Abstract
Brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase B (TrkB) may influence brain reserve, the ability of the brain to tolerate pathological changes without significant decline in function. Here, we explore whether a specifically vulnerable population of human neurons shows a compensatory response to the neuropathological changes of Alzheimer disease (AD) and whether that response depends on an upregulation of the BDNF pathway. We observed increased neuronal TrkB expression associated with early AD pathology (Braak and Braak stages I–II) in hippocampal CA1 region samples from cognitively intact Framingham Heart Study subjects (n = 5) when compared to cognitively intact individuals with no neurofibrillary tangles (n = 4). Because BDNF/TrkB signaling affects memory formation and retention through modification of the actin cytoskeleton, we examined the expression of actin capping protein β2 (Capzb2), a marker of actin cytoskeleton reorganization. Capzb2 expression was also significantly increased in CA1 hippocampal neurons of cognitively intact subjects with early AD pathology. Our data suggest that increased expression of TrkB and Capzb2 accompanies adequate brain reserve in the initial stages of AD pathology. In subsequent stages of AD, the higher levels of TrkB and Capzb2 expression achieved may not be sufficient to prevent cognitive decline.
Keywords: Alzheimer disease, Brain-derived neurotrophic factor (BDNF), Brain reserve, Capzb2, TrkB
INTRODUCTION
One in 5 persons who are currently 65 years old will develop clinical Alzheimer dementia in their lifetime. Postponing the onset of clinical disease by as little as 5 years could halve individual risk and population burden of disease (1, 2). The onset of clinical dementia is determined not only by the pace of pathological changes but also by brain reserve—the ability of the brain to tolerate the pathological changes found in Alzheimer disease (AD) without manifesting clinical signs and symptoms (3). Neurotrophic factors, most notably brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase B (TrkB) regulate synaptic plasticity and functional efficiency in adulthood (4–6), and thus may influence brain reserve.
In aged rodents and non-human primates, as well as in models of familial AD and entorhinal injury, BDNF gene delivery restores learning and memory (3). The underlying mechanisms might include reversing synapse loss, improving cell signaling independently of amyloid burden, and preventing lesion-induced neuronal death (3). Effective administration of BDNF therapy in AD requires an understanding of the dynamics of BDNF and TrkB expression during the progression of AD in human brains. In 1991, Phillips et al used in situ hybridization to study the abundance of neurotrophic factors in hippocampi of patients diagnosed with AD (4). BDNF mRNA in persons with AD was found to be decreased in comparison to controls although there was no information on the pathological (Braak and Braak staging) or clinical (dementia rating) staging of individual AD samples examined (4). A similar study found increased TrkB receptor mRNA and protein expression in AD (5). In 2010, Fujimura et al studied the expression of BDNF and TrkB in AD and control hippocampal neurons using quantitative polymerase chain reaction (qPCR) and reported lower expression of both in advanced AD (6). We recently observed that the expression of TrkB in hippocampal CA1 pyramidal neurons was upregulated during mid-stage non-familial AD (Braak and Braak stage III–IV, BBIII–IV), in comparison to neurons from a brain containing no neurofibrillary tangles (NFTs) (7).
Increased expression of endogenous BDNF after injury has been observed in rat hippocampal neurons (8), although increases are less prominent in aged than in young animals (9). BDNF/TrkB signaling may govern regeneration following neuronal injury (10, 11) and affect neurite length by modifying the neuronal cytoskeleton (12, 13). There is growing evidence that cytoskeletal abnormalities may be critically involved in the pathogenesis of neurodegeneration. For example, apolipoprotein E ε4 isoform (apoE4), the well-documented genetic risk factor for the common sporadic late-onset AD (14), inhibits neurite outgrowth in cultured neuronal cells (15) and correlates with the simplification of dendritic branching patterns in the brains of AD patients (16). Actin capping protein β2 subunit (Capzb2) knockdown in cultured hippocampal neurons results in short, poorly branched neurites similar to those seen in AD brains (17). Besides capping F-actin, Capzb2 also directly binds βIII-tubulin (17). The interaction between actin capping protein (CapZ) and β-tubulin has been uncovered in a mass spectrometry screen of alterations in protein-target binding in vivo in response to spatial learning (18), a process that requires BDNF (19). Moreover, in a rat model of dementia there is activity-dependent, synapse-specific regulation of CapZ redistribution, which may be important in both maintenance and remodeling of synaptic connections receiving spatial and temporal patterns of inputs (20). Thus, the expression of Capzb2 may represent one of the likely down-stream read-outs for BDNF-TrkB signaling in CA1 hippocampal pyramidal neurons in AD patients. In line with the previously documented increased cytoskeletal reorganization including dendritic proliferation and sprouting in neurons of AD patients (21–23), we recently demonstrated increased expression of Capzb2 and TrkB in mid-stages AD pathology (7). BDNF has been shown to promote growth of undifferentiated dendrites and axons in cultured hippocampal pyramidal neurons (24), a process that requires Capzb2 (17).
