Abstract
Thermosensitive liposomes are attractive vehicles for delivery and release of drugs to tumors. To improve targeting efficacy for breast cancer treatment, an 8.3 kDa HER2-specific Affibody molecule (ZHER2:342-Cys) was conjugated to the surface of liposomes. The effects of this modification on physical characteristics and stability of the resulting nano particles denoted “Affisomes” were investigated. Thermo-sensitive small unilamellar vesicles (SUV) liposomes of (80–100 nm) diameter consisting of dipalmitoyl phosphatidylcholine (DPPC, Tm 41 °C) as the matrix lipid and a maleimide conjugated pegylated phospholipid (DSPE-MaL-PEG2000) were prepared by probe sonication. Fluorescent probes were incorporated into liposomes for biophysical and/or biochemical analysis and/or triggered release assays. Affibody was conjugated to these liposomes via its C-terminal cysteine by incubation in the presence of a reducing agent (tributylphosphine) for 16–20 hours under argon atmosphere. Lipid conjugated affibody molecule was visible as an 11.3-kDa band on a 4–12% Bis/Tris gel under reducing conditions. Affibody conjugation yields were ~70% at a protein/lipid ratio of 20 μg/mg, with an average number of 200 affibody molecules per Affisome. Affibody conjugation to thermosensitive liposomes did not have any significant effect on the hydrodynamic size distribution of the liposomes. Thermo-sensitivity of Affisomes was determined by monitoring release of entrapped calcein (a water-soluble fluorescent probe, λex/em 490/515 nm) as a function of temperature. Calcein was released from Affisomes (thermosensitive liposomes with affibody-Targeted SUV) as well as non-targeted SUV (thermosensitive liposomes without affibody) in a temperature-dependent manner, with optimal leakage (90–100%) at 41°C. In contrast, liposomes prepared from Egg-phosphatidyl choline (Tm ~0 °C) under similar conditions released only 5–10% calcein at 41°C. Affisomes, when stored at room temperature, retained >90% entrapped calcein up to 7 days. Moreover, incubation of liposomes in PBS supplemented with 10% heat-inactivated serum (FBS) did not result in destabilization of liposomes. Therefore, Affisomes present promising and novel drug delivery candidates for breast cancer targeting.
Introduction
Liposomes (lipid-based nanoparticles) have been explored as drug delivery vehicles for decades (1;2–4). In recent years, approaches have been developed to design liposomes to promote in vivo stability (1;5–7), site-specific targeting (2;8–10) and localized drug release (11;12;13). Among these, pegylated (stealth) liposomes have proven to be successful in the clinic because of significant increase in survival times in circulation. However, these liposomes rely on uncoating of the polymer coat for drug release (1;6). The success of antibody-coated liposomes (immunoliposomes) is limited by the availability of technology for large scale production of purified single chain antibodies (14;15), and efficient antibody-conjugation methods (16;17). Recent reports describe breast cancer targeting anti-HER2 monoclonal antibodies (mAb)-conjugated doxorubicin loaded liposomes by Park and colleagues (2;14). Following endocytosis of the immunoliposomes the doxorubicin presumably enters the cell’s cytosol by permeation across the liposomal and endosomal membrane. In order to achieve a more rapid and efficient release of the liposomal payload, methods have been devised for triggering release of liposome contents by locally raising the temperature a few degrees above the human body temperature (18).
Thermosensitive liposomes for triggered drug release are based on the lipid destabilizing mechanism(s), phase transition effects, and thermal melting temperatures (Tm)(13). It has been clearly demonstrated that hyperthermia can be used to selectively enhance both the delivery and the rate of release of drugs from thermosensitive liposomes to targeted tissues (11;19). Thermosensitive liposomes are currently in clinical trials to treat Hepatocellular Carcinoma Liver Neoplasms and breast cancer (ClinicalTrials.gov Identifier #NCT00441376 and #NCT00346229, respectively). However, these nano-particles were not designed for receptor-mediated targeting.
We undertook this study to design and develop the next generation of delivery systems that will combine imaging, targeting and triggered drug release. Targeted delivery of liposomes has thus far been limited to using receptor ligands, antibodies and antibody-derived molecules. In this study, we have used a novel class of targeting agents, Affibody molecules, as an alternative to targeting antibodies. Affibody molecules are relatively small proteins (6–8kDa), and offer the advantages of being extremely stable and highly soluble α-helical proteins and can be readily expressed in bacterial systems or produced by peptide synthesis. ZHER2:342-Cys (8.3kD) affibody molecule, which binds to HER2 receptor with high affinity (22 pM) and its C-terminus cysteine is available for bio-molecule conjugation via malemide-sulfhydryl reaction was used in this study (20). The HER2 receptor is noted for its role in the pathogenesis of various types of malignancies including breast, lung, prostate, and ovarian carcinomas and is associated with poor prognosis. Therefore, it presents a viable target for therapy. We report that HER2-specific Affibody molecules-conjugated thermosensitive liposomes (Affisomes) retain their triggered release properties, without any significant modulation in their physico-chemical characteristics. Our newly designed Affisome formulations may have implications in treatment of HER2 positive tumors including breast cancer.
Materials and Methods
Reagents
Reagents were from Sigma-Aldrich Inc. (St. Louis, MO). Tributylphosphine and Biogel A-0.5m were procured from Bio-Rad Hercules, CA. All other chemicals and reagents were of analytical grade.
