher to control factors such as size of the stimuli and area covered by the stimuli in relation to the background. By controlling factors such as size, the researcher is able to calculate ratio of area and number between arrays, and can assess which cues the animal is using to make the discrimination. However, only social species have been tested in paradigms carefully controlling factors such as dot size, ratio, area and movement of the stimuli (Beran, 2008; Brannon, Cantlon & Terrace, 2006; Brannon & Terrace, 2000; Cantlon & Brannon, 2006). Therefore, it may often be the case that these species can estimate the relative size or amount of some commodity, but are not necessarily enumerating the specific items.
Some studies indicate that numerical estimation in non-human primate species may be more akin to magnitude estimation than true counting. The performance of both rhesus monkeys and capuchins declines with increased ratio between the quantity in two sets in tasks presenting two arrays of dots that vary in number, as predicted by Weber’s law, which states that the size of a just noticeable difference in stimulus intensity is a constant proportion of the original stimulus magnitude (Beran, 2008; Brannon, et al., 2006; Brannon & Terrace, 2000; Cantlon & Brannon, 2006). For instance, 3 versus 6 dots are easier to discriminate than 3 versus 4 dots. As the ratios increase, the difference between the two arrays is smaller, making it more difficult to discriminate the arrays on a perceptual basis. However, studies also show that such tasks tap into numerical abilities as monkeys’ performance remains high when amount or area are not confounded with number, and even when enumerating subsets within moving arrays (e.g., Beran, 2008). Careful control of such non-numerical factors can indicate whether species are capable of tracking and individuating items of a set, such as members of their group, and using number to do so versus some other stimulus property. Thus, there is reason to speculate that this skill might have emerged in particular in social species, such as primates, cetaceans and social birds such as corvids, and parrots (Pepperberg, 2006). However, it is possible that this is a more evolutionarily ancient capacity that serves as a foundation of numerical cognition and may be shared among other large-brained species that exhibit numerical abilities. One working hypothesis is that animals that forage over large home ranges must evolve the ability to discriminate quantities of items, such as foods, to assist them in choices regarding relative costs and benefits of travel time and energy pay-offs. However, one possibility is that they are very good at assessing quantity or magnitude (approximate amount) for static items but do not need to assess numerosity (exact number of items), and, in particular, have not evolved the capacity for enumerating dynamic stimuli. We test this possibility for the first time.
Here, three American black bears chose larger or smaller arrays of static and moving dots, showing effects of ratio and area that made their performance quite comparable to that of more well-studied, social species. One bear was able to choose a smaller subset from within a larger array of moving dots, even when area was not confounded with number and only number operated as a valid cue to the correct choice. These results from a non-social, large-brained mammal on both static and moving arrays, controlling for area and number, indicate that group- living is not a prerequisite for the capacity to make numerousness judgments and even to enumerate subsets of moving stimuli.
EXPERIMENTAL PROCEDURES
Subjects
Three captive adult American black bear siblings (one female and two males) were tested. The bears had previously participated in studies of cognitive dissonance (West, Jett, Beckman & Vonk, 2010), spatial memory (Zamisch & Vonk, in press) and concept formation (Vonk, Jett & Mosteller, in review), although they had not previously been tested on tasks assessing quantity estimation or numerosity. The research took place in an off exhibit area of the bears’ enclosure at the Mobile Zoo in Wilmer, AL. Testing of the animals complied with the institutional animal care and use review board (IACUC approval # 06091401) and the zoo was compliant with USDA regulations. The experiments provided a form of enrichment for the subjects and did not present any risks or adverse effects.
Materials
A durable Panasonic Toughbook Laptop Computer and 19” vartech armorall Capacitative touch-screen monitor welded to the front of a rolling computer cart comprised the experimental apparatus. Experiments were programmed using Visual Basic for Windows. Stimuli consisted of dots drawn inside of two outlined boxes (59.5 mm × 63.5 mm) which were centered to the left and right of center screen. The dots within the boxes (hereafter arrays) ranged in number from one to ten, and in some conditions, varied by color. Each had a randomly assigned diameter of 3 to 12 mm (Figure 1). Correct responses were followed by a melodic tone and a blank screen paired with food reinforcement, which consisted of portions of the bears’ regular zoo diet (fruits, vegetables) and special treats such as honey roasted peanuts, banana pellets, dried banana chips, yogurt covered raisins, wafer cookies etc. Food was presented by hand by the experimenter. An incorrect response was followed by a buzz tone and a brief time-out with a blank screen.
Figure 1.
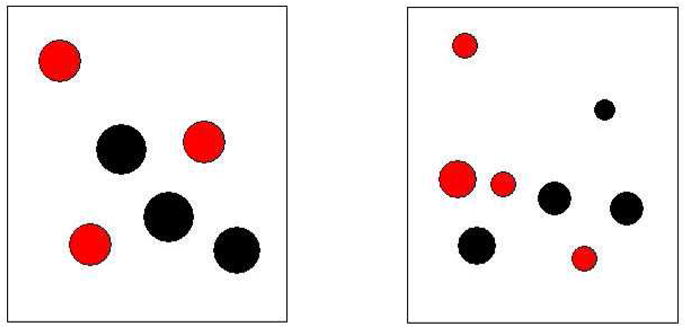
An example trial, showing the outlined boxes with dots contained within each. Shown is an incongruent trial, because the array with the larger number of dots has the smaller overall area of pixilated dots. The trial shown here also shows how the arrays could be subdivided into subsets for which only black dots were relevant for comparing the two arrays.
Procedure
Individual subjects were separated prior to testing, but were tested in the indoor area of a home-cage. Subjects could move freely in their home cages throughout testing sessions. Thus, participation was entirely voluntary. The computer cart was pushed up against the interior mesh separating the human experimenter from the bear, allowing the animal access to the touch-screen monitor. Brutus had been trained to respond by touching the monitor with his nose, while Bella and Dusty predominantly used their paws to touch the screen. The experimenter was centered behind the computer cart observing the animal’s responses on the laptop monitor which was positioned directly behind the touch-screen monitor. The experimenter could not provide any cues because she could not see the bear’s face or paws during the trial and thus did not know if a response was going to be correct or not until the program provided feedback. The experimenter presented the bear with a food reward, as described above, immediately following a correct response at a consistent location. Trials continued automatically until the end of a session.
