Abstract
Obesity adversely affects myocardial metabolism, efficiency, and diastolic function. Our objective was to determine if weight loss can ameliorate obesity-related myocardial metabolism and efficiency derangements and that these improvements directly relate to improved diastolic function in humans.
We studied 30 obese (body mass index [BMI]>30kg/m2) subjects with positron emission tomography (myocardial metabolism, blood flow) and echocardiography (structure, function) before and after marked weight loss from gastric bypass surgery (N=10) or moderate weight loss from diet (N=20).
Baseline BMI, insulin resistance, hemodynamics, left ventricular (LV) mass, systolic function, myocardial oxygen consumption (MVO2), and fatty acid (FA) metabolism were similar between the groups. MVO2/gram decreased after diet-induced weight loss (P=0.009). Total MVO2 decreased after dietary (P=0.02) and surgical weight loss (P=0.0006) and was related to decreased BMI (P=0.006). Total myocardial FA utilization decreased (P=0.03), and FA oxidation trended lower (P=0.06) only after surgery. FA esterification and LV efficiency were unchanged. After surgical weight loss, LV mass decreased by 23%, (Doppler-derived) E/E’ by 33%, and relaxation increased (improved) by 28%, and. Improved LV relaxation related significantly to decreased BMI, insulin resistance, total MVO2, and LV mass but not FA utilization. Decreased total MVO2 predicted LV relaxation improvement independent of BMI change (P=0.02).
Weight loss can ameliorate the obesity-related derangements in myocardial metabolism and LV structure and diastolic function. Decreased total MVO2 independently predicted improved LV relaxation, suggesting that myocardial oxygen metabolism may be mechanistically important in determining cardiac relaxation.
INTRODUCTION
Obesity is a major risk factor for heart failure (1). Data suggest that ‘obesity cardiomyopathy,’ is a distinct clinical entity characterized by left ventricular (LV) remodeling, reduced cardiac efficiency, and LV diastolic dysfunction, which may progress to systolic dysfunction (2-4). The mechanisms responsible for the relation between obesity and heart failure in humans are not well-understood but may, in part, involve altered myocardial substrate metabolism (4, 5). Results from studies in animal models demonstrate obesity increases myocardial fatty acid (FA) metabolism and oxygen consumption (MVO2) leading to increased oxidative stress, cardiac dysfunction and apoptosis (6-9). Interventions that reduce FA accumulation and/or oxidative stress in cardiac myocytes prevent the development of myocardial dysfunction in these models (6). Excessive amounts of FA metabolites in skeletal muscle also adversely affect ATP generation and function (10). In humans, obesity and insulin resistance are related to similar increases in MVO2 and myocardial FA metabolism and oxidative stress markers (4, 11).
Weight loss in humans ameliorates obesity-related cardiac hypertrophy and diastolic dysfunction (12, 13, 14). Weight loss also improves the excessive myocardial FA uptake and storage related to obesity (15, 16). However, the effects of weight loss on myocardial FA oxidation, MVO2, and efficiency are unknown. Furthermore, it is not known whether weight loss-induced changes in myocardial metabolism predict improvement in cardiac function in humans. We hypothesized 1) weight loss would reverse the high rates of MVO2 and FA metabolism and decrease the cardiac inefficiency associated with obesity, 2) improvements in MVO2 and/or FA metabolism would predict improved LV relaxation. To evaluate the relationship between metabolism and functional changes over a wide range of weight loss, two different modes of weight loss were studied, diet and gastric bypass surgery. Positron emission tomography (PET) was used to quantify MVO2 and myocardial FA metabolism. Echocardiography with tissue Doppler imaging was used to quantify cardiac structure, systolic and diastolic function (LV relaxation [E’] and E/E’).
METHODS AND PROCEDURES
Study subjects
Obese (body mass index [BMI]> 30 kg/m2) subjects enrolled in this prospective, interventional study. Surgery subjects were recruited from the bariatric surgery center at Barnes-Jewish Hospital, diet subjects from the Volunteer for Health office. All subjects completed screening, including a history, physical examination, and blood testing. Individuals with suggestive histories were screened for coronary disease and sleep apnea using exercise stress echocardiography and polysomnography. Subjects were sedentary upon study entry. Exclusion criteria: weight > 159kg, age <21 or >50 years (to decrease the impact of aging) (17), insulin-requiring diabetes, heart failure, a history of coronary disease, chest pain, or untreated sleep apnea, being an active smoker, pregnant, lactating, or postmenopausal. Subjects in the surgery group and the diet groups were purposefully studied after different periods of weight loss (16±5 and 8±2 months, respectively) in order to maximize weight loss and weight stability, (14, 18), and minimize weight regain and subject drop out (14). This study was designed to recruit subjects who would undergo different weight loss interventions in order to obtain a large spectrum of weight loss, and hence, metabolic and functional changes for comparison. Both groups were screened and imaged over the same 5-year time frame by the same research team, using the same equipment. Written, informed consent was obtained before subject participation.
Gastric bypass surgery
The same surgeon (J.C.E.) performed all bypass procedures at Barnes-Jewish Hospital using standard surgical techniques. Briefly, a small (~20 mL) proximal gastric pouch was created by stapling the stomach, and a 75 cm Roux-en-Y limb constructed by transecting the jejunum distal to the ligament of Treitz and creating a jejunojejunostomy 75 cm distal to the transection.