Differences in the criteria for defining controls and in categorizing the neuropathological stage of the disease have made it difficult to integrate the findings from the various human studies cited earlier. The present study represents a first attempt to correlate the expression of established modulators of synaptic plasticity and actin cytoskeletal reorganization, including BDNF, TrkB, and Capzb2, with a clinical dementia rating (CDR) score, a reliable indicator of human cognitive performance level in a sample of persons with varying degrees of neurodegenerative changes (25). We analyzed laser-dissected CA1 pyramidal neurons rather than whole brain homogenates because of specific vulnerability of hippocampal neurons in early AD (26), We compared expression of BDNF, TrkB and Capzb2 in samples of neuropathologically `normal' and cognitively intact subjects (controls), with samples of persons with AD-related pathological changes who were cognitively intact prior to death (CDR0), and samples of persons with AD-related pathological changes as well as early clinical dementia (CDR0.5–1). This approach was possible due to the existence of a unique sample of Framingham Heart Study (FHS) participants who had undergone repeated ante-mortem cognitive testing and brain imaging.
MATERIALS AND METHODS
We examined human hippocampal CA1 region available from FHS participants harvested by the Brain Bank of the Boston University Alzheimer Disease Center (NIA P30 AG13846; BVAX cases), Massachusetts Alzheimer Disease Research Center (NIA P50 AG05134; MADRC case) and autopsy service at Boston Medical Center cases 1–4 (BM1–4) (Table). The tissues were used in accordance with the policies of Boston University Medical Center's Institutional Review Board.
Table.
Case Data
| Case | Braak and Braak Stage | CERAD plaque density/presence of senile plaques in hippocampus* | Cognitive status | Age (y) | Sex | PMI (h) | RIN |
|---|---|---|---|---|---|---|---|
| BM1 | 0 | None/0 | No neurological history and no evidence of dementia | 70 | M | 24 | 6.2 |
| BM2 | 0 | None/0 | No neurological history and no evidence of dementia | 73 | F | 25 | 4.5 |
| BM3 | 0 | None/0 | No neurological history and no evidence of dementia | 75 | F | 36 | 5.4 |
| BM4 | 0 | None/0 | No neurological history and no evidence of dementia | 77 | F | 46 | 4.5 |
| BVAX1 | I | Sparse/1+ (D) | CDR 0 | 77 | F | 6 | 4.8 |
| BVAX2 | I | None/0 | CDR 0 | 91 | F | 16 | 6.1 |
| BVAX3 | I | Sparse/1+ (N) | CDR 0 | 94 | M | 1.5 | 6.7 |
| BVAX4 | II | Sparse/1+ (D) | CDR 0 | 90 | M | 15 | 5.7 |
| BVAX5 | II | None/0 | CDR 0.5 | 96 | F | NA | 5.8 |
| BVAX6 | IV | Frequent, temporal only/ 2+ (N) | CDR 1 | 92 | M | NA | 5.7 |
| BVAX7 | IV | Moderate/ 2+ (N) | CDR 1 | 96 | F | 3.5 | 7.4 |
| MADRC1 | IV | Sparse/ 1+ (D & N) | Mild dementia | 103 | F | 5 | 6.0 |
0 = none; 1+ = mild; 2+ = moderate, 3+ = severe, 4+ = very severe.
BM1 – 4= Boston Medical Center cases 1–4; BVAX1-6 = Brain Bank of the Boston University Alzheimer Disease Center cases 1–6; CERAD = The Consortium to Establish a Registry for Alzheimer's Disease; CDR 0–1 = clinical dementia rating scores 0 – 1; D = diffuse plaques; F = female; M = male; MADRC1= Massachusetts Alzheimer Disease Research Center case 1; N = neuritic plaques; PMI = postmortem interval; RIN= RNA integrity number.
FHS Specimens
All FHS participants in the FHS had undergone screening cognitive tests (a mini-mental state examination) once in 2 years and also had a more detailed cognitive assessment examining multiple cognitive domains once in 1974–1975, once in 1999–2004 and at least twice thereafter. The presence or absence of dementia in all FHS participants is defined using DSM-IV criteria that require impairment in memory and in at least 1 other area of cognitive function, as well as documented functional disability. AD is defined using NINCDS-ADRDA criteria for definite, probable or possible AD (27). A dementia-free inception cohort was identified in 1975 (28). Since then, this cohort has been under surveillance for the development of incident dementia through biennial mini-mental status examinations, annual telephone interviews, linkage with participant's primary care providers and records. All participants are invited to become brain donors and the nearly 700 persons who have accepted this invitation undergo additional periodic brain MRI and a detailed neuropsychological assessment at least once every 2 years beyond age 75 years. In addition, twice in the past 10 years, all consenting participants regardless of whether or not they were prospective brain donors were administered a neuropsychological test battery using standard administration protocols and trained examiners. Details of the tests administered and normative values have been previously published (28, 29). These tests cover all major cognitive domains, including most tests from the Alzheimer Disease Center's Uniform Data Set (30, 31), and are comprised of multiple tests measuring related cognitive domains of premorbid vocabulary, verbal memory, visuospatial memory, new learning, abstraction, attention and executive function, and language. Persons who screen positive or are otherwise referred (by self, family or treating physicians) undergo detailed neurological and neuropsychological assessment, informant interview (with a physician-administered CDR) and a review of hospital records, nursing home notes, brain imaging and laboratory tests. Brain imaging is available in >90% of participants. A structured family interview (including Blessed Dementia and Hachinski scales) (32, 33) is conducted with the next-of-kin based on which a retrospective CDR score is assigned after the participant dies. The retrospective CDR is very similar to the retrospective collateral dementia interview validated by Davis et al (34). We combined this information with all ante-mortem medical records and lifelong FHS records, including at least 2 brain MRI exams and multiple cognitive batteries administered over decades. The sensitivity (77%) and specificity (92%) of our clinical diagnosis have been described (35). A final clinical decision regarding the presence or absence of dementia, diagnosis of dementia type and date of onset/diagnosis is made by a clinical consensus panel including behavioral neurologists and neuropsychologists who review all available records including records at the time of death. All deaths are reviewed to assign a cause of death and to determine if dementia was present or absent at the time of death.