Training
Bears were trained to discriminate between two arrays of dots, in order to choose the array containing the larger (Brutus) or smaller number of dots (Dusty and Bella). During training, the bears received eight to ten sessions a day, three days a week. The bears had previously been trained to perform two-choice discrimination tasks on a touch-screen computer [see Vonk et al., in review). In order to train them to choose larger or smaller numerical arrays of dots, they were first presented with 20-trial sessions in which they were required to choose accurately between arrays of one dot and arrays of three dots for five out of six consecutive trials within a session. If this criterion was not met, these were the only two arrays that were presented during this session. Once this criterion was met within a session, the bears were presented with arrays of two dots versus arrays of six dots until they performed this discrimination correctly for five out of six consecutive trials within a session. Thus, they could meet both criteria within a single 20-trial session if they were correct on five consecutive trials with each discrimination. It would thus take them a minimum of ten trials to achieve this goal if they were correct on all ten of these trials. Having passed both criteria, arrays of a varying and randomly determined number of dots between one and ten were presented where one set was always numerically larger than the other. These trials could occur within the same 20-trial session if the criterion was met on both simpler discriminations within the same session. When the bears reached this point, the start of each new session involved a new criterion for the warm-up discriminations (1 versus 3 and 2 versus 6) so that instead of having to make 5 of 6 correct responses on these comparisons, the bears were required to meet a criterion of only two consecutive correct trials of 1 versus 3 and two consecutive correct trials of 2 versus 6 dots on each session before the variable arrays began to appear. This procedure was implemented to remind the bears of the task at hand before moving on to the more difficult variable trials. The bears were required to reach a criterion of 80% correct (16/20 correct trials) on four consecutive 20-trial sessions that included both the warm-up trials and more difficult variable trials before moving to 100-trial sessions of variable dot arrays, which comprised the formal testing sessions.
Brutus was trained to choose the array containing the larger number of dots, whereas Dusty and Bella were trained to choose the array containing the smaller number of dots. Because bears have never been tested for numerical abilities, we did not make assumptions a priori about the ease of performing these discriminations. Although prior research has indicated difficulty choosing smaller rather than larger sets (Boysen & Berntson, 1995), such studies have typically involved real food items. When abstract stimuli are used in paradigms where animals are trained to point to smaller arrays of real items, chimpanzees can overcome this bias (Boysen, et al., 1999). Researchers have not explicitly compared the performance of non-humans when choosing larger versus smaller arrays with static, abstract and non-ecologically relevant stimuli. Thus, it was of interest to test the assumption that animals generally are predisposed to choose larger arrays, despite the small sample size.
Brutus was trained to initiate trials by touching a start button, but this procedure led to frustration behaviours for Dusty, so Dusty and Bella were trained without the start button, as this was more similar to prior testing for them (Vonk et al., in review). Given that Dusty experienced difficulty and exhibited an extreme side bias, after 25 sessions with one versus three dots he was presented with a discrimination consisting of one versus eight dots until he acquired this discrimination (24 sessions). However, having acquired this discrimination he was rapidly able to meet the criterion of five out of six correct trials with 1 versus 3 and 2 versus 6 dots, and required only two such sessions before moving on to the formal testing phase.
Testing
Static Sets
Given that the bears had no experience with numerical stimuli, each bear began testing with static stimuli. On each trial, the bears were presented with two arrays of dots contained within a border, identical in size (as in Figure 1), that ranged in number from one to ten and that remained stationary on the screen throughout the trial. The dot arrays were randomly determined on each trial with the constraint that the two arrays could not contain equal numbers of dots. On some trials, the dot number was congruent with its overall amount of pixilated area (i.e., the array containing the larger number of dots was also larger in area). On other trials, the dot number was incongruent with its amount of pixilated area; that is the array containing the larger number of dots contained a smaller area of pixilation. For Brutus, who was trained to choose a larger number of dots, using either number or area as a cue would lead to high levels of performance on congruent trials, but using area as a cue would lead to lower levels of performance on incongruent trials. However, for the bears that were trained to choose the smaller number, on incongruent trials, choosing the larger area would lead them to choose correctly, even though this would be in conflict with the rule that was being reinforced - choose smaller number. Of course, if they were operating on the basis of the rule that was being reinforced, but using area rather than number as a cue, they should have encountered greater difficulty on incongruent trials where area and number were incongruent.
The bears received one to three 100-trial sessions two to three times a week over a period of six months. Brutus and Dusty were given 30 100-trial sessions in this static condition. Bella was given 20 100-trial sessions with static stimuli, as she was already performing at over 75% accuracy on both congruent and incongruent trials at this point.
Moving Sets
The basic procedure was the same, except that, in this experiment, the dots moved on the screen throughout the trial. Each dot was given a randomly selected trajectory and began to move around the screen within its perimeter area (i.e. the boxes that contained each array of dots within a delineated border) as soon as it appeared. The movement took place at one of four randomly selected speeds, and a dot moved in a straight line until it contacted one of the walls of the outline of the stimulus array, at which point it was redirected, as if it had been deflected. Thus, the movement appeared chaotic as dots passed through each other. All dots appeared at once and were moving simultaneously. Movement continued until the subject made a response by touching one of the arrays. Brutus and Dusty completed 30 100-trial sessions with moving stimuli. Bella completed 20 sessions, after which she was dropped from testing, given that she showed no signs of improvement with either congruent or incongruent sets.
Moving Subsets
In order to test whether bears could individuate subsets of moving items, in this experiment the bears were required to enumerate only a subset of dots within each set of moving dots. Each array contained 1 to 12 dots with all dots moving at randomly determined speeds and directions as in the condition above. Each dot was randomly assigned a size and to either the target set (black dots) or a distracter set (red dots; see Figure 1). Both black and red dots moved within the array. On the basis of previous work on color discrimination in bears (Bacon & Burghardt, 1976; Kelling et al., 2006) it was believed that the bears should be easily able to discriminate the black and red dots. The target sets that were paired on each trial could not contain the same number of dots. Once again, the target sets could be either congruent or incongruent in terms of their number and total area. Brutus completed 30 100-trial sessions and Dusty completed 20 100-trial sessions, after which testing was terminated, given that he showed no signs of improvement.