Diet
Diet subjects participated in 20 group behavior modification sessions, led by a behaviorist, a registered dietitian, and a physical therapist. The meal plans ranged from 1200 to 1500 kcal/day, depending on subject sex and BMI. The plans were designed to achieve a ≤1% body weight/week. Macronutrient content of the plan followed the Expert Panel’s recommendations on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, convened by the NIH and the Dietary Guidelines for Americans (19). Subjects completed daily food records. Subjects were taught a variety of weight-management skills. The exercise approach included a balance of strength, flexibility, balance, and endurance. Goals gradually increased to 30 minutes of exercise, 5 days/week. Two weeks prior to follow-up imaging studies, calorie intake was adjusted to maintain stable body weight.
Cardiac PET imaging and image analysis
The evening before the imaging study, at a standardized time, all subjects were given a standard meal containing 12 kcal/kg adjusted body weight (= ideal body weight +[(actual body weight-ideal body weight) × 0.25]). After this, subjects remained fasted until their imaging studies were completed. All imaging started at 8 AM.
Imaging studies were performed with a Siemens tomograph (ECAT 962 HR+; Siemens Medical Systems, Iselin, NJ). Subjects underwent positioning and transmission scans. Myocardial blood flow (needed for myocardial FA metabolism calculation), MVO2, and FA utilization, oxidation, and esterification were obtained using PET imaging after injections of 15O-water, 1-11C-acetate, and 1-11C-palmitate, using well-validated kinetic models as previously described (4, 17, 20-22). The calculations that describe the relationship between the different measures of FA metabolism are: FA utilization/gram = blood flow/gram × FA uptake/gram × [average plasma free FA at the time of the 1-11C-palmitate injection]; FA utilization/gram = FA oxidation/gram + esterification/gram. The intra-class correlation coefficient for the MVO2 image analysis is 0.96. Total MVO2, FA utilization, and oxidation were calculated by multiplying each by LV mass. One subject did not undergo FA imaging due to technical difficulties.
Measurement of plasma insulin and substrates
Subjects underwent phlebotomy throughout the PET study. Samples were drawn before the study and after each tracer injection during the imaging that followed. Plasma insulin and substrate levels presented here are averages of those measurements. Plasma insulin levels were measured by radioimmunoassay, glucose by automated hexokinase assay. Plasma free FA was measured using an enzymatic method (NEFA C kit, WAKO Chemicals USA, Inc). The homeostasis model assessment of insulin resistance (HOMA) was used to calculate insulin resistance, using the first AM, fasting glucose and insulin levels (23).
Echocardiography
Immediately following MVO2 measurement, all subjects underwent a complete two-dimensional, M-mode, and Doppler echocardiographic study using second harmonic imaging. Two-dimensional guided M-mode measurements were obtained from the parasternal cross-sectional view for LV diameter and wall thickness measurements. Ejection fraction was calculated using the modified Simpson method. LV end-diastolic and left atrial volumes were measured using the method of discs. Left atrial volumes were indexed to body surface area. LV mass was measured using the area-length method. LV mass was also indexed to height2.7. Pulsed-wave Doppler-derived transmitral inflow measurements were obtained from the apical four-chamber view with the sampling gate at the mitral leaflet tips. The early diastolic (E) and late atrial (A) velocities were measured and the E/A ratio, a load-dependent measure of diastolic function was calculated. The relatively load-independent tissue Doppler-derived measures of LV systolic function and relaxation, S’ and E’, respectively, were measured at the septal and lateral annulus and averaged to obtain one S’ and one E’ for each echocardiogram (24). E/E’(septal) ratio was also obtained (25). LV mass, E’, and E/E’ were not obtained on 1, 1, and 5 subjects, respectively. Calculations: cardiac output = time-velocity integral of the LV outflow tract × its area; cardiac work = cardiac output × mean arterial pressure; efficiency = work/MVO2 (work and MVO2 were converted to joules/gram/minute as previously described) (26, 27). A single investigator (ADW) blinded to all clinical parameters, measured all echocardiograms. The intra-observer correlation coefficient [ICC] was obtained to assess reproducibility of measurements by re-measuring 50% of the studies in a random fashion. All reported measurements represent the average of three consecutive cardiac cycles. Reproducibility measurements (ICC and [95% confidence intervals]) for the primary outcomes of left ventricular diastolic function and left ventricular mass were as follows: E-wave, (0.987 [0.968-0.995]), E’ (0.919 [0.726-0.952]), and LV mass, (0.856 [0.620-0.929]).
Statistical analyses
All data are expressed as mean ± standard deviation. SAS software (version 9.2, SAS Institute, Inc; Cary, North Carolina) was used. Between-group comparisons used unpaired Student’s t-tests. Within-group comparisons used paired Student’s t tests. Fisher’s exact tests were used to compare proportions between groups. Linear regression was used to evaluate the relations between continuous variables. All cardiac endpoints and independent variables were predetermined. The tissue Doppler measure of left ventricular relaxation (E’) was the primary measure of LV relaxation because of its load-independence. A priori it was decided to use the continuous variables mean arterial pressure and HOMA were used rather than categorical variables hypertension, diabetes, or metabolic syndrome status. If more than one variable correlated with an endpoint, analysis of covariance was used to determine its independent predictor(s). To enter the multivariate analyses, an independent variable had to have a P<0.10 in the univariate analyses. In the multivariate models each independent variable was adjusted for all other independent variables. A P<0.05 was considered significant.