The neuropathological assessment is conducted without any knowledge of the subject's age or clinical history and includes a detailed assessment of Alzheimer, vascular and other pathologies. Neuropathological evaluation of all autopsied brains is performed by a single neuropathologist (A.C.M.) who is blinded to all demographic and clinical information. Briefly, the brains are received fresh and the gross neuropathological findings are recorded. One hemisphere is snap frozen at −80°C. The other hemisphere is fixed in 4% periodatelysine-paraformaldehyde at 4°C for at least 2 weeks. Ten-μ-thick paraffin-embedded sections from 30 brain regions were evaluated. The density of NFTs was rated semiquantitatively in 14 regions using AT8-immunostained and Bielschowsky silver impregnated sections. The neuropathological report is generated prior to a final clinico-pathological conference during which the clinical diagnoses and pathological findings are discussed.
MADRC Specimens
Brain tissue from the selected autopsies performed at Massachusetts General Hospital is accompanied by the reports generated by the Neuropathology Core of the MADRC. These reports include neuropathology diagnosis of AD-associated changes and descriptive clinical information on the severity of neurological signs and symptoms of AD-associated cognitive decline.
Laser Capture Microdissection
In preparation for laser capture microdissection (LCM), frozen blocks of the hippocampal tissue (level of the lateral geniculate) were oriented and affixed to cryotome chucks with optimal cutting temperature embedding compound (Sakura Finetek, Torrance, CA). Ten-μm-thick cryosections were stained to visualize cells. We found that approximately 2,000 CA1 pyramidal neurons yielded enough cDNA for qPCR of 4 genes (our genes of interest - BDNF, Capzb2, TrkB, and, as control, a housekeeping gene 18S rRNA). A Veritas LCM system (Arcturus Veritas, Mountain View, CA) was used to collect CA1 pyramidal neurons onto optically clear collecting caps that contain a thin plastic film impregnated with a laser sensitive dye. Caps were placed over the area of interest and illuminated with a monochromatic IR laser pulse. The pulse selectively heats the plastic/dye combination at a set size (diameter range: 4–50 μm) and collects precisely microdissected underlying tissue when the cap is lifted. The isolated material was viewed to verify its integrity before RNA extraction. For the visualization of neurons, each section was lightly fixed in 70 % ethanol, rinsed with RNase-free dH2O, incubated in HistoGene stain solution (Applied Biosystems, Foster City, CA), and dehydrated in ethanol solutions and xylene. After neurons were collected, RNA was prepared using the Arcturus picopure RNA isolation system (Applied Biosystems). Briefly, plastic LCM collecting caps with captured neurons were incubated at 42°C for 30 minutes in 25 μl of guanidinium thiocyanate-containing extraction buffer. After brief centrifugation, tubes containing the extraction buffer were frozen at −80°C. Samples are thawed and pooled prior to purification on Picopure RNA columns (Applied Biosystems). The RNAs from the cell homogenate were attached to the column filter. After DNase (Qiagen Sciences, Gaithersburg, MD) treatment to remove genomic DNA, total cellular RNA from each column was eluted in 12 μl of elution buffer. RNA was converted into cDNA using a 20-μl total reaction volume via the Standard Superscript III (Invitrogen, Carlsbad, CA) protocol for qPCR analysis.
RNA Quality Control
We examined the RNA quality of the hippocampal blocks used for LCM and subsequent qPCR analysis by the commonly accepted quantitative digital analysis of Agilent 2100 Bioanalyzer (Agilent Technologies, Böblingen, Germany) generated electropherograms. The resulting RNA integrity number (RIN) of 1 (poor) to 10 (best) is obtained based on the presence and ratio of the 5S, 18S and 28S ribosomal RNA peaks in a sample of total cellular RNA. We found that even partially degraded RNA with a RIN value of 3.1 shows fairly well-preserved mRNA profile (unpublished data). The hippocampi used for qPCR displayed RIN values from 4.5 to 7.4, and thus within international tissue banks human brain mRNA integrity standards (36).
Primer Design and qPCR Analysis
PCR primers were designed using Primer3 software (http://fokker.wi.mit.edu/primer3/) and synthesized by Invitrogen. All the primers were tested using standard cDNA from normal human brain as template provided by Advanced Tissue Resource Center Reagent Bank. The Bank also provided primers for PCR of 18S rRNA, a housekeeping gene often used as an internal control. The PCR products obtained with these primers were checked on an Agilent Bioanalzyer using the DNA 1000 assay. The Capzb2 primers (forward: gcaaatcgagaaaaacctca; reverse: ctccaagggagggtcatact), BDNF primers (forward: cagctgccttgatggttact; reverse: ccaatgatgtcaagcctctt) and TrkB primers (forward: ggacgtgtacagcactgact; reverse: tgccataggtgaaaatctcc) were used for qPCR analysis. qPCR analysis of the cDNA from each dissected tissue was carried out on a Bio-Rad iCycler. qPCR was performed using 1 μl of input cDNA in full-strength and dilutions of 1:2 and 1:4. Each experiment was run in duplicate. Cycle threshold (CT) values for the duplicates were averaged. The averages of CT values for 18S were subtracted from TrkB, BDNF, and Capzb2 CTs to obtain a value of change in CT (ΔCT). Three ΔCT values (from 3 cDNA concentrations) for each gene were obtained and then averaged.