Lipids
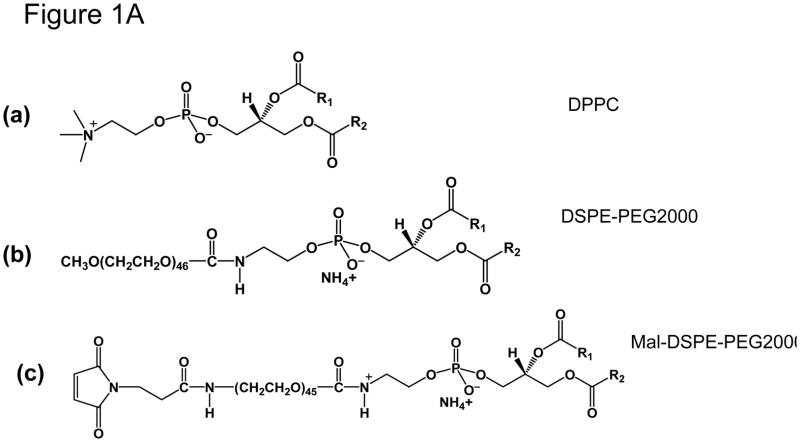
Chemical Structure of lipids used in this study is shown in Figure 1A. Egg Phosphatidyl choline (Egg PC), Dipalmitoylphosphatidylcholine (DPPC), 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Methoxy(Polyethylene glycol)-2000] (DSPE-PEG2000), 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Maleimide (Polyethylene Glycol)2000] (DSPE-MaL-PEG2000) were purchased from Avanti Lipids, Inc. (Alabaster, AL). Lipid purity and analysis was routinely conducted by thin layer chromatography (TLC) using silica gel as a stationary phase and chloroform:methanol:water (65:25:4 by volume) as a mobile phase (21). Molybdenum Blue Spray (Sigma, USA) was used to visualize lipids (22). The purity of lipids used in these studies was >99%.
Figure 1.
(A) Chemical structure of phospholipids used for HER2-specific Affibody conjugated Thermo-sensitive liposomes. (a) DPPC., (b) DSPE-PEG2000., (c) DSPE-MaL-PEG2000-PE. R1 and R2 represent the fatty acyl chains at sn-1 and sn-2-positions, respectively.
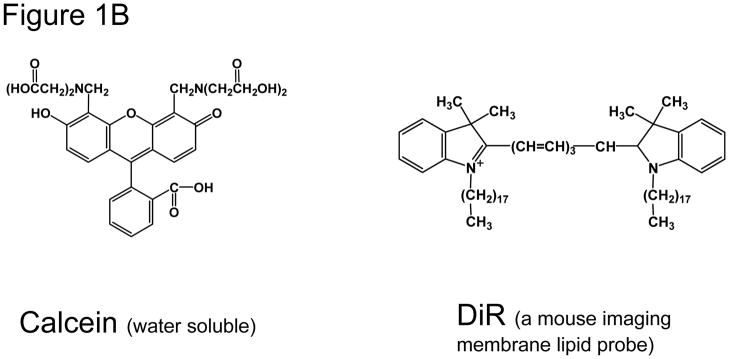
(B) Fluorescent Probes loaded into HER2-specific Affibody conjugated Thermo-sensitive liposomes: (a) Calcein., (b) DiR.
Fluorescent Markers
The chemical structures of fluorescent probes used in this study are shown in Figure 1B. Calcein (λEx/Em 494/517 nm), and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (‘DiR’; DiIC18λEx/Em 748/780 nm) were from Invitrogen Inc (Carlsbad, CA).
Affibody
HER2-specific Affibody, ZHER2:342-Cys was obtained from Affibody Biotechnology (Solna, Sweden) through the Collaborative Research and Development Agreement (CRADA).
Preparation of Small Unilamellar Liposomes (SUV)
Liposomes were prepared by probe sonication. Lipids (including any fluorescent lipids) were mixed at desired molar ratios in a glass tube. The formulations used in this study are described in Table I. A lipid film was formed by removing chloroform solvent under nitrogen at room temperature. Any residual chloroform was removed by placing the films overnight in a vacuum desiccator. Multilamellar vesicles (MLVs) were formed by reconstituting the lipid film with HBSE buffer (10mM HEPES, 150mM NaCl, 9.1mM EDTA, pH 7.5) by vigorous vortexing. To entrap calcein, lipid film was reconstituted in HBSE buffer containing self-quenched concentration of calcein (0.1 M at pH 7.5). MLVs were sonicated at 4 °C using a Probe Sonicator (W-375 Heat Systems-Ultrasonics, New York, USA). Usually 10 minutes’ sonication (1 min pulses and 1 min rest), yielded liposomes in the size range of 100 – 150 nm diameter as determined by dynamic light scattering. SUV were centrifuged at 2000 xg for 5–10 minutes to remove any titanium particles and larger aggregates. Calcein-loaded liposomes were separated from un-entrapped calcein using size exclusion gel chromatography column (Biogel-A0.5m), pre-equilibrated with HBSE buffer, pH 7.4. Presence of liposomes in the column fractions was analyzed by measuring entrapped calcein (water soluble solute) or DiR fluorescence (lipid). Liposome rich fractions were pooled and total phospholipid content was determined by inorganic phosphate analysis (23), to an average concentration of final lipid at 2.5–3.5 mg/ml. The resulting liposomes were characterized for their size and thermosensitivity, and Affibody conjugation (see below).
TABLE I.