RESULTS
Subject characteristics (Tables 1A and 1B)
Table 1.
A. Pre- and Post-Weight Loss (Diet Group, N=20)
| Pre-weight loss | Post-weight loss | P value | |
|---|---|---|---|
| Age (yrs) | 35±5 | ||
| Female (%) | 60% | ||
| Race (%white) | 75% | ||
| Body mass index (kg/m2) | 39±6 | 36±7 | <0.0001 |
| Total cholesterol (mg/dL) | 182±38 | 153±35 | 0.02 |
| Triglycerides (mg/dL) | 153±112 | 118±73 | 0.09 |
| HOMA | 3.8±2.0 | 2.7±1.5 | 0.005 |
|
| |||
| Medication use | |||
| Metformin | 0% | 0% | |
| Sulfonylureas | 0% | 0% | |
| ACE inhibitors | 0% | 0% | |
| Angiotensin receptor blockers | 0% | 0% | |
| Hydrochlorothiazide | 0% | 0% | |
| Statins | 0% | 0% | |
|
| |||
| Hemodynamics | |||
| Heart rate (bpm) | 69±11 | 61±12 | 0.01 |
| Mean arterial pressure (mmHg) | 89±9 | 89±10 | 0.83 |
|
| |||
| Cardiac structure | |||
| LV mass (g) | 186±34 | 186±32 | 0.95 |
| LV mass/height2.7 (g/m2.7) | 44±7 | 44±8 | 0.9 |
| LV end-diastolic volume (mL) | 124±25 | 114±24 | 0.10 |
| Left atrial volume (mL) | 52±14 | 48±14 | 0.21 |
| Left atrial volume index (mL/m2) | 24±6 | 22±6 | 0.43 |
|
| |||
| Systolic function | |||
| LV ejection fraction (%) | 60±5 | 60±6 | 0.75 |
| S’ (cm/s) | 8.4±1.2 | 8.1±0.9 | 0.28 |
| Cardiac minute work (mmHg*[L/min]) | 453±99 | 421±135 | 0.24 |
|
| |||
| Diastolic function | |||
| E’ (cm/s) | 14.0±2.7 | 14.1±2.2 | 0.92 |
| E/E’ | 5.9±1.1 | 6.1±1.1 | 0.44 |
| E/A ratio | 1.65±0.43 | 1.81±0.44 | 0.24 |
| B. Pre- and Post-Weight Loss (Gastric Bypass Surgery Group, N=10)
| |||
| Pre-weight loss | Post-weight loss | P value (pre- to post comparison) | |
|
| |||
| Age (yrs) | 44±7a | ||
| Female (%) | 100%b | ||
| Race (%white) | 100% | ||
| Body mass index (kg/m2) | 44±7 | 29±5 | <0.0001 |
| Total cholesterol (mg/dL) | 167±33 | 136±27 | 0.04 |
| Triglycerides (mg/dL) | 168±90 | 72±21 | 0.005 |
| HOMA | 5.5±5.3 | 0.9±0.4 | 0.02 |
|
| |||
| Medication use | |||
| Metformin | 10% | 0% | |
| Sulfonylureas | 10% | 0% | |
| ACE inhibitors | 20% | 20% | |
| Angiotensin receptor blockers | 20% | 20% | |
| Hydrochlorothiazide | 20% | 20% | |
| Statins | 10% | 10% | |
|
| |||
| Hemodynamics | |||
| Heart rate (bpm) | 72±13 | 65±17 | 0.16 |
| Mean arterial pressure (mmHg) | 89±6 | 86±9 | 0.38 |
|
| |||
| Cardiac structure | |||
| LV mass (g) | 180±24 | 141±20 | 0.0008 |
| LV mass/height2.7 (g/m2.7) | 47±8 | 37±7 | 0.001 |
| LV end-diastolic volume (mL) | 98±23c | 69±11 | 0.02 |
| Left atrial volume (mL) | 45±13 | 37±14 | <0.05 |
| Left atrial volume index (mL/m2) | 20±6 | 20±9 | 0.97 |
|
| |||
| Systolic function | |||
| LV ejection fraction (%) | 61±6 | 61±10 | 0.83 |
| S’ (cm/s) | 7.9±0.9 | 7.9±0.6 | 0.87 |
| Cardiac minute work (mmHg*[L/min]) | 445±90 | 347±81 | 0.10 |
|
| |||
| Diastolic function | |||
| E’ (cm/s) | 8.2±1.2d | 10.4±1.8 | 0.004 |
| E/E’ | 12.5±2.1d | 8.1±0.9 | 0.0006 |
| E/A ratio | 1.18±0.44 | 1.45±0.52 | 0.2 |
ACE = angiotensin converting enzyme; HOMA = homeostasis model index; LV = left ventricular. One subject did not have a post-intervention cholesterol panel.
P<0.0005,
P<0.05
P<0.01,
P<0.0001 for baseline differences between the diet (see Table 1A) and surgery groups. One subject did not have an ejection fraction available post-weight loss.
There were few differences between groups at baseline. Most importantly, the groups’ BMI, plasma triglycerides, LV mass, hemodynamics, HOMA-IR, and systolic function were similar. The only significant baseline differences between the group were that the surgery group was slightly older and had a higher fraction of women, diabetic (3 of 10), hypertensive (5/10), and treated sleep apnea (3/10) subjects than the diet group; LV end-diastolic volume was lower in the surgery group, likely because there was a higher percentage of women in this group, and women are known to have smaller LV cavity size than men.