Analyses
Separate binary logistic regressions were conducted for each subject. Performance (correct/incorrect) was regressed on the predictors; difference in number (difference), ratio of number between arrays (ratio), and ratio of area between arrays (area), congruence (congruent/incongruent), and the interaction of congruence with each of the other predictors. Prior to being entered into the regression, each continuous variable was standardized. The same analyses were performed for each discrimination (static, moving and moving with subsets). Alpha was set to .05 for all tests.
RESULTS AND DISCUSSION
Training
Brutus and Bella required 22 and 36 20-trial sessions, respectively, to complete training. Dusty persisted with a left side bias and was moved to training with a discrimination of one versus eight dots, with 24 20-trial sessions consisting solely of this discrimination until he finally seemed to spontaneously acquire this discrimination. After this point, he rapidly reached criterion on one versus three and two versus six dots after only two sessions and proceeded to testing.
Testing
Static Stimuli
If the bears could truly use number as a cue we would predict no or little effect of congruence, although one might expect the congruent trials to be easier, because on these trials they could use both area and number as a cue to guide performance. For Bella and Dusty, performance might be better on incongruent trials if they used larger area to guide performance, although they were reinforced for choosing smaller number. We also expected that ratio would be negatively correlated with performance, given prior work with primates (Beran, 2007; Beran, 2008; Brannon, et al., 2006; Brannon & Terrace, 2000; Cantlon & Brannon, 2006). That is, as the ratio of difference between the arrays increases, performance should worsen.
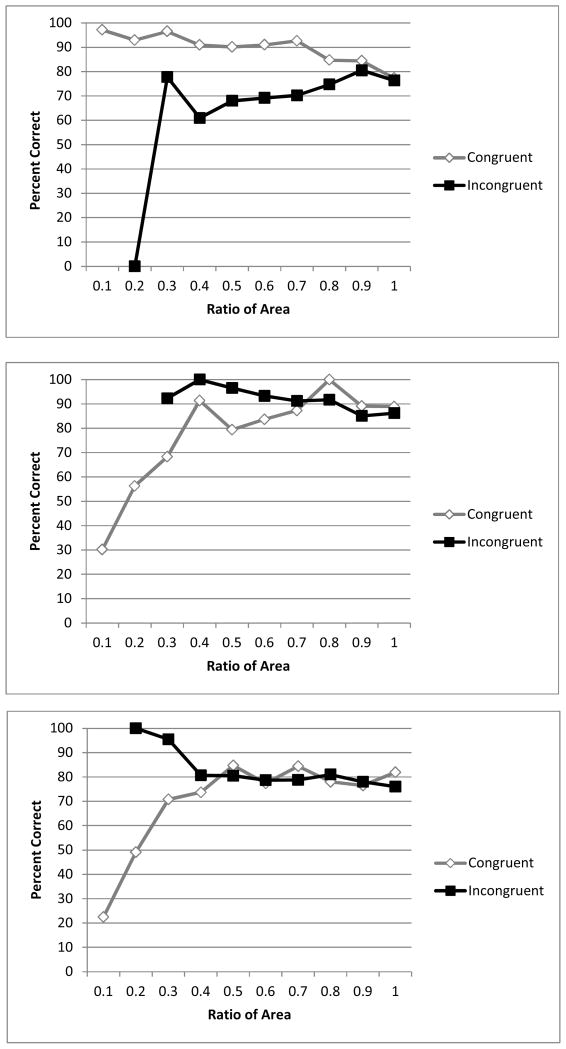
Figure 2 depicts performance as a function of ratio between area in the arrays. Brutus performed better, on average, with congruent trials, as one might expect, (B = .35, Wald = 3.74, p = .05, CI = .05 – 1.42). Area affected Bella’s performance differently on congruent and incongruent trials. For Bella, there was a main effect of congruence (B = 2.70, Wald = 30.65, p < .001, CI = .03 – .18), and congruence interacted with area, (B = 2.95, Wald = 75.77, p < .001, CI = 9.85 – 37.20). When separate logistic regressions were conducted for congruent and incongruent trials, area significantly predicted whether trials would be correct or not on both types of trials, but greater area led to worse performance on incongruent trials (B = −1.13, Wald = 17.62, p < .001, CI = .19 – .55) and better performance on congruent trials (B = 1.83, Wald = 77.60, p < .001, CI = 4.13 – 9.31). Dusty tended to perform better on incongruent trials, and when ratio was smaller, as with Bella, area affected Dusty’s performance differently on incongruent and congruent trials. There was a main effect of congruence (B = −1.42, Wald = 52.50, p < .001, CI = .17 – .36), and ratio, (B = − .65, Wald = 9.45, p =.002, CI = .35 – .79), and congruence interacted with area, (B = 1.55, Wald = 94.36, p < .001, CI = 3.45 – 6.44). When separate logistic regressions were conducted for congruent and incongruent trials, larger area led to worse performance on incongruent trials (B = −.44, Wald = 12.65, p < .001, CI = .51 – .82) and better performance on congruent trials (B = 1.11, Wald = 120.78, p < .001, CI = 2.49 – 3.70). Figure 2 depicts performance as a function of ratio between area in the arrays.
Figure 2.
Percentage correct as a function of the ratio between area between arrays, on both congruent and incongruent trials, for Brutus, who chose larger (top), Bella (middle) and Dusty (bottom), who chose smaller, with static stimuli.
Bella and Dusty’s pattern of responding suggests that they were choosing based on larger area, which allowed them to perform better on incongruent trials when the smaller number of dots contained the larger area of pixilation. However, all three bears reached above chance levels of performance on both congruent and incongruent trials, so the use of area was not the whole story, and the bears often relied on number rather than area to make correct responses (Binomial tests, all ps < .001). With these static data, only Dusty showed significant effects of ratio, which, according to Weber’s law, indicates analogue magnitude estimation (see also Beran, 2008; Dehane, 1992; Feigenson, Dehaene, & Spelke, 2004). Analogue magnitude estimation is thought to operate when individuals must represent large approximate quantities. Errors generally increase in proportion to the size of the set of items, for which quantity is being estimated (Feigenson et al., 2004).