Liposome Formulations Used in this Study
| Liposomes | Lipids | Fluorescent Markers | Affibody Conjugation |
|---|---|---|---|
| Affisomes (thermo-sensitive) | DPPC (88.5 mol%) Mal-DSPE-PEG2000 (5.5 mol%) DSPE-PEG2000 (5.5 mol%) |
Calcein (0.1M, aqueous) DiR (0.5 mol%, lipid tracer) |
YES |
| Control-I (non-thermo-sensitive) | Egg PC (88.5 mol%) Mal-DSPE-PEG2000 (0.0 mol%) DSPE-PEG2000 (11 mol%) |
Calcein (0.1M, aqueous) DiR (0.5 mol%, lipid tracer) |
NO |
| Control-II (thermo-sensitive NO Affibody) | DPPC(88.5 mol%) Mal-DSPE-PEG2000 (0.0 mol%) DSPE-PEG2000 (11 mol%) |
Calcein (0.1M, aqueous) DiR (0.5 mol%, lipid tracer) |
NO* |
| Control-III (thermo-sensitive NO Affibody) | DPPC (88.5 mol%) Mal-DSPE-PEG2000 (5.5 mol%) DSPE-PEG2000 (5.5 mol%) |
Calcein (0.1M, aqueous) DiR (0.5 mol%, lipid tracer) |
NO** |
affibody was added to Control-II liposomes to assess non-specific association of affibody with liposomes
L-cysteine was added to block free malemide groups of Mal-DSPE-PEG2000 on the liposome surface
Liposome Characterization
A Malvern Zetasizer Nano ZS instrument (Southborough, MA) with back scattering detector was used for measuring the hydrodynamic size (diameter) in batch mode (no fractionation) at 25°C in a quartz cuvette. Hydrodynamic size is reported as the volume-weighted diameter and its percentage over all present size populations. Sample concentration was 2–5 mg/mL in HBSE buffer. Three measurements were made per sample and the averaged data is reported.
Thermosensitivity Assay
Thermosensitivity of liposomes was determined by temperature-induced leakage of calcein from the liposomes. Calcein was loaded into liposomes at its self-quenching concentration; therefore the release of contents results in an increase of fluorescence due to dequenching (13). Calcein-loaded liposomes (200 μL) were added to 1.8 ml of HBSE-buffered saline in a cuvette at 37°C under constant stirring. After a 5-min equilibration at 37 °C, the temperature was increased to 39 or 42°C, and the resulting increase in calcein fluorescence was measured on continuously (λEx/Em 490/515 nm respectively) using an Fluoromax 3 Fluorimeter (HORIBA Jobin Yvon Inc, NJ, USA). Complete leakage (100%) was achieved by the addition of Triton X-100 (final concentration 1%). The percentage of calcein leakage was calculated using the following formula: ((Fx−Fo)/(Ft))×100%, where Fx is the fluorescence of calcein before the addition of Triton X-100, Fo is the fluorescence at 37 °C, and Ft is the fully dequenched calcein fluorescence after addition of Triton X-100.
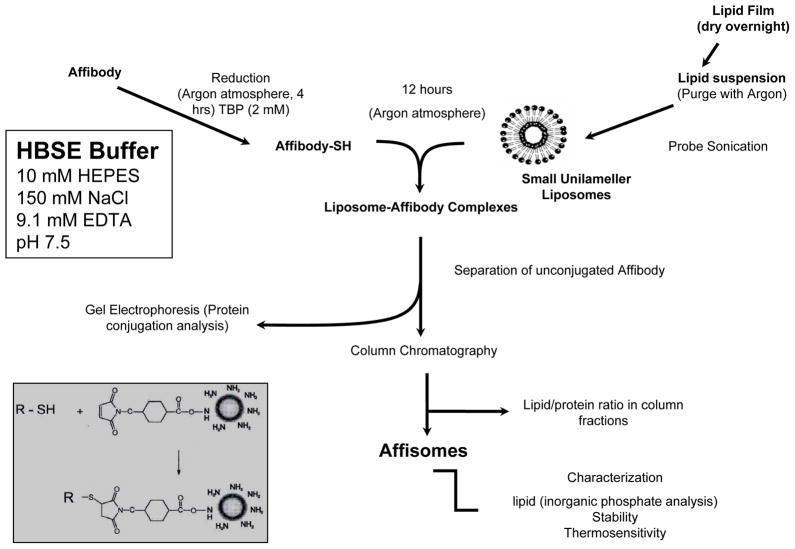
Affibody Conjugation to Liposomes
The steps involved in affibody conjugation are shown in Figure 2. Prior to conjugation with liposomes, Affibody molecules (175μg) were incubated with tributylphosphine (2 μM final concentration) in a glass tube under argon for 4 hours to ensure complete reduction of any disulfide bonds. The reduced Affibody was then added to the liposomes (7.5 mg phospholipid), and incubated for 10–16 hours at room temperature under argon. At the end of incubations, L-cysteine (1 mM final concentration) was added to the samples and incubations were for 15 minutes at room temperature to block any free maleimide functional groups on the liposome surface. Unconjugated Affibody was separated from liposomes on a Biogel A-0.5m column pre-equilibrated with HBSE buffer. Dir and calcein fluorescence in each fraction was determined as above. Liposome-rich fractions were pooled together and filtered through a Millex-HB 0.45 μm filter unit. Phospholipid content was determined by inorganic Pi analysis. Covalent coupling of Affibody to Affisomes was determined by gel electrophoresis, with or without treatment of liposomes with NuPage reducing agent. Samples were loaded on a NuPage 4–12% Bis/Tris gel (NuPage), and the gel was run in MES buffer at 200V, 115 mA for approximately 35 minutes. The gel was stained with protein staining agent (MicrowaveBlue, Protiga Inc, Frederick, MD)). Quantification of lipid-conjugated Affibody molecules was done by determination of grey level intensity of affibody bands on the gels using the Odyssey software. Known concentrations of free affibody (0.25–1.0 μg) were used for calibration. Liposomes prepared without the conjugation lipid DSPE-MaL-PEG2000 (Liposome Control-II) were treated with affibody under identical conditions to assess specificity of Mal-SH reaction. The preparations were filtered through a Millex-HB 0.45 μm filter unit (Fisher scientific). Effect of affibody conjugation on average size of liposomes was determined as described above.
Figure 2. Effect on Inclusion of Pegylated lipids on Thermosensitivity of Liposomes.