After weight loss, the only medication change made was the 2 subjects in the surgery group taking oral diabetic medications discontinued their use. Tables 1A and B also show weight loss-induced changes. The surgery group had a greater decrease in BMI than the diet group (P<0.0001). Surgery decreased plasma triglycerides and insulin resistance. Three subjects were diabetic pre- but not post-surgery. The diet group’s insulin resistance also decreased. Heart rate decreased in the diet group.
Weight loss effects on MVO2 and LV efficiency
MVO2
Baseline total MVO2 or MVO2 indexed to heart weight, did not differ between groups. After weight loss total MVO2 decreased in both groups (Figure 1). In the surgery group this was mostly due to the decrease in LV mass since MVO2/gram did not change. In the diet group, this was mostly due to decreased MVO2/gram (P=0.009) since LV mass did not change. Total MVO2 change correlated strongly with the decrease in BMI (Figure 1C, Table 2). Change in MVO2/gram trended toward a correlation with a change in cardiac work (P=0.10).
Figure 1.
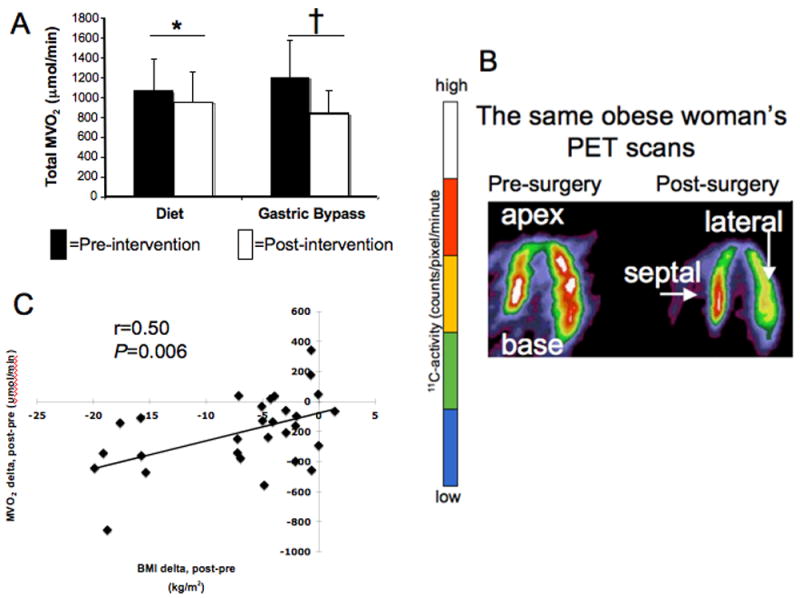
A. Total MVO2 decreased after diet and surgery (*P=0.02, †P=0.0006).
B. Typical positron emission tomography (PET)-derived myocardial images in the horizontal long-axis. The pre-surgery image (left) illustrates higher MVO2 (higher C-11 activity accumulation after 1-11C-acetate injection) than in the post-surgery image (right) in the same subject. The color scale graphically depicts C-11 activity: higher counts represented as white and red; lower counts (and lower MVO2) represented in blue and green.
C. Relation between MVO2 change and BMI change.
Table 2.
Correlations with cardiac endpoints in all subjects.
| ΔBMI | ΔMAP | ΔHOMA | |
|---|---|---|---|
| ΔMVO2/gram | r=0.09, P=0.63 | r=-0.23, P=0.22 | r=-0.32, P=0.09 |
| ΔTotal MVO2 | r=0.50, P =0.006 | r=-.06, P=0.77 | r=0.17, P=0.39 |
| ΔTotal myocardial FA utilization | r=0.35, P=0.07 | r=0.21, P=0.29 | r=0.31, P=0.12 |
| ΔLV mass | r=0.52, P=0.004 | r=0.33, P=0.08 | r=0.36, P=0.06 |
| ΔE/E’ | r=0.77, P<0.0001 | r=0.14, P=0.51 | r=0.43, P=0.03 |
BMI = body mass index, MAP = mean arterial pressure, HOMA = homeostasis model index, MVO2 = myocardial oxygen consumption, LV = left ventricular.
Efficiency
Baseline myocardial efficiency (work/MVO2) was similar between groups and did not change in either group after intervention despite decreases in MVO2, likely due to a trend towards a decreased cardiac work in the surgery subjects (P=0.10).
Weight loss effects on myocardial FA metabolism and its components
Plasma substrates and hormones
Baseline plasma free FAs (Table 3) and insulin were similar between groups (data not shown). Plasma glucose was different between the surgery and the diet groups (6.4±1.8 vs. 5.0±0.6μmol/mL, P=0.004). Post-surgery, glucose decreased to 4.9±0.4μmol/mL (P=0.02); insulin decreased from 16.2±10.3 to 4.2±2.5μU/mL (P=0.004). Insulin decreased in the diet group (13.9±5.9 to 10.6±6.8μU/mL, P=0.009).
Table 3.