Statistical Analysis
Student t-test was performed in statistical analysis where 2 groups/samples were compared; oneway ANOVA analysis was performed in multiple group comparisons together with Student t-test (Prism5, GraphPad, La Jolla, CA). Non-parametric tests, although less sensitive than Student t-test, are designed to evaluate statistical significance under circumstances of low sample sizes, and thus we submitted our ΔCT values to Wilcoxon test (MS Excel plug-in) (Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348). This test assesses significance based on ordinal ranking rather than raw sample values (Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348).
Immunohistochemistry
Hippocampal blocks were fixed in 4% paraformaldehyde and embedded in paraffin. For detection of Capzb2 and BDNF signal in hippocampal CA1 neurons, 5-μm sections were incubated with monoclonal anti-Capzb2 antibody produced in mouse (Developmental Studies Hybridoma Bank, Iowa City, IA, 1:100) and polyclonal rabbit anti-BDNF (Santa Cruz Biotechnology, Santa Cruz, CA, 1:100) for 3 hours at room temperature, washed in Tris-buffered saline, pH 7.4, incubated with a secondary antibody (universal cocktail of horseradish peroxidase-labeled goat anti-mouse and goat anti-rabbit antibodies) and visualized with diaminobenzidine ([DAB], Sigma, St. Louis, MO). For the detection of TrkB, sections were incubated with a rabbit polyclonal anti-TrkB antibody (Santa Cruz Biotechnology, 1:100) overnight at 4°C and processed as above. Immunohistochemical staining with each antibody was performed with the same antibody solution for all of the cases to assure identical staining conditions. Images of 50 CA1 neurons per representative case were analyzed using ImageJ 1.42q to obtain mean signal intensities for TrkB, BDNF, and Capzb2. For the detection of neurofibrillary changes, paraffin sections were incubated with anti-tau antibody (rabbit polyclonal, Dako, Glostrup, Denmark, 1: 1000) overnight at 4°C, washed with Tris-buffered saline, incubated with a secondary antibody (Super Sensitive Link Label IHC Detection Systems kit, Biogenex, San Ramon, CA) and visualized with DAB. Slides were counterstained with hematoxylin and dehydrated with ethanol and xylene. Images were taken from the CA1 region under 10× magnification.
RESULTS
TrkB and Capzb2 mRNAs Are Significantly Upregulated in CA1 Hippocampal Neurons in Initial Stages of AD Pathology (Braak and Braak Stage I-II)
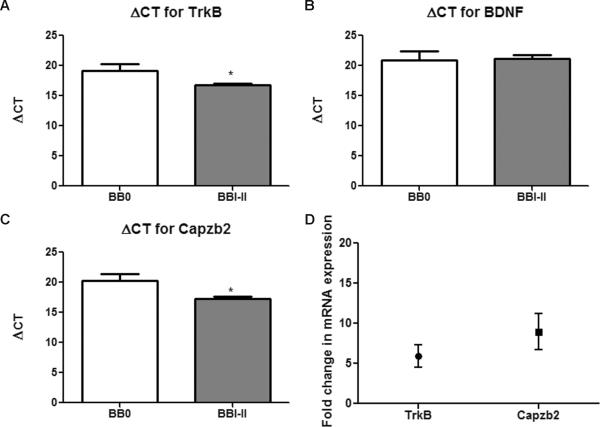
We examined the relationship between NFT formation and the expression of modulators of neuronal cytoskeleton important for synaptic plasticity, BDNF, TrkB, and Capzb2. To generate cDNA for qPCR analysis, we obtained RNA from approximately 2000 CA1 pyramidal neurons from FHS specimens with Braak and Braak stage I-II (BVAX1–5) and from neuropathology-absent controls (BM1–4). All specimens had satisfactory RNA integrity numbers (Table). The PCR quantification curves for control, the housekeeping gene 18S rRNA, and for TrkB, BDNF, and Capzb2 were generated from cDNA of each LCM dissectate. ΔCT between each gene of interest and the house keeping gene 18S rRNA was calculated. Three cDNA concentrations (full-strength,1:2 dilution, and 1:4 dilution) were used to calculate ΔCT in order to confirm that the obtained ΔCT values were consistent with the cDNA concentrations. Because duplicate samples were run, the average CT value for each gene of interest was subtracted from the average CT value for 18S rRNA, giving the average ΔCT value. The cDNAs in BBI-II group show lower ΔCT for TrkB and Capzb2 than cDNAs from control group, indicating significantly higher expression of TrkB (5.4-fold increase) and Capzb2 (7.7-fold increase) in neurons from the BBI-II group (Fig. 1A, C, D; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348). Because amplicons are doubled each cycle, the fold change was calculated using the equation 2×, where × is the difference between the average ΔCT of BBI-II and the average ΔCT of controls (Fig. 1D). ΔCT for BDNF was similar in BBI-II and control groups, indicating unchanged levels of BDNF mRNA in CA1 hippocampal neurons at the onset of AD pathology (Fig. 1B; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348).
Figure 1.