Moving Stimuli
Once again, separate binary logistic regressions were conducted for each subject. Performance (correct/incorrect) was regressed on the predictors; difference in number (difference), ratio of number between arrays (ratio), and ratio of area between arrays (area), congruence (congruent/incongruent), and the interaction of congruence with each of the other predictors. Here we expected the same effects as before; negative relationships between ratio and performance and perhaps effects of area on performance. Brutus might be expected to perform slightly better on congruent trials, while the opposite might be true for Bella and Dusty, as choosing larger area and smaller number were in conflict on congruent trials.
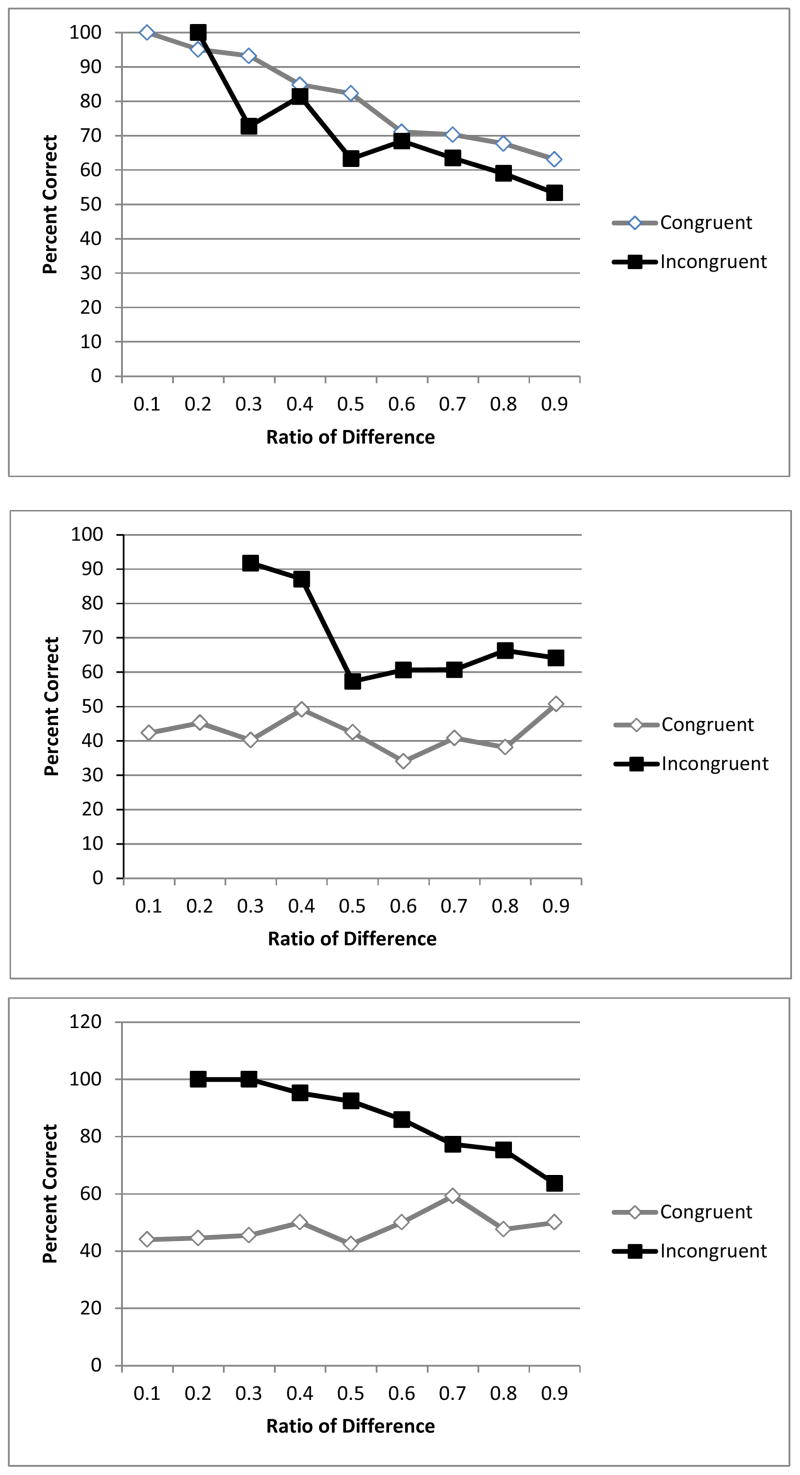
As with static dot arrays, Brutus performed better on congruent trials when he could use both number and area as a cue, and, like primates tested previously, he performed better with smaller ratios between the arrays, main effect of congruence (B = .63, Wald = 22.66, p < .001, CI = 1.45 – 2.44), and ratio, (B = − .44, Wald = 13.88, p < .001, CI = .51 – .81), and an interaction of congruence and area (B = − .71, Wald = 25.48, p < .001, CI = .38 – .65). When separate logistic regressions were conducted for congruent and incongruent trials, larger area predicted better performance on incongruent trials (B = .25, Wald = 6.68, p = .01, CI = 1.06 – 1.56) and worse performance on congruent trials (B= −.45, Wald = 20.51, p < .001, CI = .52 – .77). These results indicate that, with moving stimuli, Brutus relied more heavily on area as a cue. Also, ratio now was significantly related to his performance, suggesting that he was using magnitude estimation to perform the task. Brutus showed the expected patterns in that he performed better with larger differences in number and smaller ratios between number. Figure 3 depicts performance as a function of ratio between numbers of dots in the arrays.
Figure 3.
Percentage correct as a function of ratio between number in the two arrays, on both congruent and incongruent trials, for Brutus, who chose larger (top), Bella (middle) and Dusty (bottom), who chose smaller, with moving stimuli.