Myocardial fatty acid (FA) metabolism/gram and its contributors
| Pre-diet | Post-diet | Pre-gastric bypass | Post-gastric bypass | |
|---|---|---|---|---|
| Plasma FA (μmol/mL) | 666±143 | 644±131 | 734±257 | 571±135 |
| Myocardial blood flow (mL/g/min) | 0.96±0.21 | 1.03±0.22 | 1.22±0.24* | 1.10±0.18 |
| FA utilization (nmol/g/min) | 148±38 | 144±36 | 166±48 | 148±79 |
| FA oxidation (nmol/g/min) | 134±37 | 128±37 | 141±47 | 127±50 |
| FA esterification (nmol/g/min) | 14±20 | 14±12 | 25±29 | 15±17 |
P<0.01 baseline differences between groups. No post-weight loss changes
Myocardial blood flow
Baseline myocardial blood flow (mL/g/min) was higher in the surgery than the diet group and did not change in either (Table 3).
Myocardial FA metabolism
Baseline myocardial FA metabolism measures/gram were similar between groups and did not change with weight loss (Table 3). However, myocardial FA oxidation/gram change correlated with myocardial oxygen consumption/gram change (r=0.39, P=0.04). Baseline total myocardial FA utilization and oxidation were similar between groups. After surgery, total myocardial FA utilization decreased, mirroring the post-surgical LV mass decrease, and total myocardial FA oxidation trended lower (Figures 2A, 2B). Total and per gram FA esterification did not change with either intervention (Table 3). Total FA utilization change trended towards relating to BMI change (Table 2). No FA metabolism measure correlated with diastolic function change (Table 4A).
Figure 2.
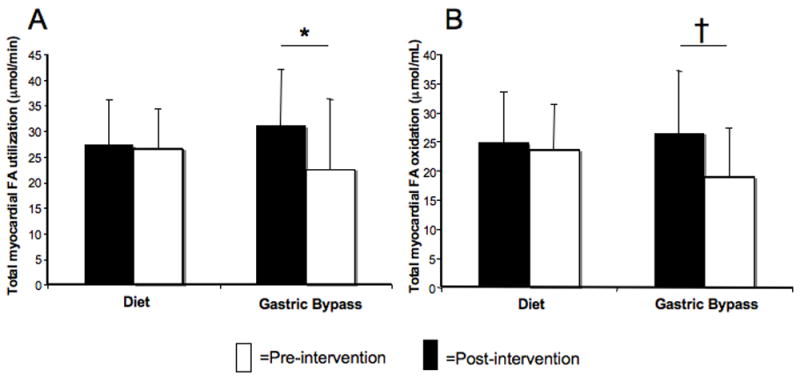
A. Total myocardial fatty acid (FA) utilization decreased after surgery (*P<0.05).
B. Total FA oxidation change, †P=0.06.
Table 4.
A. Correlations with left ventricular relaxation improvement (ΔE’).
| ΔBMI | ΔMAP | ΔHOMA | ΔTotal MVO2 | ΔLV mass | ΔTotal myocardial fatty acid utilization | |
|---|---|---|---|---|---|---|
|
| ||||||
| ΔE’ | r=-0.46 | r=-0.01 | r=-0.41 | r=0.56 | r=0.56 | r=0.18 |
| P=0.01 | P=0.95 | P=0.03 | P=0.002 | P=0.002 | P=0.37 | |
| B. Independent predictors of LV relaxation improvement. | |||||||
| Multivariate analyses | |||||||
| All subjects | Women only | ||||||
| R2 | ΔBMI | ΔTotal MVO2 | R2 | ΔBMI | ΔTotal MVO2 | ||
| ΔE’ | 0.34 | P=0.33 | P<0.03 | ΔE’ | 0.55 | P=0.38 | P=0.003* |
| ΔBMI | LV mass change | ΔBMI | ΔLV mass | ||||
| ΔE’ | 0.35 | P=0.32 | P<0.03 | ΔE’ | 0.33 | P=0.20 | P=0.14 |
| ΔBMI | HOMA change | ΔBMI | ΔHOMA | ||||
| ΔE’ | 0.26 | P=0.08 | P=0.19 | ΔE’ | 0.32 | P=0.11 | P=0.19 |
HOMA = homeostasis model index, LV = left ventricular, MVO2 = myocardial oxygen consumption
Weight loss effects on diastolic function and relation to myocardial metabolism
LV relaxation
Baseline E’ was worse in the surgery group than in the diet group, but LV relaxation improved markedly after surgery (Table 1B, Figure 3A, 3B). Improved relaxation correlated with weight loss amount, increased insulin sensitivity, an LV mass decrease, and a total MVO2 decrease (Table 4A, Figure 3C). In multivariate modeling, in all subjects, total MVO2 predicted LV relaxation improvement independent of BMI change (Table 4B). LV mass decrease also independently predicted LV relaxation improvement. However, because of sex-related differences in MVO2 and LV mass,(26, 27) we repeated the multivariate analysis in only the women, and decreased MVO2 was the sole independent predictor of LV relaxation. In this model, BMI change and total MVO2 change accounted for 55% of the improvement in LV relaxation. The multivariate analysis was not repeated in the men given their limited numbers.
Figure 3.