TrkB and Capzb2 mRNAs are significantly upregulated in CA1 hippocampal neurons of brains with initial Alzheimer disease (AD) pathology (Braak and Braak [BB] stages I–II) in comparison to neuropathology-absent brains. (A–C) BBI–II brains (BVAX1–5) show increased mRNA expression (lower ΔCT values) for TrkB (A) and Capzb2 (C) vs. control brains (BM1–4). Calculations for ΔCT were made by subtracting the average CT for housekeeping gene 18SrRNA from average CT from gene of interest; *p < 0.05, Student t test (error bars indicate SEM). Brain-derived neurotrophic factor (BDNF) mRNA expression is similar in both groups (B). (D) Fold changes of mean mRNA expression of TrkB (5.4-fold increase) and Capzb2 (7.7-fold increase) between the 2 groups. CT = cycle threshold.
TrkB and Capzb2 mRNAs Are Significantly Upregulated in Cognitively Intact FHS Subjects (CDR0) with Initial AD Pathology (BBI-II)
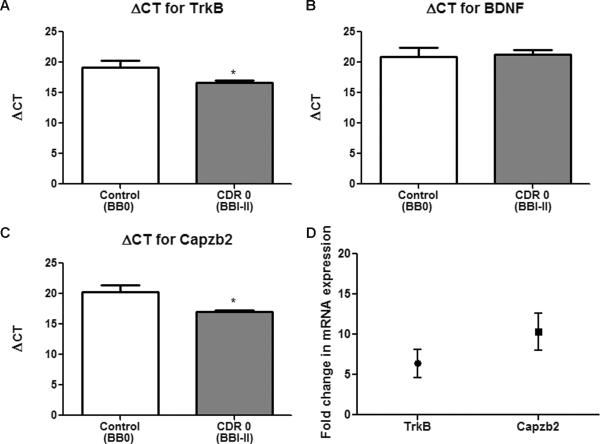
We next examined the mRNA expression of BDNF, TrkB, and Capzb2 in individuals who were cognitively intact despite having early AD pathology. FHS subjects BVAX1–3 had BBI and BVAX4 had BBII stage of NFT formation although they all were cognitively intact (CDR score 0). On average, their CA1 hippocampal neurons showed significantly lower ΔCT value, i.e. higher mRNA expression for TrkB (5.7-fold increase) and Capzb2 (9.4-fold increase) than control neurons from cognitively intact individuals with no NFTs (BM1–4, all BB0) (Fig. 2A, C, D; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348). BDNF mRNA was, again, similarly expressed in neurons of both groups (Fig. 2B; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348).
Figure 2.
TrkB and Capzb2 mRNAs are significantly upregulated in cognitively intact Framingham Heart Study (FHS) subjects clinical dementia rating score 0 (CDR0) with initial Alzheimer disease (AD) pathology (Braak and Braak [BB] stages I–II)) in comparison to individuals with no cognitive abnormalities and no neurofibrillary tangles (controls). (A–C) CDR0/BBI brains (BVAX1–3) and a BBII brain (BVAX4) show lower ΔCT (meaning higher mRNA expression) for TrkB (A) and Capzb2 (C) vs. control brains (BM1–4); *p < 0.05, Student t test (error bars indicate SEM). Brain-derived neurotrophic factor (BDNF) mRNA expression is similar in both groups (B). Calculations for ΔCT were made by subtracting the average CT for housekeeping gene 18SrRNA from average CT from gene of interest. (D) Fold changes of mean mRNA expression of TrkB (5.7-fold increase) and Capzb2 (9.4-fold increase) between the 2 groups. CT = cycle threshold.
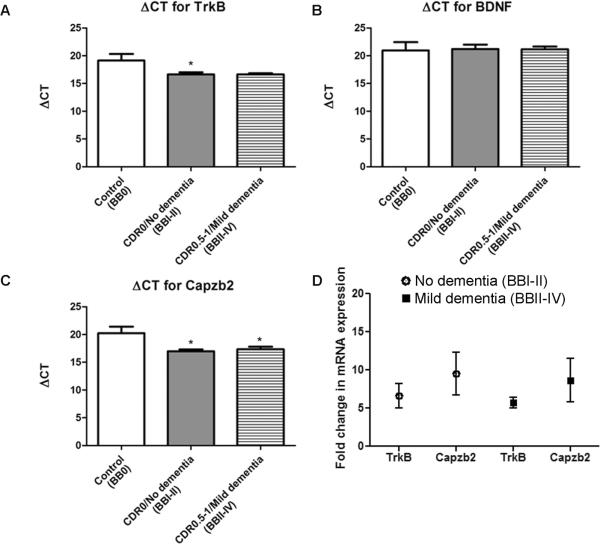
TrkB and Capzb2 mRNAs Remain Upregulated in Demented Subjects
Importantly, we wanted to establish the relationship between the level of expression of BDNF, TrkB, and Capzb2 in hippocampal neurons and presence of dementia. We compared neuronal populations from subjects with no dementia (CDR0) that included FHS BBI (BVAX1–3) and BBII (BVAX4) cases to neuronal populations from patients with dementia (CDR0.5–1, mild to moderate dementia): BBII (BVAX5) and BBIV (BVAX6 and 7 and MADRC1) (Table). Interestingly, whereas the expression of TrkB mRNA continues the increasing trend observed in CDR0 cases, the average expression in cases with dementia was not significantly different from that of controls (BM1–4) (Fig. 3A; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348). BDNF mRNA expression levels in BBI-II and BB II-IV cases were similar to those in the controls (Fig. 3B; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348). Capzb2 mRNA expression in dementia cases (BBII-IV) was significantly increased in comparison to controls (Fig. 3C, D; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A348). Thus, as neuropathological changes progress, Capzb2 mRNA expression levels remain significantly elevated, likely reflecting cytoskeletal reorganization underlying degenerative and regenerative morphological changes as earlier suggested (7).