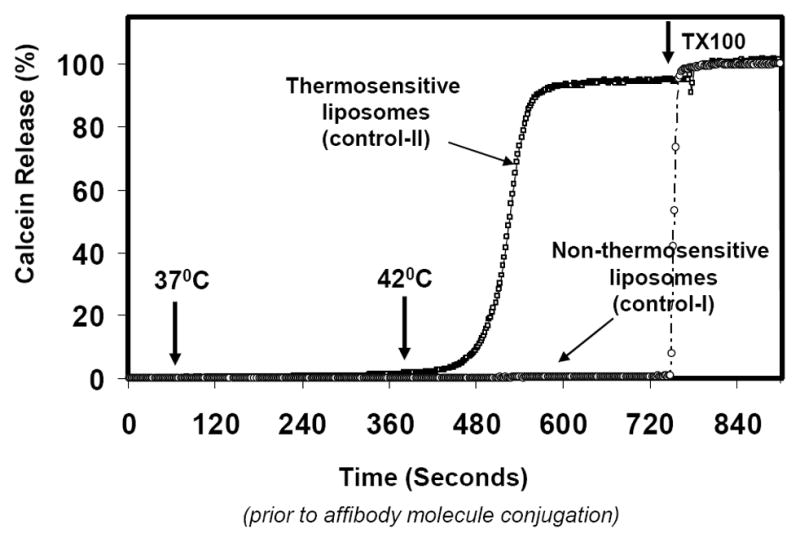
Calcein loaded liposomes were placed in stirred cuvette containing 2 ml PBS at 37°C and effect on inclusion of pegylated lipids on thermo-sensitivity of liposomes was measured. The temperature was increased to 41°C and calcein release from liposomes (due to relief of self-quenching) was monitored in a Horiba Fluoromax-3 cuvette reader (Edison, NJ) at an excitation/emission of 490/515nm,. Complete (100%) leakage was obtained by addition of 20 μl TX100 (10% w/v). Thermo-sensitive liposomes show almost 100% release of encapsulated calcein at temperatures at and above 41°C.
Analysis of Affibody Molecules Per Liposome
Average number of affibody molecules per liposome were calculated as described elsewhere (24). The following parameters are used: (a) affibody to lipid ratio 20 μg/mg phospholipid (=0.02gr/gr);(b), Liposome size, 100 nm diameter;(c), M.W. of a single lipid molecule=103; (d) M.W. of affibody = 8.300, and (e) Outer surface area of the liposomes=3.8×104 nm2. Based on 9×104 lipid molecules per liposome (Zhu 2005), the M.W. of liposomes=9×107 and Affibody molecules/liposome (mol/mol) = (0.02/8300)/(1/9×107) =216 mol/mol.
Results and Discussion
Our initial liposome preparations were composed of DPPC: Lyso PC: DSPE-MaL-PEG2000 and DiR, a lipophilic probe with excitation/emission in near infrared region was incorporated into liposomes (Fig. 1B, Table I), for future in vivo imaging application. Similar lipid compositions have been reported previously for thermo-sensitive liposomes (19;25;26). However, we were unable to reproducibly entrap calcein (a water-soluble fluorescent probe, λex/em 490/515 nm) into these liposomes. Therefore, we tested formulations without lyso-PC (a destabilizing lipid). We entrapped calcein at its self-quenched concentration (0.1–0.15 M) in liposomes containing DPPC/DSPE-MaL-PEG2000/DiR (Fig. 1, Table I and Methods section). Kinetics of temperature-induced release of entrapped calcein from liposomes was determined by monitoring increase in fluorescence due to relief of self-quenching by spectrofluorometry. Calcein was released from Thermosensitive liposomes (Control-II and Control-III, Table I) in a temperature-dependent manner, with 90–100% leakage at 41 °C (Fig. 2). Non-thermosensitive liposomes prepared from egg PC (Control-I, Table I), however, under similar conditions released only 5–10% calcein at 41°C. These data confirmed suitability of our liposomes for triggered release application. Analysis of hydrodynamic size distribution of these liposome preparations was conducted by dynamic light scattering measurements (see below, Figure 4C for details). All our formulations exhibited an average size distribution of 80–100 nm. We also observed that incorporation of the DSPE-MaL-PEG2000 lipid in liposomes neither altered thermosensitivity nor calcein entrapment efficiency of our liposomes (data not shown). Next, these liposomes were tested for conjugation of ZHER2:342-Cys Affibody molecules.