Overall, Bella performed very well on incongruent trials when she could choose larger area, but not on congruent trials when area conflicted with number, significant main effect of congruence (B = −1.15, Wald = 51.58, p < .001, CI = .23 – .43) and significant interactions of congruence and each of the other predictors; difference (B = −.55, Wald = 6.56, p = .01, CI = .38 – .89), ratio (B = −.60, Wald = 9.98, p =.002, CI = .38 – .80), and area, (B = .79, Wald = 25.94, p < .001, CI = 1.63 – 3.00). When separate logistic regressions were conducted for congruent and incongruent trials, larger area predicted poorer performance on incongruent trials (B = −.31, Wald = 6.66, p= .01, CI = .58 – .93) and better performance on congruent trials (B = .49, Wald = 23.29, p < .001, CI = 1.34 – 1.99). A greater difference between number in the two arrays predicted better performance only on incongruent trials, (B = .47, Wald = 6.16, p= .01, CI = 1.10 – 2.32). A greater ratio predicted worse performance only on congruent trials, (B = −.45, Wald = 11.20, p = .001, CI = .49 – .83).
Dusty generally performed better on incongruent trials and his performance was affected differently by difference, ratio and area depending on whether the trials were incongruent or congruent. There was a main effect of congruence (B= −2.02, Wald = 132.68, p < .001, CI = .09 – .19), and significant interactions of congruence and each of the other predictors; difference (B = .89, Wald = 13.28, p <.001, CI = 1.51 – 3.92), ratio (B = .79, Wald = 11.07, p =.001, CI = 1.38 – 3.51), and area, (β = 2.38, Wald = 193.26, p < .001, CI = 7.75 – 15.18). When separate logistic regressions were conducted for congruent and incongruent trials, greater area led to worse performance on incongruent trials (B = −.79, Wald = 35.63, p < .001, CI = .35 – .59) and better performance on congruent trials (B = 1.59, Wald = 214.64, p < .001, CI = 3.97 – 6.08). A greater difference tended produce lower performance on incongruent trials, (B = −.38, Wald = 2.89, p = .09, CI = .44 – 1.06) and higher performance on congruent trials (B = .51, Wald = 26.06, p < .001, CI = 1.31 – 2.03). A greater ratio led to lower performance on both incongruent (B = −1.31, Wald = 42.66, p < .001, CI = .18 – .40) and congruent trials, (B= −.52, Wald = 16.13, p < .001, CI = .46 – .77), which is the expected result based on past research.
That Bella and Dusty continued to perform very well on incongruent trials but very poorly on congruent trials indicated that they were continuing to use area as a cue, which they could do more easily on trials when there was a larger numerical difference, and a smaller ratio. However, if area was the only cue they were using, they should have been as far below chance on congruent trials as they were above chance on incongruent trials, and this was not the case. It may have been the case that they relied on area when it could be used as a cue, but attempted to use number to some degree on some other trials, although they failed to do so reliably. All bears showed the expected patterns of performing better with greater differences in numerical distance, and smaller ratios between the stimuli.
Moving Stimuli with Subsets
Bella was discontinued in the experiment based on her overall low level of performance with moving stimuli, and the need to continue testing her in other experiments. Once again, separate binary logistic regressions were conducted for both Brutus and Dusty. Performance (correct/incorrect) was regressed on the predictors; difference in number of subsets (difference), ratio of number between subsets in arrays (ratio), and ratio of area between subsets in arrays (area), congruence (congruent/incongruent), and the interaction of congruence with each of the other predictors.
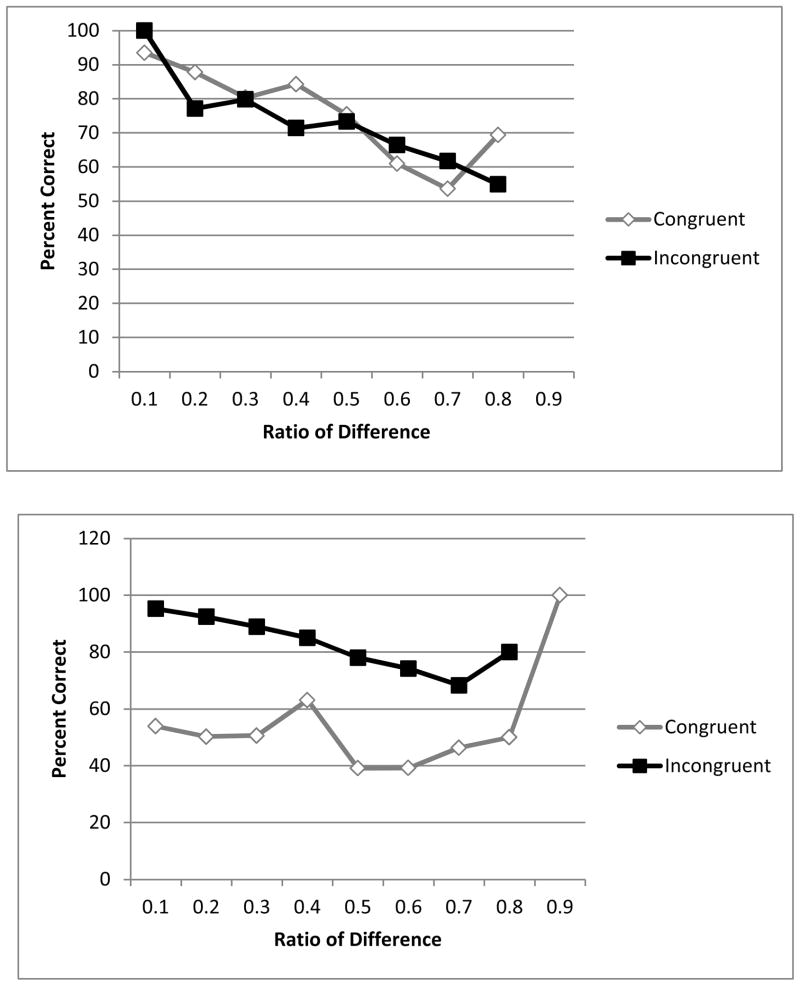
Brutus was able to accurately enumerate a target set of moving stimuli from among a larger set of moving stimuli, without relying exclusively on area as a cue. Regression analysis revealed a main effect of ratio (B = −.46, Wald = 30.27, p < .001, CI = .54 – .75), and an almost significant interaction of congruence and difference (B = .34, Wald = 3.52, p = .06, CI = .99 – 2.01). A greater numerical difference between arrays led to better performance on only the congruent trials, (B = .34, Wald = 11.38, p = .001, CI = 1.15 – 1.70). The lack of a relationship on incongruent trials may have been an artifact of the anomalous poor performance with a difference of six dots between sets on incongruent trials where Brutus was incorrect on the single trial of this type. Importantly, there was no overall difference between Brutus’ performance on congruent and incongruent trials in enumerating a subset among moving stimuli. Binomial tests reveal that Brutus was above chance on both congruent and incongruent trials (both ps < .001). In addition, although his performance showed the expected trends for difference and ratio, there was no longer an effect of area.