Figure 3.
TrkB and Capzb2 mRNAs remain upregulated in dementia. (A–C) In dementia cases ((Braak and Braak [BB] stages II–IV), as opposed to subjects with no dementia (BBI–II), the average TrkB mRNA expression is not significantly increased when compared to controls (BB0); however, there is a trend towards upregulation (lower ΔCT values) (A). Brain-derived neurotrophic factor (BDNF) mRNA expression in cases BBI–II (no dementia) and BBII–IV (dementia) is similar to BB0 subjects (controls) (B). Both BBI–II subjects with no dementia (1–4) and BBII–IV dementia cases (BVAX5–7; MADRC1) show significantly increased mRNA expression (lower ΔCT values) for Capzb2 compared to control subjects (BM1–4); *p < 0.05, One-Way ANOVA (C). Calculations for ΔCT were made by subtracting the average CT for housekeeping gene 18SrRNA from average CT from gene of interest. (D) Fold-increases of mean mRNA expression of TrkB (5.7-fold increase in No Dementia / BBI–II group, and 5.6-fold increase in Dementia / BBII–IV group) and Capzb2 (9.4-fold increase in No Dementia / BBI–II group, and 7.5-fold increase in Dementia / BBII–IV group) vs. controls. CT = cycle threshold.
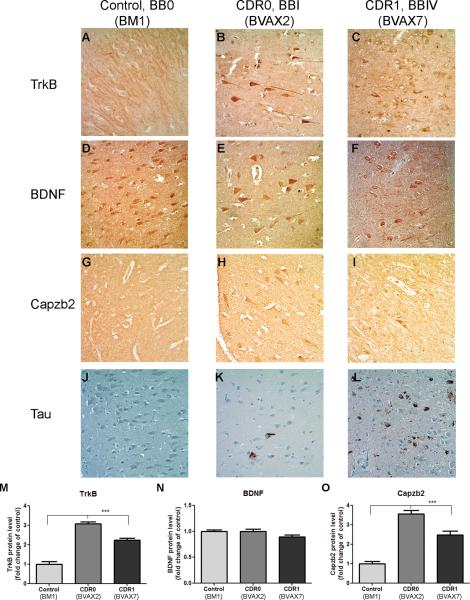
TrkB, BDNF, and Capzb2 Protein Immunoreactivities in CA1 Neurons Correlate with mRNA Expression
We performed immunohistochemistry for TrkB, BDNF, Capzb2, and tau on all of the patient specimens (Table). CA1 region sections of a representative case from each of the 3 groups studied were analyzed for the presence/intensity of signals for TrkB, BDNF, Capzb2, and for the evidence of neurofibrillary changes. In accordance with mRNA analysis, CA1 pyramidal neurons of CDR0/BBI and CDR1/BBIV cases showed significant upregulation of TrkB and Capzb2 protein expression in comparison to control (Fig. 4A–C, G–I, M, O); all 3 had similarly intense BDNF immunoreactivity (Fig. 4D–F, N). Tau immunohistochemistry highlighted rare neurofibrillary changes in CDR0/BBI case and substantial neurofibrillary pathology in CDR1/BBIV case whereas control cases were free of any tau signal (Fig. 4J–L).
Figure 4.
Immunohistochemistry for TrkB, brain-derived neurotrophic factor (BDNF), and Capzb2 in representative cases from control, clinical dementia rating score 0 (CDR0), and CDR score 1 (CDR1) group. (A–C, M) TrkB signal in CA1 pyramidal neurons of a CDR0 Framingham Heart Study (FHS) subject (Braak and Braak [BB] stage I) (B) and of a CDR1 FHS subject (BBIV) (C) is stronger than that in a control (BB0) (A), p ≤ 0.0001; One-way ANOVA followed by Tukey multiple comparison test. Fold-change increase of TrkB in CDR0 and CDR1 FHS cases are 3.08 and 2.25, respectively (M). (D–F, N) In these same hippocampi, BDNF signal in CA1 pyramidal neurons of CDR0 (E) and CDR1 individual (F) is comparable to the signal in a control (D, N). (G–I, O) Capzb2 signal in CA1 neurons of the CDR0 subject (H) and of the CDR1 subject (I) is stronger than that of the control (G) p ≤ 0.0001; One-way ANOVA followed by Tukey multiple comparison test. Fold-change increase of Capzb2 in CDR0 and CDR1 FHS cases are 3.55 and 2.49, respectively (O). (J–L) Immunohistochemistry for tau highlights intensity of neurofibrillary changes in the CDR0 BBI subject (K) and in the CDR1 BBIV subject (L); the control is free of tau immunostaining (J). Signals were blindly quantified in 50 CA1 neurons per experimental condition using Image J 1.42q.