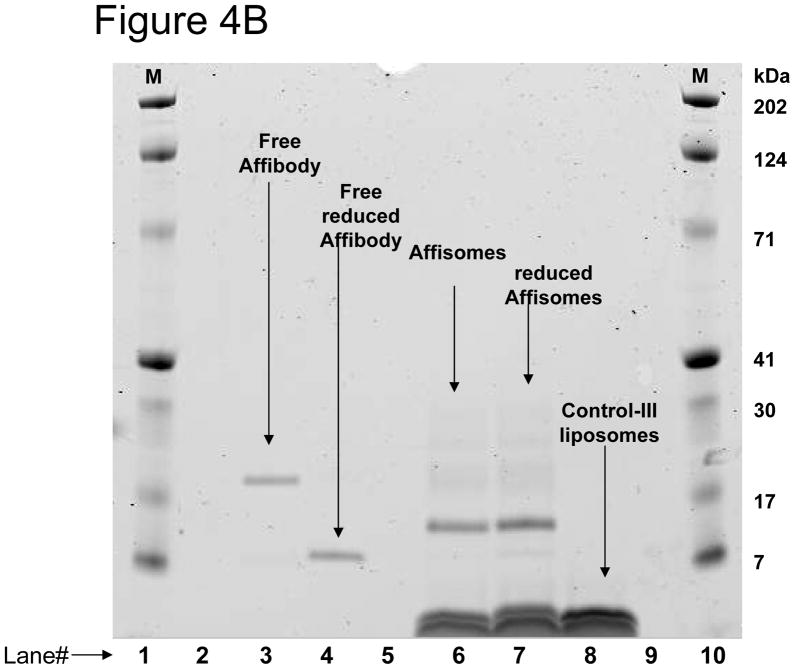
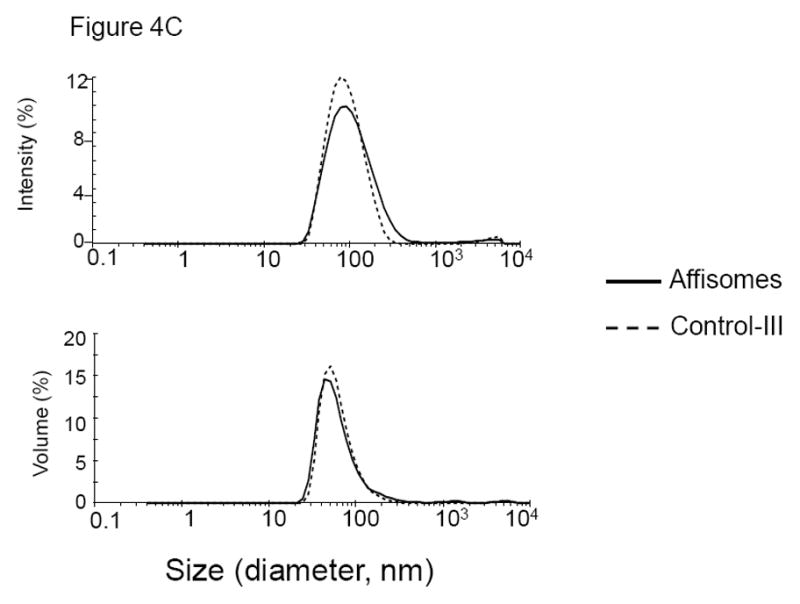
Figure 4. ZHER2:342-Cys Affibody Conjugation to Thermosensitive Liposomes.
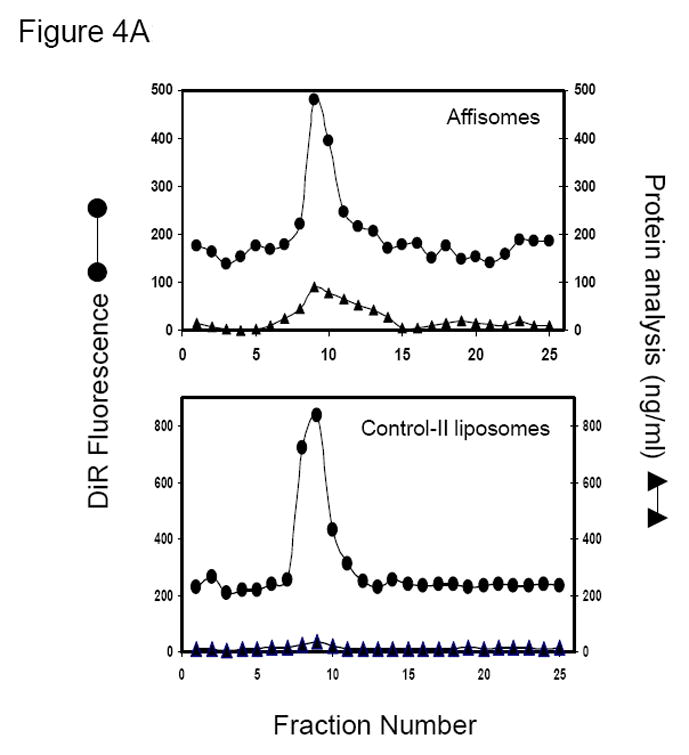
(A) Elution profile of Affisomes on Bio-Gel A0.5m Column. Elution profiles of anti-HER2 Affibody conjugated liposomes and control II liposomes on a BioGel A0.5-m column was detected to determine which fractions contained liposomes by monitoring DiR fluorescence (excitation/emission of 740/760nm) and protein determination as described in methods section. (B) Determination of HER2-Specific Affibody Conjugation to Liposomal Mal-DSPE-PEG2000:Fractions collected from bio-Gel column (Figure 4A) rich in liposomes were pooled together and were analyzed for the presence of affibody containing by gel electrophoresis (described in details in methods section). M (Markers) =Mark12 Unstained Standard (Invitrogen, USA).
(C) Effect of Affibody-conjugation on hydrodynamic size distribution of thermo-sensitive liposomes: Size analysis Affisomes and thermosensitive liposomes (Control III) were characterized at Malvern Zetasizer Nano ZS instrument. The data are plotted as intensity (top panel) and volume (bottom panel) weighted distributions.
Steps involved in liposome-affibody conjugation are summarized in Figure 3. The conjugation of affibody to the liposome surface was based on chemical reaction between maleimide of the lipid with the thiol group on the C-terminus of Affibody, as previously described for conjugation for anti-HER2 antibodies to the liposome surface (16;17). We used a ratio of 1:5 or 1:10 of Affibody:DSPE-MaL-PEG2000 for conjugation (see Methods section) to achieve optimal affibody coupling. Initial analysis of affibody-liposome conjugation was done by separation of unconjugated affibody from the conjugated product on a size exclusion column. Elution profile of affibody-conjugated liposomes (Affisomes) on a BioGel A0.5m column (1×40 cm) is shown in Figure 4A. All fractions were analyzed for entrapped calcein and DiR fluorescence, representing liposomes that are eluted into the void-volume (fractions 8–12) of the column. On the other hand, free affibody (even in its dimer form) was fractionated in the included volume of the column (fraction 20 and beyond, data not shown).