Dusty continued to perform above chance on only incongruent trials (Binomial test, p < .001; on congruent trials, p = .14), suggesting that he continued to choose on the basis of area rather than number. Regression analysis supported this conclusion, main effect of congruence (B = −2.11, Wald = 110.52, p < .001, CI = .08 – .18), and ratio, (B = −.47, Wald = 14.12, p < .001, CI = .49 – .80) and a significant interaction of congruence and area, (B = 2.05, Wald = 131.06, p < .001, CI = 5.48 – 11.07). When separate logistic regressions were conducted for congruent and incongruent trials, larger area predicted worse performance on incongruent trials (B = −.75, Wald = 28.52, p < .001, CI = .36 – .62) and better performance on congruent trials (B = 1.30, Wald = 136.68, p < .001, CI = 2.96 – 4.58). Figure 4 depicts performance as a function of ratio between the number of dots in the arrays.
Figure 4.
Percentage correct as a function of ratio between number in the two arrays, on both congruent and incongruent trials, for Brutus, who chose larger (top), and Dusty (bottom), who chose smaller, with subsets of moving stimuli.
Conclusion
Many nonhuman species can discriminate smaller or larger amounts or even smaller or larger numbers of items when static stimuli are presented in the visual domain. Less evidence is available for moving stimuli, but some primates seem capable in discriminating these sets (e.g., Beran, 2008). However, very few attempts have been made to assess the quantitative skill of carnivores, and there is no evidence as to whether bears can make these kinds of judgments. The present results suggest that they can, and at least one of the bears showed a pattern of results that matches nicely that reported for nonhuman primates – namely that this bear was using the number of dots to control responding rather than some other stimulus property. But, despite showing proficiency in using number as a critical cue in making these discriminations and comparisons, other stimulus properties were highly salient for bears as well.
These results suggest that it is easier for bears to choose the larger amount rather than the smaller amount, even with two-dimensional abstract stimuli, and even when they are reinforced for choosing smaller. This is consistent with previous work with primates, which have been found to have difficulty inhibiting responses to select smaller amounts of food (Boysen & Berntson, 1995; Boysen, Berntson & Mukabi; 2001), although this bias can sometimes be overcome when abstract symbols replace the food items (Boysen, Mukobi & Berntson, 1999; Shumaker, Palkovich, Beck, Guagnano, & Morowitz, 2001). The data suggest that bears are attracted naturally to focus on choosing a larger number over a smaller one, even when number was in conflict with area. One bear maintained that pattern even for the most difficult task, in which subsets of moving stimulus arrays had to be compared while the bear ignored the distractor’s contribution to the overall number in that set. Thus, choosing a greater amount or a greater number of items when comparing two or more sets may be more intuitive than choosing a smaller amount or smaller number - even with arbitrary two-dimensional stimuli. This is a finding that also matches that found with human children, who also prefer to “go for more” (Estes, 1976).
The bears seemingly found it easier to enumerate static rather than moving stimuli. This may not be surprising, given that they have not evolved to live in social groups and thus would not need to use number naturally to track individual members of a group, but rather to enumerate quantities of food items. Even live food items, such as fish, do not need to be individuated and may be quantified by estimating size or area. In comparison, for social animals, such as primates, it may be important to track the presence of individual members of a moving group; thus they may have evolved the ability to rely more heavily on number than area when performing such tasks (see Beran, 2008). Overall, however, bears showed similar trends to those found in primates previously tested in analogous tasks (Beran, 2008), in that they were more likely to choose correctly on trials with a smaller ratio between number and area between arrays. Thus, group-living is not a necessary prerequisite for the capacity to enumerate static or moving stimuli, or subsets of moving stimuli. Perhaps such an ability has evolved in order to assist predators in discriminating among groups of prey that contain more vulnerable members, or more appetizing members. In this case, perhaps it is an ability of predator but not prey species among non-social animals.
These data are among the first to show that bears, an understudied species in comparative psychology and biology, may have evolved cognitive mechanisms equivalent to their distant primate relatives, at least within the quantitative domain. Only further research can determine where their capacities lie within the social reasoning domain, and other areas of physical reasoning, such as causal reasoning, tool use, understanding of time and space, and categorization of natural stimuli. Such research will be critical for determining the necessary social and physical ecological factors for shaping an organism’s cognitive development. Too often, researchers focus exclusively on species most closely related to humans, or those most amendable to testing, in examining particular cognitive traits or using standard methodology. Other species must be tested for comparative psychology to be truly comparative, and for a fuller understanding of the evolution of cognition to occur. More research with bears, along with other carnivores and non-primate species will provide exactly this result.
Demonstrating that important research questions can be addressed through the use of touch-screen technologies in this species for the first time is an important methodological advance. Bears are of particular interest because of their large brains, their relatively solitary lifestyles and flexible foraging patterns within the order Carnivore. Important comparisons to other species such as wild and domestic canines will have much to tell us about the evolution of social and physical reasoning. In conjunction with other work underway (Vonk et al., in review), this work suggests that bears are rapid problem-solvers, capable of abstract concept formation, and may change the bias of researchers to focus on group-living as the driving force for complex cognition. That touch screens can be used effectively with bears to test a wide range of cognitive abilities now provides the means to examine more closely their cognitive competence in comparison to other more widely studied species, such as primates, that have been performing computerized tasks for decades. This is an exciting possibility that such divergent species can be given identical tests toward the end of promoting a fuller picture of comparative cognition and the diverse forces giving rise to both similar and distinct traits.
Highlights.
This is the first demonstration of quantity estimation in bears.
A nonsocial species can enumerate moving stimuli and subsets of stimuli.
Bears predominantly appeared to use area but could also use number as a cue.
Bears showed effects of ratio and difference comparable to those of primates.