DISCUSSION
In this study, we found greater expression of TrkB and Capzb2 in CA1 hippocampal neurons of individuals with preserved cognitive status and initial NFT formation than in cognitively intact individuals without any NFTs. In contrast, BDNF expression remained unchanged, raising the possibility that the upregulated TrkB expression in CDR0 individuals is responsible for the increase in BDNF/TrkB signaling tapping the brain reserve. In the group of individuals with more advanced tangle formation and dementia (CDR0.5–1), the increase in TrkB expression and the unchanged expression of BDNF might have been insufficient to provide adequate brain reserve. In light of the reported restoration of learning and memory functions in AD animal models upon BDNF gene delivery (3), exogenous intervention to boost BDNF/TrkB signaling would appear to be a compelling therapy in early AD. However, the experiments by Frank et al suggested that the exposure of developing and adult rodent hippocampal neurons to BDNF in vitro and in vivo results in long-term functional desensitization to BDNF and downregulation of TrkB mRNA (37). It is possible that BDNF/TrkB signaling is regulated differently in healthy vs. diseased hippocampal neurons. Nevertheless, the reported increase in TrkB mRNA expression in astrocytes occasionally associated with senile plaques in hippocampi of AD brains raises concerns that the administration of neurotrophic factors could promote gliosis and plaque formation (5). Importantly, if the observed increase in TrkB expression in cognitively intact FHS subjects with initial formation of NFTs constitutes brain reserve, downregulation of TrkB might represent a potentially harmful side effect of exogenous BDNF delivery.
Because of its implication in neuroprotection, BDNF has been studied as a possible candidate gene conferring risk of AD (38–40). BDNF Val66Met polymorphism carriers have been previously reported to have smaller hippocampal volumes and reduced gray matter in the frontal cortex (41). Consistent with the evidence that Val66Met polymorphism may affect activity-dependent secretion of BDNF and hippocampal functioning, Antal et al observed differences in plasticity induction between Val66Val and Val66Met carriers (42). N-methyl-D-aspartate receptor activation, a crucial step towards synaptic plasticity, was recently shown to derepress BDNF promoters 1- and 4-mediated transcription (43). In parallel, each of these BDNF promoter regions exhibited reduced occupancy by histone deacetylase 1 and methyl cytosine binding protein 2 in cultured hippocampal neurons (43). This epigenetic regulation of BDNF expression was further corroborated by the involvement of RACK1, a scaffolding protein important for regulation of signaling pathways, in derepression of BDNF exon 4 transcription via chromatin remodeling upon cAMP pathway activation (44). Thus, while the relationships between BDNF gene polymorphisms and AD are not yet fully understood, there is compelling evidence that epigenetic regulation, i.e. chromatin remodeling, connects BDNF with the molecular processes involved in learning and memory. In vivo studies in rodents confirm the data obtained in vitro. Docosahexaenoic acid dietary supplementation increased levels of pro-BDNF and mature BDNF, further boosted by exercise (45). Exercise also restored TrkB in ApoE ε4 mice to the level observed in ε3 mice and increased synaptophysin, a marker of synaptic function, in ε4 mice. Hippocampal BDNF levels were similarly increased in both ε3 and ε4 mice after exercise (46). Exposure to an enriched environment for 3 to 4 weeks also caused dramatic increase in BDNF mRNA in mouse hippocampus (47). Human data corroborate these experimental paradigms, i.e. exercise (48) and leisure activities (49), may reduce the risk for clinical dementia in the elderly. Our data suggest that designing strategies to enhance BDNF/TrkB signaling epigenetically in persons with early cognitive changes associated with AD pathology (mild cognitive impairment, MCI, due to AD pathology) (50), and in persons with no clinical symptoms but with biomarker evidence of AD pathology (so-called preMCI due to AD pathology) (51), may be beneficial in delaying the onset of clinical dementia. Chromatin remodeling via histone acetylation in aging mice (52) and in a mouse model of neurodegeneration (53) appears critical for the regulation of learning and memory. We plan further assessment of epigenetic modification of the BDNF pathway in FHS brains.
In conclusion, our study of CA1 hippocampal neurons in FHS participant brain donors adds to the emerging evidence that the BDNF/TrkB pathway may be involved in the compensatory response to early AD pathology, i.e. it may underlie the biology of cognitive reserve. We predict that the prevention of clinical AD will require a multi-dimensional approach and that modulation of the BDNF/TrkB pathway, calibrated to each individual's needs, might be one facet of this multi-dimensional approach.
Supplementary Material
ACKNOWLEDGMENTS
We thank V. Alvarez, J. Himali, K. Fitch, and C. Spencer for technical support, J. Kozubek for statistical expertise, and Z. Xie, J.K. Blusztajn, and A. Fischer for critical reading of the manuscript.