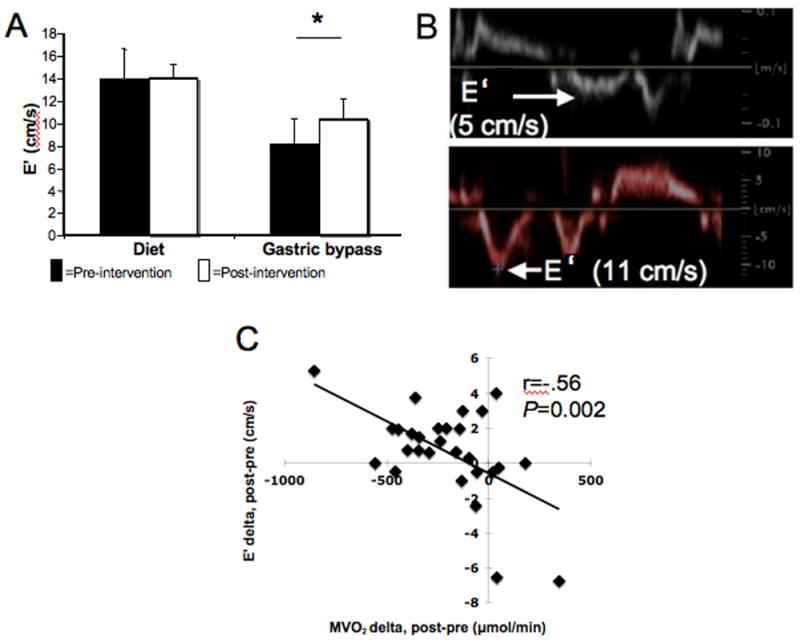
A. LV relaxation improved post-surgery by 28% (*P<0.005). E’ was different between groups at baseline (P<0.0001).
B. A sample tissue Doppler-derived E’ pre-surgery (top), which improved post-surgery in the same subject (bottom).
C. Decreased total MVO2 after weight loss correlated with improved LV relaxation.
E/E’
Baseline E/E’ was worse in the surgery group than in the diet group but improved (decreased) after surgery (Tables 1A and 1B). The improvement correlated robustly with weight loss amount (Table 2), insulin resistance improvement (Table 2), total MVO2 decrease (r=0.54, P=0.005), LV mass decrease (r=0.73, P<0.0001), and end-diastolic volume change (r=0.54, P=0.005). Weight loss independently predicted E/E’ improvement.
Weight loss effects on LV remodeling
Notwithstanding the constancy in blood pressure before and after weight loss in both groups, LV mass decreased after surgery (Table 1B). Weight loss amount correlated with decreased LV mass (Table 2). LV end-diastolic volume also decreased significantly after weight loss, which together with the aforementioned decrease in E/E’ suggests a decrease in preload and/or plasma volume.
DISCUSSION
The present study is the first to demonstrate, in men and women, that weight loss can ameliorate both the myocardial oxygen and FA oxidation derangements related to obesity and that the change in myocardial oxygen consumption predicts improved LV relaxation. Even moderate, diet-induced weight loss decreased MVO2 (total and per gram) while marked weight loss decreased total MVO2. The decrease in total MVO2 related to decreased BMI. Marked weight loss decreased total FA utilization, and total FA oxidation trended lower. LV relaxation and E/E’ (an estimate of LV filling pressure) (25), both improved after surgery-induced weight loss. In all subjects, LV relaxation improvement correlated with amount of weight loss, LV mass change, insulin resistance improvement, and a decrease in total MVO2. In multivariate analyses, total MVO2 decrease independently predicted improved LV relaxation in all subjects and also in women.
The first novel finding of this study is that weight loss decreased MVO2 requirements. This is similar to the finding that whole body oxygen consumption decreases with weight loss (28). In our study, myocardial FA oxidation/gram decreases contributed to MVO2/gram change, which is in agreement with studies showing that FAs are the major substrate oxidized in the post-absorptive heart (4), and that FAs “cost” relatively more oxygen to burn than glucose (29). There is also a suggestion that part, but not all, of the decrease in MVO2/gram is due to a decrease in work (P=0.10), consistent with the fact that MVO2 is used for both mechanical and non-mechanical processes. The absence of LV mass change in the presence of this MVO2/gram change after diet therapy suggests that metabolism changes may precede, and thus contribute to significant structural and functional remodeling, consistent with a study in animals demonstrating that metabolism changes precede trophic changes (30). The present study’s finding that total MVO2 changes post-surgery (after an LV mass change, i.e., remodeling) and those of previous studies also demonstrate that the converse is true: that LV structural remodeling influences metabolism (31). The lack of a significant change in MVO2/gram in the surgery group may result from smaller numbers of surgery subjects, or it may be that there is a continuum of metabolic and structural changes that are not perfectly matched as weight loss progresses. E.g., MVO2/gram may change first, then after significant structural remodeling occurs and the denominator decreases, the ratio becomes similar to baseline but total MVO2 is decreased. A study with multiple sampling points is needed to prove this; however, subject radiation exposure and costs currently limit many repeated samplings using PET.