Bears performed ‘better’ when choosing ‘larger’ relative to ‘smaller’ amounts.
Acknowledgments
We are indebted to the Mobile Zoo, especially its director, John Hightower. Without his support and assistance these experiments could not have been conducted. In addition, special thanks to Stephanie Jett for her assistance during data collection and Dr. Joan Sinnott for her support. The research was supported by the Aubrey Keith Lucas and Ella Ginn Lucas Endowment for Faculty Excellence Awards to JV and by NSF Grant #0924811 and NIH Grant #060563 to MJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer Vonk, Email: jenvonk@gmail.com.
Michael J. Beran, Email: mjberan@yahoo.com.
References
- Agrillo C, Dadda M, Serena G, Bisazza A. Use of number by fish. PLoS ONE. 2009;4:e4786. doi: 10.1371/journal.pone.0004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson US, Stoinski TS, Bloomsmith MA, Marr MJ, Smith AD, Maple TS. Relative numerousness judgment and summation in young and old Western Lowland gorillas. Journal of Comparative Psychology. 2005;119:285– 295. doi: 10.1037/0735-7036.119.3.285. [DOI] [PubMed] [Google Scholar]
- Bacon ES, Burghardt GM. Learning and color discrimination in the American black bear. International Conference on Bear Research and Management. 1976b;3:27–36. [Google Scholar]
- Bentley-Condit VK, Smith EO. Animal tool use: Current definitions and an updated comprehensive catalog. Behaviour. 2010;147:185–221. [Google Scholar]
- Beran MJ. Summation and numerousness judgments of sequentially presented sets of items by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 2001;115:181–191. doi: 10.1037/0735-7036.115.2.181. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Monkeys (Macaca mulatta and Cebus apella) track, enumerate and compare multiple sets of moving items. Journal of Experimental Psychology, Animal Behaviour Processes. 2008;34:63–74. doi: 10.1037/0097-7403.34.1.63. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Rhesus monkeys (Macaca mulatta) enumerate sequentially presented sets of items using analog numerical representations. Journal of Experimental Psychology: Animal Behaviour Processes. 2007;33:42– 54. doi: 10.1037/0097-7403.33.1.42. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Beran MM. Chimpanzees remember the results of one-by-one addition of food items to sets over extended time periods. Psychological Science. 2004;15:94–99. doi: 10.1111/j.0963-7214.2004.01502004.x. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG. Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behaviour Processes. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG, Mukabi KL. Size Matters; Impact of item size and quantity on array choice by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 2001;115:106–110. doi: 10.1037/0735-7036.115.1.106. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Mukobi KL, Berntson GG. Overcoming response bias using symbolic representations of number by chimpanzees (Pan troglodytes) Animal Learning and Behaviour. 1999;27:229–235. [Google Scholar]
- Brannon EM, Cantlon JF, Terrace HS. The role of references points in ordinal numerical comparisons by rhesus macaques (Macaca mulatta) Journal of Experimental Psychology: Animal Behaviour Processes. 2006;32:120–134. doi: 10.1037/0097-7403.32.2.120. [DOI] [PubMed] [Google Scholar]
- Brannon EM, Terrace HS. Representation of the numerosities 1–9 by rhesus macaques (Macaca mulatta) Journal of Experimental Psychology: Animal Behaviour Processes. 2000;26:31–49. doi: 10.1037//0097-7403.26.1.31. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM. Semantic congruity affects numerical judgments similarly in monkeys and humans. Proceedings of the National Academy of Sciences, USA. 2006;102:16507–16511. doi: 10.1073/pnas.0506463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deecke V. Tool-use in the brown bear (Ursus arctos) Animal Cognition. 2012 doi: 10.1007/s10071-012-0475-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Varieties of numerical abilities. Cognition. 1992;44:1–42. doi: 10.1016/0010-0277(92)90049-n. [DOI] [PubMed] [Google Scholar]
- Dungl E, Schratter D, Huber L. Discrimination of face-like patterns in the giant panda (ailuropoda melanoleuca) Journal of Comparative Psychology. 2008;122:335–343. doi: 10.1037/0735-7036.122.4.335. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Clayton N. The Mentality of crows: Convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–1907. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- Emmerton J. Numerosity differences and effects of stimulus density on pigeons’ discrimination performance. Animal Learning and Behaviour. 1998;26:243– 256. [Google Scholar]
- Emmerton J, Lohmann A, Niemann J. Pigeons’ serial ordering of numerosity with visual arrays. Animal Learning and Behaviour. 1997;25:234– 244. [Google Scholar]
- Estes KW. Nonverbal discrimination of more and fewer elements by children. Journal of Experimental Child Psychology. 1976;21:393–405. [Google Scholar]
- Feigenson L, Dehaene S, Spelke E. Core systems of number. Trends in Cognitive Sciences. 2004;8:307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Fiorito G, Biederman GB, Davey VA, Gherardi F. The role of stimulus preexposure in problem solving by octupus vulgaris. Animal Cognition. 1998;1(2):107–112. doi: 10.1007/s100710050015. [DOI] [PubMed] [Google Scholar]
- Gittleman JL. Carnivore brain size, behavioural ecology, and phylogeny. Journal of Mammalogy. 1986;67:23–36. [Google Scholar]
- Gomez-Laplaza LM, Gerlai R. Can Angelfish (Pterophyllum scalare) count? Discrimination among different shoal sizes follows Weber’s law. Animal Cognition. 2011;14:1–9. doi: 10.1007/s10071-010-0337-6. [DOI] [PubMed] [Google Scholar]
- Hare B. From nonhuman to human mind: What changed and why? Current Directions in Psychological Science. 2007;16:60–64. [Google Scholar]