This work was supported by NIH 5T32AG000115-25 (PFK, PRP), R01AG031287, R01AG08122, R01AG033193, R01AG16495 and P30AG013846 (SS), and Mallory funds at BUSM Pathology Department (ID).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 2.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–14. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 3.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–7. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips HS, Hains JM, Armanini M, et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 5.Connor B, Young D, Lawlor P, et al. Trk receptor alterations in Alzheimer's disease. Brain Res Mol Brain Res. 1996;42:1–17. doi: 10.1016/s0169-328x(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 6.Fujimura RK, Reiner T, Ma F, et al. Changes in the expression of genes associated with intraneuronal amyloid-beta and tau in Alzheimer's disease. J Alzheimers Dis. 2010;19:97–109. doi: 10.3233/JAD-2010-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao PF, Davis DA, Banigan MG, et al. Modulators of cytoskeletal reorganization in CA1 hippocampal neurons show increased expression in patients at mid-stage Alzheimer's disease. PLoS One. 2010;5:e13337. doi: 10.1371/journal.pone.0013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Q, Rosenfeld RD, Matheson CR, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–48. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- 9.Shetty AK, Rao MS, Hattiangady B, et al. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–32. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- 10.Goutan E, Marti E, Ferrer I. BDNF, and full length and truncated TrkB expression in the hippocampus of the rat following kainic acid excitotoxic damage. Evidence of complex time-dependent and cell-specific responses. Brain Res Mol Brain Res. 1998;59:154–64. doi: 10.1016/s0169-328x(98)00156-9. [DOI] [PubMed] [Google Scholar]
- 11.Avwenagha O, Campbell G, Bird MM. Distribution of GAP-43, beta-III tubulin and F-actin in developing and regenerating axons and their growth cones in vitro, following neurotrophin treatment. J Neurocytol. 2003;32:1077–89. doi: 10.1023/B:NEUR.0000021903.24849.6c. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto Y, Yamauchi J, Tanoue A, et al. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103:10444–9. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–80. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Grupe A. Genetics of late-onset Alzheimer's disease: progress and prospect. Pharmacogenomics. 2007;8:1747–55. doi: 10.2217/14622416.8.12.1747. [DOI] [PubMed] [Google Scholar]
- 15.Nathan BP, Chang KC, Bellosta S, et al. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–9. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- 16.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer's disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516–29. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis DA, Wilson MH, Giraud J, et al. Capzb2 interacts with beta-tubulin to regulate growth cone morphology and neurite outgrowth. PLoS Biol. 2009;7:e1000208. doi: 10.1371/journal.pbio.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson TJ, Backlund PS, Jr, Alkon DL. Hippocampal protein-protein interactions in spatial memory. Hippocampus. 2004;14:46–57. doi: 10.1002/hipo.10152. [DOI] [PubMed] [Google Scholar]
- 19.Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–7. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 20.Kitanishi T, Sakai J, Kojima S, et al. Activity-dependent localization in spines of the F-actin capping protein CapZ screened in a rat model of dementia. Genes Cells. 2010;15:737–47. doi: 10.1111/j.1365-2443.2010.01411.x. [DOI] [PubMed] [Google Scholar]
- 21.Scheibel AB, Tomiyasu U. Dendritic sprouting in Alzheimer's presenile dementia. Exp Neurol. 1978;60:1–8. doi: 10.1016/0014-4886(78)90164-4. [DOI] [PubMed] [Google Scholar]
- 22.Scheibel AB. Dendritic changes in senile and presenile dementias. Res Publ Assoc Res Nerv Ment Dis. 1979;57:107–24. [PubMed] [Google Scholar]
- 23.McKee AC, Kowall NW, Kosik KS. Microtubular reorganization and dendritic growth response in Alzheimer's disease. Ann Neurol. 1989;26:652–9. doi: 10.1002/ana.410260511. [DOI] [PubMed] [Google Scholar]
- 24.Labelle C, Leclerc N. Exogenous BDNF, NT-3 and NT-4 differentially regulate neurite outgrowth in cultured hippocampal neurons. Brain Res Dev Brain Res. 2000;123:1–11. doi: 10.1016/s0165-3806(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 25.Olde Rikkert MG, Tona KD, Janssen L, et al. Validity, reliability, and feasibility of clinical staging scales in dementia: a systematic review. Am J Alzheimers Dis Other Demen. 2011;26:357–65. doi: 10.1177/1533317511418954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging. 1997;18:S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Farmer ME, White LR, Kittner SJ, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60:1023–40. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 29.Au R, Seshadri S, Wolf PA, et al. New norms for a new generation: cognitive performance in the Framingham offspring cohort. Exp Aging Res. 2004;30:333–58. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 33.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–7. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 34.Davis PB, White H, Price JL, et al. Retrospective postmortem dementia assessment. Validation of a new clinical interview to assist neuropathologic study. Arch Neurol. 1991;48:613–7. doi: 10.1001/archneur.1991.00530180069019. [DOI] [PubMed] [Google Scholar]
- 35.Au R, Seshadri S, Knox K, et al. The Framingham Brain Donation Program: Neuropathology Along The Cognitive Continuum. Curr Alzheimer Res. 2012 Apr 2; doi: 10.2174/156720512801322609. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durrenberger PF, Fernando S, Kashefi SN, et al. Effects of antemortem and postmortem variables on human brain mRNA quality: a BrainNet Europe study. J Neuropathol Exp Neurol. 2010;69:70–81. doi: 10.1097/NEN.0b013e3181c7e32f. [DOI] [PubMed] [Google Scholar]
- 37.Frank L, Ventimiglia R, Anderson K, et al. BDNF down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur J Neurosci. 1996;8:1220–30. doi: 10.1111/j.1460-9568.1996.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 38.Riemenschneider M, Schwarz S, Wagenpfeil S, et al. A polymorphism of the brain-derived neurotrophic factor (BDNF) is associated with Alzheimer's disease in patients lacking the Apolipoprotein E epsilon4 allele. Mol Psychiatry. 2002;7:782–5. doi: 10.1038/sj.mp.4001073. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Fukumoto H, Orne J, et al. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–6. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Forlenza OV, Diniz BS, Teixeira AL, et al. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11:774–80. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 41.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 42.Antal A, Chaieb L, Moliadze V, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–7. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Tian F, Marini AM, Lipsky RH. NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons. Amino Acids. 2010;38:1067–74. doi: 10.1007/s00726-009-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He DY, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285:19043–50. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–9. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichol K, Deeny SP, Seif J, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–94. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuzumaki N, Ikegami D, Tamura R, et al. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus. 2011;21:127–32. doi: 10.1002/hipo.20775. [DOI] [PubMed] [Google Scholar]
- 48.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 49.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 50.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peleg S, Sananbenesi F, Zovoilis A, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–6. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 53.Fischer A, Sananbenesi F, Wang X, et al. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–82. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.