Figure 3.
Steps involved in Conjugation of Affibody Molecule to Liposome Surface.
Our initial attempts to directly analyze liposome-conjugated affibody quantity in various fractions by using protein assay kits were unsuccessful because of high background and extremely low protein ratios in the liposomes. Therefore, presence of affibody in various liposome fractions was assayed by analyzing the fractions by gel electrophoresis (see Methods section). For quantitation of affibody, various concentrations (0.25 –1 μg) of reduced affibody were used and a calibration curve was generated (not shown, see Methods section). It is clear from the elusion profile of liposomal lipid and affibody (Figure 4A) that affibody co-eluted in the liposome fraction only when DSPE-MaL-PEG2000 was incorporated in liposomes (Affisomes, see Table I). Non-targeted-SUV prepared without DSPE-MaL-PEG2000-lipid (control-II, see Table I), only co-eluted a very small concentration of affibody, presumably due to non-specific association of affibody with liposomes. The affibody conjugation in the liposome fractions was further confirmed by gel electrophoresis (Figure 4B). A ~3 kDa increase in molecular weight of affibody (M.W. 8.3 kDa) in Affisomes due to the conjugation with DSPE-MaL-PEG2000 (M.W. 2.9 kDa) was present (Figure 4B, lane 6). Treatment of Affisomes with dithiothreitol (DTT) did not affect the mobility of conjugated affibody (Figure 4B, lane 7). In contrast, free affibody (lane 3, Figure 4B) upon treatment with DTT was reduced to monomer (Figure 4B, Lane 4). Control-III liposomes (without affibody conjugation) did not show any bands corresponding to the affibody (Figure 4, Lane 8). Additional controls (lipid compositions without maleimide functional group (Control-II) or incubation of Affisomes with non-reduced affibody) were included to determine specificity of affibody-conjugation via the maleimide-SH reaction. In either case we did not observe any association of affibody to the liposomes above background levels confirming the specificity of this reaction (data not shown). Analysis of population size distribution of the liposomes before and after conjugation with affibody showed that conjugation did not have any significant effect (Figure 4C).
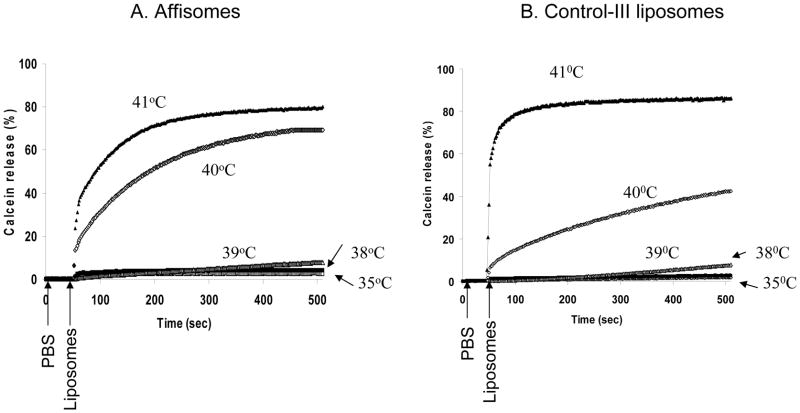
Since we aimed at developing affibody-conjugated liposomes to improve targeting potential without compromising their thermosensitivity, we evaluated the effect of affibody-conjugation on temperature-dependent release of entrapped calcein (see Figure 5). Calcein loaded Affisomes and non-conjugated Thermosensitive liposomes (Control III) were examined for temperature-triggered calcein release from liposomes (due to relief of self-quenching) at λexcitation/emission of 490/515nm in a Fluorimeter (see Methods section). The results presented in Figure 5 show that Control-III liposomes show ~100% release of encapsulated calcein at temperatures at and above 40°C, with only a slight calcein release at 39°C. We made similar observations when Affisomes were tested. Both liposomes released calcein only at or above 40°C, confirming that affibody conjugation did not alter thermosensitivity of liposomes.
Figure 5. Effect of Affibody Conjugation on thermo-sensitivity of Liposomes.
HER2-specific Affibody molecules-conjugated thermo sensitive liposomes (Affisomes) and thermosensitive liposomes (Control III) were Calcein loaded and were placed in stirred cuvette containing 2 ml PBS at 35°C and liposomes thermo-sensitivity was measured. The temperature was increased to 41°C and calcein release from liposomes (due to relief of self-quenching) was monitored in a Horiba Fluoromax-3 cuvette reader (Edison, NJ) at an excitation/emission of 490/515nm,. Complete (100%) leakage was obtained by addition of 20 μl TX100 (10% w/v). Thermo-sensitive liposomes show almost 100% release of encapsulated calcein at temperatures at and above 41°C.