- Humphrey NK. The social function of intellect. In: Bateson PPG, Hinde RA, editors. Growing Points in Ethology. Cambridge UK: Cambridge University Press; 1976. pp. 303–317. [Google Scholar]
- Hvorecny LM, Grudowski JL, Blakeslee CJ, Simmons TL, Roy PR, Brooks JA, Boal JG. Octopuses (octopus bimaculoides) and cuttlefishes (sepia pharaonis, S. officinalis) can conditionally discriminate. Animal Cognition. 2007;10(4):449–459. doi: 10.1007/s10071-007-0085-4. [DOI] [PubMed] [Google Scholar]
- Ikeda Y. A perspective on the study of cognition and sociality of cephalopod mollusks, a group of intelligent marine invertebrates. Japanese Psychological Research. 2009;51(3):146–153. doi: 10.1111/j.1468-5884.2009.00401.x. [DOI] [Google Scholar]
- Jaakkola K, Fellner W, Erb L, Rodriguez M, Guarino E. Understanding of the concept of numerically “less” by bottlenose dolphins (Tursiops truncatus) Journal of Comparative Psychology. 2005;119:286–303. doi: 10.1037/0735-7036.119.3.296. [DOI] [PubMed] [Google Scholar]
- Jolly A. Lemur social behaviour and primate intelligence. Science. 1966;153:501–506. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- Judge PG, Evans TA, Vyas DK. Ordinal representation of numeric quantities by brown capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behaviour Processes. 2005;31:79–94. doi: 10.1037/0097-7403.31.1.79. [DOI] [PubMed] [Google Scholar]
- Kelling AS, Synder RJ, Jackson M Marr, Bloomsmith MA, Gardner W, Maple TM. Color vision in the panda (Ailuropoda melanoleuca) Learning and Behaviour. 2006;34:154–161. doi: 10.3758/bf03193191. [DOI] [PubMed] [Google Scholar]
- Kilian A, Yaman S, von Fersen L, Gunturkun O. A bottlenose dolphin discriminates visual stimuli differing in numerosity. Learning and Behaviour. 2003;31:133–142. doi: 10.3758/bf03195976. [DOI] [PubMed] [Google Scholar]
- Kubinyi E, Virányi Z, Miklósi A. Comparative social cognition: From wolf and dog to humans, Comparative Cognition and Behaviour Review. 2007;2:26–46. [Google Scholar]
- Mather JA. Behaviour development: A cephalopod perspective. International Journal of Comparative Psychology. 2006;19(1):98–115. [Google Scholar]
- Miklósi A, Topál J. Review comparative social cognition: What can dogs teach us? Animal Behaviour. 2004;67:995–1004. [Google Scholar]
- Milton K. Distribution patterns of tropical plant foods as an evolutionary stimulus to primate mental development. American Anthropologist. 1981;83:534–548. [Google Scholar]
- Milton Katharine. Foraging behaviour and the evolution of primate intelligence. In: Byrne Richard W, Whiten Andrew., editors. Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans. New York, NY, US: Clarendon Press/Oxford University Press; 1988. pp. 285–305. [Google Scholar]
- Murofushi K. Numerical matching behavior by a chimpanzee (pan troglodytes): Subitizing and analogue magnitude estimation. Japanese Psychological Research. 1997;39(3):140–153. doi: 10.1111/1468-5884.00050. [DOI] [Google Scholar]
- Pepperberg IM. Grey parrot numerical competence: A review. Animal Cognition. 2006;9:377– 391. doi: 10.1007/s10071-006-0034-7. [DOI] [PubMed] [Google Scholar]
- Perdue BM, Synder RJ, Zhihe Z, Marr MJ, Maple T. Sex differences in spatial ability: a test of the range size hypothesis in the order Carnivora. Biology Letters. 2011;7:380–383. doi: 10.1098/rsbl.2010.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue BM, Talbot CF, Stone A, Beran MJ. Putting the elephant back in the herd: Elephant relative quantity judgments match those of other species. Animal Cognition. under review doi: 10.1007/s10071-012-0521-y. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Mitchell S. Can a pigeon simultaneously process temporal and numerical information? Journal of Experimental Psychology: Animal Behaviour Processes. 1994;20:66–78. [Google Scholar]
- Rosati AG, Santos LR, Hare B. Primate social cognition: Thirty years after Premack and Woodruff. In: Ghazanfar A, Platt M, editors. Primate Neuroethology. Oxford: Oxford University Press; 2010. pp. 117–143. [Google Scholar]
- Santos LR, Barnes JL, Mahajan N. Expectations about numerical events in four lemur species Eulemur fulvus, Eulemur mongoz, Lemur catta and Varecia rubra, Animal Cognition. 2005;8:253– 262. doi: 10.1007/s10071-005-0252-4. [DOI] [PubMed] [Google Scholar]
- Seed A, Emery N, Clayton N. Intelligence in corvids and apes: A Case of convergent evolution? Ethology. 2009;115:401–420. [Google Scholar]
- Shumaker RW, Palkovich AM, Beck BB, Guagnano GA, Morowitz H. Spontaneous use of magnitude discrimination and ordination by the orangutan (Pongo pygmaeus) Journal of Comparative Psychology. 2001;115:385–391. [PubMed] [Google Scholar]
- Tarou LR. An examination of the role of associative learning and spatial memory in foraging of two species of bear (family: Ursidae) (Ailuropoda melanoleuca, Tremarctos ornatus), Dissertation Abstracts International: Section B: The Sciences and Engineering. 2004;64:5260. [Google Scholar]
- Thomas RK, Chase L. Relative numerousness judgments by squirrel monkeys. Bulletin of the Psychonomic Society. 1980;16:79–82. [Google Scholar]
- Tomasello M, Call J. Primate Cognition. Oxford University Press; New York NY: 1997. [Google Scholar]
- Uller C, Jaeger R, Guidry G, Martin C. Salamanders (Plethodon cinereus) go for more: Rudiments of number in an amphibian. Animal Cognition. 2003;6:105–112. doi: 10.1007/s10071-003-0167-x. [DOI] [PubMed] [Google Scholar]
- Vonk J, Jett SE, Mosteller KW. Concepts in American black bears. Cognition. In Revision 2011 Feb 18; Submitted to. [Google Scholar]
- West S, Jett SE, Beckman T, Vonk J. The Phylogenetic roots of cognitive dissonance. Journal of Comparative Psychology. 2010;124:425–432. doi: 10.1037/a0019932. [DOI] [PubMed] [Google Scholar]
- Zamisch V, Vonk J. Spatial memory in captive American black bears (Ursus Americanus) Journal of Comparative Psychology. doi: 10.1037/a0028081. In Press. [DOI] [PubMed] [Google Scholar]