Another major finding of this study was the strong correlation between change in MVO2 and improvement in LV relaxation, independent of weight loss amount or blood pressure or myocardial FA metabolism change. The relaxation improvement after surgery (28%) is greater than that in surgical weight loss studies with shorter-term follow-up (~18% after 3 months) (12, 32). Although it may appear counterintuitive that a decrease in MVO2 could increase in LV relaxation, there are possible mechanisms for this salutary link. First, MVO2 as measured in our study by C-11 acetate injection and PET imaging is almost exclusively from the function of mitochondria, a known source of free radical generation (33), which contributes to cardiac dysfunction development in animal models of obesity (6, 7). Thus, it is logical to propose that the decrease in MVO2 found in the current study in humans may relate to decreased reactive oxygen species generation and hence, functional improvement (see proposed working model, Figure 4). Second, decreased MVO2 may improve LV relaxation if there is better coupling of MVO2 to ATP generation, and of ATP generation to relaxation. I.e., if less energy generated from oxidative metabolism is dissipated (by uncoupling protein and/or adenine nucleotide transporter activity) and/or if less oxygen use is “wasted”(33) by the creation of free radicals, more ATP should be available for relaxation. This is supported by a study in adipose tissue, which showed that uncoupling proteins 2 and 3 mRNA expression decreases after weight loss (34). With this improved coupling, we had expected efficiency would improve. However, the lack of a change in efficiency (cardiac minute work/MVO2) may be due to the fact that this common measure of cardiac work used does not directly incorporate the work of LV sarcomeric relaxation (moving actin and myosin apart), an ATP-requiring process (as evidenced by rigor mortis rather than relaxation when metabolism stops) (35).
Figure 4.
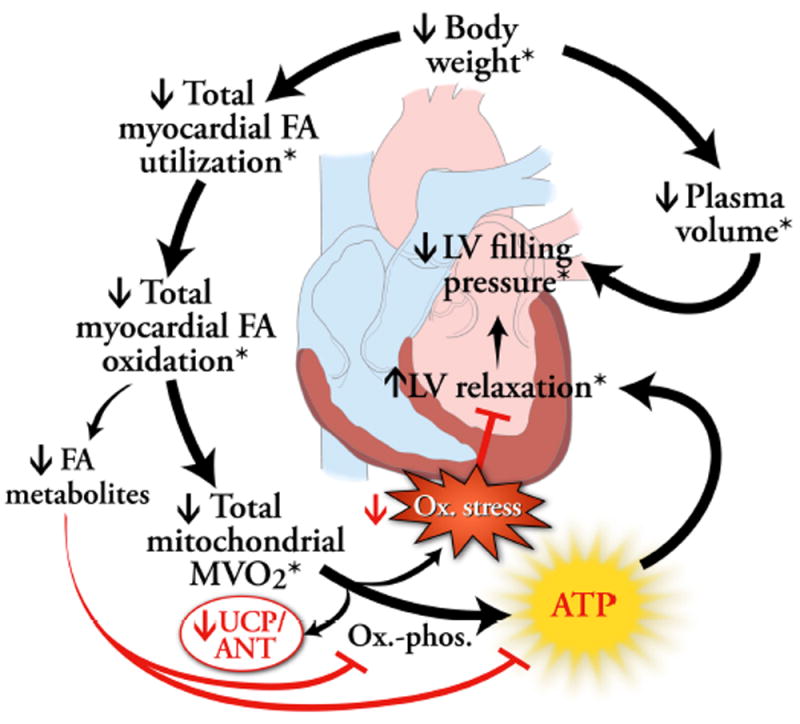
A potential model of how weight loss-induced changes in myocardial fatty acid (FA) and oxygen metabolism may reverse obesity-related impaired left ventricular (LV) relaxation. Weight loss results in decreased myocardial FA utilization and oxidation. This may result in decreased intracellular FA metabolites. These metabolites can impair oxidative phosphorylation, so their diminishment should improve adenosine triphosphate (ATP) production and hence, relaxation. Because FA oxidation is a major contributor to overall mitochondrial oxygen consumption, total MVO2 then decreases. Total MVO2 may also decrease as a result of decreased uncoupling protein (UCP) or adenine nucleotide transporter (ANT) activity. A decrease in total MVO2 should lead to improved left ventricular (LV) relaxation, (an ATP-requiring process), if there is improved flow of energy directed toward ATP production and subsequent LV relaxation rather than dissipation by UCP or ANT. A decrease in MVO2 may also lead to diminished free radical production and consequent oxidative stress (Ox. Stress), which can negatively affect relaxation. Text with an asterisk (*) indicates findings of this study.
We also hypothesized that myocardial FA metabolism would decrease with weight loss and that this would also relate to improved LV diastolic function. We did find a decrease in total myocardial FA utilization and a trend toward a decrease in total FA oxidation but no difference in FA utilization or oxidation/gram. This is partially consistent with the findings of Viljanen et al., who found a decrease in myocardial FA uptake (μmol/100g/min) after diet therapy (15). Differences in tracers, the sex of the study subjects, and types of weight loss may account for the differences in our results. For example, the study by Viljanen was in men (15), and the current study included both men and women. Sex has a major affect on myocardial metabolism at baseline, (26, 27), and it may also affect myocardial responses to weight loss. The current study was not powered to evaluate the effects of sex on the heart’s metabolic response to weight loss and requires future study. The study by Viljanen also had a marked weight loss within a much shorter period of time, and it may be that there is a continuum of myocardial FA metabolic responses depending on rapidity of weight loss and whether there is a weight stabilization period or not (15). Our study further showed that decreased myocardial FA oxidation contributes to an overall decrease in MVO2 thereby potentially indirectly contributing to an improvement in relaxation. Our finding that LV relaxation abnormalities are reversible in obese humans, suggests that at this stage of obesity-related heart disease, mechanisms besides or in addition to FA-induced apoptosis likely contribute to diastolic dysfunction.