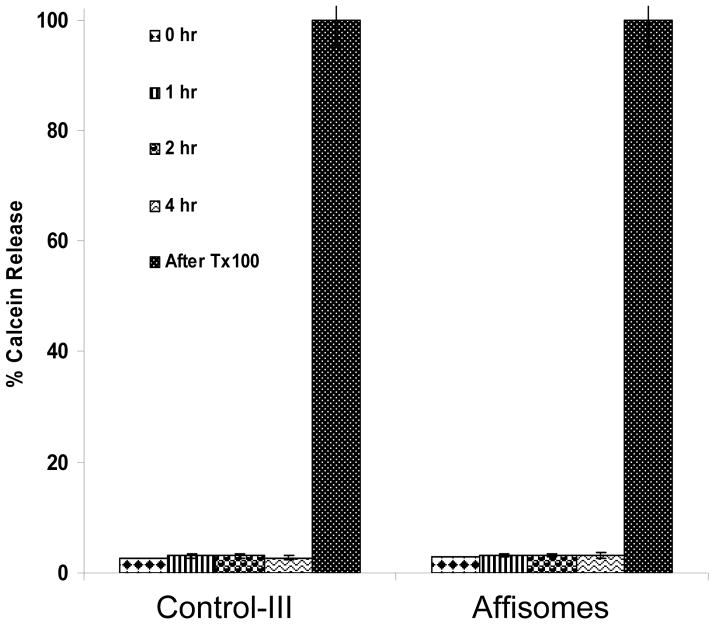
For future medical applications, it is critical that physical properties of Affisomes are not altered upon storage or upon interactions with plasma components prior to targeting to permissive cells and/or tissues. Therefore, we examined stability of Affisomes in the presence of serum by monitoring release of calcein. Affisomes were incubated in PBS supplemented with 10% heat-inactivated serum at 37°C for various time periods, and extent of calcein release was measured. Results are shown in Figure 6. We did not observe any significant release of calcein from Affisomes or non-conjugated thermo-sensitive liposomes (Control-III) in the presence of serum up to 4 hours incubations. Therefore, affibody-conjugation to thermosensitive liposomes had no significant effect on the natural leakage of entrapped calcein. In addition, we also did not observe any change in thermosensitivity of liposomes following incubation with 10% serum (data not shown).
Figure 6. Stability of Affisomes in the presence of serum at 37°C.
HER2-specific Affibody molecules-conjugated thermo sensitive liposomes (Affisomes) and thermosensitive liposomes (Control III) were incubated with 10% FBS and Calcein release was measured at various time periods till 4h. Calcein release from liposomes was monitored in a Horiba Fluoromax-3 cuvette reader (Edison, NJ) at an excitation/emission of 490/515nm. Complete (100%) leakage was obtained by addition of 20 μl TX100 (10% w/v). Thermo-sensitive liposomes show stability till 24h and release of encapsulated calcein at temperatures at and above 41°C occurred after 24 h incubation with serum at 37°C
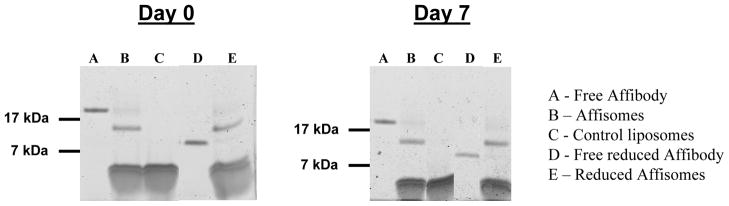
The susceptibility to aggregation of Affisomes and control liposomes was assessed after incubation for 0, 5 and 7 days at 25 °C. The inorganic Pi, average diameter and calcein release was determined following a low speed spin to remove any aggregated material (see Methods section). The results are summarized in Table II. We observed only slight differences (±7.5%) in the amounts of lipids in the supernatants at days 0, 5 or 7 for Affisomes and Control-III liposomes. Similarly, there was no significant difference in the population size distribution (data not shown) and average diameter of Affisomes (86–94 nm) or control-III liposomes (82–86 nm). Next, we assayed the amount of entrapped calcein in these liposomes. It is evident from the data (shown in Table II) that affibody-conjugation did not have an adverse effect on the time-dependent integrity of liposomes (control-III liposomes, 2.8–3.73%, Affisomes, 3.0–4.0%, days 0–18 respectively). Stability of affibody-DSPE-MaL-PEG2000 conjugates, was further analyzed by gel electrophoresis (see Methods section). The results presented in Figure 7 show that there was no difference in the mobility of 11.3 kDa band for Affisomes at day 0 (Lane B, left panel) or day 7 (Lane B, right panel). The free affibody, before (Lane A) and after (treatment with reducing agent (Lane D) and Control-III liposomes (Lane C) are also shown in Figure 7 for comparison.
Table II.
Physical Properties of Affisomes: Effect of incubation at 25°C
| Liposome Incubation At 25°C | Lipid analysis (ml)* | Hydrodynamic Size distribution (average diameter)** | Calcein Release (% of total entrapped)*** | |||
|---|---|---|---|---|---|---|
| Control-III Liposomes | Affisomes | Control –III Liposomes | Affisomes | Control –III Liposomes | Affisomes | |
| Day 0 | 1978 nmol (1.91mg) | 2219 nmol (2.15 mg) | 81.26 | 86.53 | 35.69 | 33.97 |
| Day 5 | 1832 nmol (1.777) | 2542 nmol (2.46 mg) | 86.46 | 94.22 | 32.00 | 30.33 |
| Day 7 | 1714 nmol (1.663) | 1952 nmol (1.89 mg) | 83.42 | 90.07 | 26.80# | 24.80# |
Lipid analysis was done by measuring inorganic Pi (see Methods section)
Affisomes or control liposomes were diluted 1:10 and measured as described in Methods section. Size distribution is presented as Z-Average (nm).
Liposomes were diluted 1:10 and the calcein florescence was measured before and after adding Tx100 as described in Methods section. The results are reproducible from at least three independent batches.
This measurement was done at day 18.
Figure 7. Stability of Protein Conjugation of Affisomes.
Gel electrophoresis analysis of Affisomes was done on 0 and 7 days after liposome production. M (Markers) =Mark12 Unstained Standard (Invitrogen, USA).