Limitations
While this study does show the strength of the relationships between myocardial FA metabolism and MVO2 and relaxation, it does not prove cause and effect, direction of the association, or the potential influence of unmeasured factors. More studies are needed to further test the proposed working model shown in Figure 4. Given the short-term risk of mortality and insurance issues with surgery, subjects were not randomized to treatment, and so the groups were heterogeneous. Despite this, the groups were similar in many respects. In the surgery arm, only white women enrolled because they represent ~80% of surgery patients, similar to other studies (36). The surgery group was slightly older at baseline than the diet group, however, a significant aging effect would be expected to decrease FA metabolism, which was not seen (17). The size of the groups were relatively small, which increases the possibility of a type 2 error in the analyses that did not show a difference from pre- to post-intervention. E.g., the surgery group’s size may have contributed to the lack of a significant change in MVO2/gram; however, the primary focus of this study was to evaluate the effects of a spectrum of weight loss on heart metabolism, structure, and function rather than to compare the effect of different methods. Measurement of local, cardiac reactive oxygen species and changes in myocardial metabolism of other substrates and energetics was not done.
Acknowledgments
We gratefully acknowledge our subjects’ participation, Kristin O’Callaghan’s editorial assistance, and Ava Ysaguirre’s assistance with manuscript preparation. This work was funded by grants RO1-HL073120, UL1 RR024992 (CTSA), 5 P60 DK020579, and DK 56341 from the National Institutes of Health, and a grant from the Barnes-Jewish Hospital Foundation, St. Louis, Missouri.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
Clinical implications. Our data support an association between excessive MVO2 and diastolic dysfunction in humans, suggesting that altering MVO2 might be a target for new therapies aimed at improving diastolic dysfunction. Our results also demonstrate that patients with significant diastolic function who lose marked amounts of weight can have dramatic improvements in diastolic dysfunction. Thus, patients with obesity-related LV hypertrophy, decreased LV relaxation, and/or estimated increased filling pressures may reap the most cardiac benefits from surgery surgery-induced weight loss. Whether these particular changes result in a long-term decrease in cardiovascular morbidity and mortality deserves further study.
References
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Peterson LR. Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr Cardiol Rep. 2007;9:143–149. doi: 10.1007/BF02938341. [DOI] [PubMed] [Google Scholar]
- 4.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 5.Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 8.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 9.Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord. 2001;25:378–388. doi: 10.1038/sj.ijo.0801536. [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Muller FL, Liu Y, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E678–85. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- 11.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 12.Leichman JG, Wilson EB, Scarborough T, et al. Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery. Am J Med. 2008;121:966–973. doi: 10.1016/j.amjmed.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CY, Byrne NM, O’Moore-Sullivan T, Hills AP, Prins JB, Marwick TH. Effect of weight loss due to lifestyle intervention on subclinical cardiovascular dysfunction in obesity (body mass index >30 kg/m2) Am J Cardiol. 2006;98:1593–1598. doi: 10.1016/j.amjcard.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 14.de las Fuentes L, Waggoner A, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376–2381. doi: 10.1016/j.jacc.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viljanen APM, Karmi A, Borra R, et al. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. Am J Cardiol. 2009;103:1721–1726. doi: 10.1016/j.amjcard.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–1012. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 17.Kates AM, Herrero P, Dence C, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 18.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 19.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 20.Herrero P, Markham J, Bergmann SR. Quantitation of myocardial blood flow with H2 15O and positron emission tomography: assessment and error analysis of a mathematical approach. J Comput Assist Tomogr. 1989;13:862–873. doi: 10.1097/00004728-198909000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Buck A, Wolpers HG, Hutchins GD, et al. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med. 1991;32:1950–1957. [PubMed] [Google Scholar]
- 22.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37:1723–1730. [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 25.Dokainish H, Gonzalez R, Hartley WB, et al. Usefulness of B-type natriuretic peptide levels to predict left ventricular filling pressures in patients with body mass index >35, 31 to 35, and < or =30 kg/m2. Am J Cardiol. 2007;100:1166–1171. doi: 10.1016/j.amjcard.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol. 2007;14:573–581. doi: 10.1016/j.nuclcard.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson LR, Soto PF, Herrero P, et al. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey DG, Pliego GJ, Raymond RL, Skau KB. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate. Obes Surg. 2006;16:469–477. doi: 10.1381/096089206776327378. [DOI] [PubMed] [Google Scholar]
- 29.Vik-Mo H, Mjos OD. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol. 1981;48:361–365. doi: 10.1016/0002-9149(81)90621-4. [DOI] [PubMed] [Google Scholar]
- 30.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 31.Taegtmeyer H. Genetics of energetics: transcriptional responses in cardiac metabolism. Ann Biomed Eng. 2000;28:871–876. doi: 10.1114/1.1312187. [DOI] [PubMed] [Google Scholar]
- 32.Willens HJ, Chakko SC, Byers P, et al. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95:1521–1524. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Sawyer DB, Colucci WS. Mitochondrial oxidative stress in heart failure: “oxygen wastage” revisited. Circ Res. 2000;86:119–120. doi: 10.1161/01.res.86.2.119. [DOI] [PubMed] [Google Scholar]
- 34.Vettor R, Mingrone G, Manco M, et al. Reduced expression of uncoupling proteins-2 and -3 in adipose tissue in post-obese patients submitted to biliopancreatic diversion. Eur J Endocrinol. 2003;148:543–550. doi: 10.1530/eje.0.1480543. [DOI] [PubMed] [Google Scholar]
- 35.Baliga RR, Young JB. Energizing diastole. Heart Fail Clin. 2008;4:ix–xiii. doi: 10.1016/j.hfc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]