Conclusion
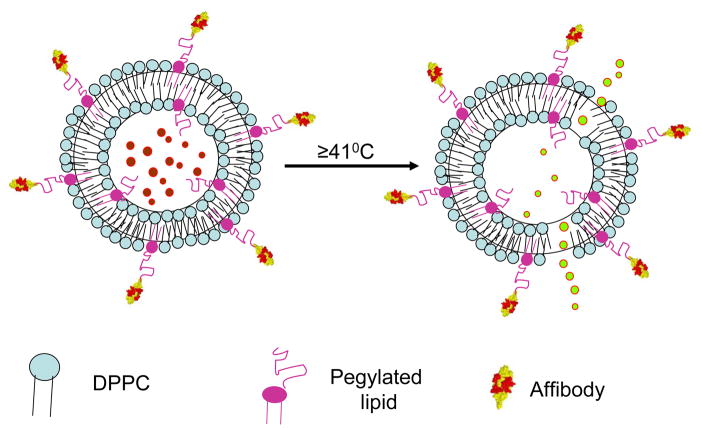
Successful application of liposomes for delivery of therapeutics is subject to availability of suitable ligands for site-specific targeting, associated with in vivo drug release mechanisms on site. To date, single chain antibodies have been proven to be promising biomolecules to carry the cargo of liposomes to desired site (27). Among in situ-triggerable liposomes available, thermosensitive liposomes bear the potential to be successful due to the availability of modalities for localized hyperthermia (12;26). To improve breast cancer treatment, HER2-specific affibody (ZHER2:342-Cys) a novel small and highly stable biomolecule, was covalently linked to thermosensitive liposomes (HER2-specific Affisomes). Affisomes are generated by a covalent coupling reaction between reduced ZHER2:342-Cys and the functional malemide group of pegylated lipids on pre-formed liposomes. Affisomes retain their thermosensitivity and maintain their integrity for an extended period of time. Design principle and composition of Affisomes is shown in a cartoon form (Figure 8). Temperature-triggered local defects in the Affi-some membrane result in controlled release of encapsulated drugs/markers. Further experiments are needed to demonstrate in vitro and in vivo drug delivery potential of these particles. The newly designed liposomes described in this report may serve as viable carriers for on-demand delivery of chemotherapeutic agents to treat breast cancer.
Figure 8.
A cartoon depicting configuration and Thermo-sensitivity of Affisomes.
Acknowledgments
“This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.” This research was supported by the Center for Cancer Research, an Intramural Research Program of the National Cancer Institute, and by Breast Cancer Research Stamp proceeds awarded through competitive peer review. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Abbreviations
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (16:0 PC)
- Egg PC
L-α-phosphatidyl choline
- DSPE-PEG2000
1,2-disteaoryl-sn-glycero-3-phosphatidylethanolamine-N-[Methoxy(Polyethylene glycol)-2000](Ammonium Salt) (18:0 (PEG2000) PE)
- DSPE-MaL- PEG2000
1,2-disteaoryl-sn-glycero-3-phosphatidylethanolamine-N-[Maleimide-(Polyethylene glycol)-2000](Ammonium Salt) (DSPE-PEG(2000) Maleimide)
- DiR
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide
- HBSE
10mM HEPES, 150mM NaCl, 9.1mM EDTA, pH 7.5
- PBS
phosphate buffered saline
Reference List
- 1.Allen TM. Trends Pharmacol Sci. 1994;15:215–220. doi: 10.1016/0165-6147(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 2.Park JW, Benz CC, Martin FJ. Semin Oncol. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 4.Allen TM. Drugs. 1997;54(Suppl 4):8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cattel L, Ceruti M, Dosio F. Tumori. 2003;89:237–249. doi: 10.1177/030089160308900302. [DOI] [PubMed] [Google Scholar]
- 6.Gabizon A, Martin F. Drugs. 1997;54(Suppl 4):15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hussein M. Clin Lymphoma. 2003;4(Suppl 1):S18–S22. doi: 10.3816/clm.2003.s.004. [DOI] [PubMed] [Google Scholar]
- 8.Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. Expert Opin Ther Targets. 2004;8:335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 9.Sapra P, Tyagi P, Allen TM. Curr Drug Deliv. 2005;2:369–381. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 10.Torchilin VP. Crit Rev Ther Drug Carrier Syst. 1985;2:65–115. [PubMed] [Google Scholar]
- 11.Needham D, Dewhirst MW. Adv Drug Deliv Rev. 2001;53:285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 12.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Int J Hyperthermia. 2006;22:205–213. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 13.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 14.Park JW. Breast Cancer Res. 2002;4:95–99. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 16.Nellis DF, Giardina SL, Janini GM, Shenoy SR, Marks JD, Tsai R, Drummond DC, Hong K, Park JW, Ouellette TF, Perkins SC, Kirpotin DB. Biotechnol Prog. 2005;21:221–232. doi: 10.1021/bp049839z. [DOI] [PubMed] [Google Scholar]
- 17.Nellis DF, Ekstrom DL, Kirpotin DB, Zhu J, Andersson R, Broadt TL, Ouellette TF, Perkins SC, Roach JM, Drummond DC, Hong K, Marks JD, Park JW, Giardina SL. Biotechnol Prog. 2005;21:205–220. doi: 10.1021/bp049840y. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein JN, Magin RL, Zaharko DS, Yatvin MB, Blumenthal R. Biophysical Journal. 1979;25:A291. [Google Scholar]
- 19.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 20.Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandstrom M, Nilsson FY, Wennborg A, Abrahmsen L, Feldwisch J. Cancer Res. 2007;67:2178–2186. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- 21.Gupta CM, Bali A. Biochim Biophys Acta. 1981;663:506–515. doi: 10.1016/0005-2760(81)90178-8. [DOI] [PubMed] [Google Scholar]
- 22.Goswami SK, Frey CF. J Lipid Res. 1971;12:509–510. [PubMed] [Google Scholar]
- 23.AMES BN, DUBIN DT. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 24.Zhu JM, Yan F, Guo ZW, Marchant RE. Journal of Colloid and Interface Science. 2005;289:542–550. doi: 10.1016/j.jcis.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 25.Mills JK, Needham D. Biochim Biophys Acta. 2005;1716:77–96. doi: 10.1016/j.bbamem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 27.Torchilin VP. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]