Abstract
The neuregulin 1 (NRG1) receptor ErbB4 is involved in the development of cortical inhibitory GABAergic circuits and NRG1-ErbB4 signaling has been implicated in schizophrenia (SCZ). A magnetic resonance spectroscopy (1H-MRS) study has demonstrated that a single-nucleotide polymorphism in ERBB4, rs7598440, influences human cortical GABA concentrations. Other work has highlighted the significant impact of this genetic variant on expression of ERBB4 in the hippocampus and dorsolateral prefrontal cortex in human post mortem tissue. Our aim was to examine the association of rs7598440 with cerebrospinal fluid (CSF) GABA levels in healthy volunteers (n=155). We detected a significant dose-dependent association of the rs7598440 genotype with CSF GABA levels (G-allele standardized β=−0.23; 95% CIs: −0.39 to −0.07; P=0.0066). GABA concentrations were highest in A homozygous, intermediate in heterozygous, and lowest in G homozygous subjects. When excluding subjects on psychotropic medication (three subjects using antidepressants), the results did not change (G-allele standardized β=−0.23; 95% CIs: −0.40 to −0.07; P=0.0051). The explained variance in CSF GABA by rs7598440 in our model is 5.2% (P=0.004). The directionality of our findings agrees with the aforementioned 1H-MRS and gene expression studies. Our observation therefore strengthens the evidence that the A-allele of rs7598440 in ERBB4 is associated with increased GABA concentrations in the human central nervous system (CNS). To our knowledge, our finding constitutes the first confirmation that CSF can be used to study genotype–phenotype correlations of GABA levels in the CNS. Such quantitative genetic analyses may be extrapolated to other CSF constituents relevant to SCZ in future studies.
Keywords: GABA, ErbB4, neuregulin, cerebrospinal fluid, rs7598440, schizophrenia
INTRODUCTION
Single-nucleotide polymorphism (SNP) and haplotype analyses have implicated neuregulin 1 (NRG1) in schizophrenia (SCZ) (Jaaro-Peled et al, 2009; Li et al, 2006). A genome-wide copy number analysis found evidence of structural ERBB4 (OMIM 600543) abnormalities in SCZ (Walsh et al, 2008). In addition, enhanced signaling of NRG1-ErbB4 in SCZ patients has been reported (Hahn et al, 2006). Converging evidence thus pleads for a role of the NRG1-ErbB4 pathway in SCZ, although the underlying mechanisms are currently unknown.
While the physiological ramifications of this pathway in the healthy human brain have yet to be fully understood, preclinical studies indicate that NRG1 is involved in neurodevelopment and brain plasticity, acting via ERBB receptor tyrosine kinases, such as ErbB4 (Mei and Xiong, 2008). ERBB4 expression in the brain is largely restricted to parvalbumin-expressing GABAergic cortical interneurons (basket and chandelier cells) in humans, rodents and non-human primates (Fazzari et al, 2010; Neddens et al, 2011). NRG1-induced GABA release depends on ErbB4 (Woo et al, 2007), resulting in suppression of firing of pyramidal neurons (Wen et al, 2010) and affecting interneuronal long-term potentiation and contextual fear conditioning (Chen et al, 2011).
Recently, a study highlighted the effect of a common variant in ERBB4 on cortical GABA concentrations in healthy volunteers using proton magnetic resonance spectroscopy (1H-MRS) (Marenco et al, 2011). The authors reported that A allele carriers of SNP rs7598440 within the ERBB4 gene possessed higher anterior cingulate cortical GABA concentrations than G-allele homozygous subjects. This suggests that genetic variation in ERBB4 signaling affects in vivo cortical GABA levels. As in vivo GABA concentrations in the prefrontal cortex measured by 1H-MRS reflect a fraction of whole-brain GABAergic neurons (Barnard et al, 1998), it remains unclear how this finding applies to other brain regions. GABA concentrations in the cerebrospinal fluid (CSF) have been proposed to reflect overall central GABA activity (Grove et al, 1983), which is supported by a rodent study demonstrating a high correlation (r=0.92) between CSF and brain GABA (Bohlen et al, 1979) and the rostrocaudal CSF GABA gradient hinting at a central nervous system (CNS) origin of CSF GABA (Grove et al, 1982).
In another study, rs7598440 was found to be associated with gene expression levels of ERBB4 in the dorsolateral prefrontal cortex (DLPFC) and hippocampus (Law et al, 2007), suggesting a cis-effect of this variant (or one that is tagged by it) on CNS ERBB4 expression. We therefore hypothesized that the effect of the ERBB4 rs7598440 genotype on GABA concentrations would not be limited to the anterior cingulate cortex but that the SNP mediates overall central GABA turnover. Additionally, our aim was to assess the validity of CSF to study genotype–phenotype correlations of central GABA activity. To our knowledge, no genetic linkage or association study on CSF GABA has been published to date. We thus investigated the association of rs7598440 with CSF GABA levels in healthy volunteers.
MATERIALS AND METHODS
Subjects
The ethics committee at the University Medical Center Utrecht (UMCU) and all local ethics committees approved this study. Volunteers were recruited at outpatient pre-operative screening services in four hospitals in and around Utrecht, The Netherlands, from August 2008 until March 2010: UMCU, the Central Military Hospital, Sint Antonius Hospital, and Diakonessenhuis. We included patients (i) undergoing spinal anesthesia for minor elective surgical procedures, (ii) ranging between 18–60 years of age, and (iii) with four grandparents born in The Netherlands or other northwestern European countries (Belgium, Germany, UK, France, and Denmark). Written informed consent was obtained from the participants. Each candidate participant received a personal telephone interview by JL (a psychiatry resident) or a medical student trained by JL. During this non-standardized interview, subjects with psychotic or neurological disorders were excluded and any use of psychotropic medication was assessed. A history of unipolar affective and anxiety disorders was allowed. To gauge the possible association of anxiety with CSF GABA levels, a Pearson's correlation between the State and Trait Anxiety Inventory and GABA was computed (α=0.05) (Spielberger, 1989).
CSF Collection
Subjects had fasted at least 6 h before lumbar puncture (LP). Before administration of medication (either pre-medication or compounds for the purpose of anesthesia), a 25–27 G needle was inserted into the L1/L2, L2/L3, L3/L4, or L4/L5 interspace (estimated by the anaesthesiologist). A single sample of 6 ml of CSF was obtained from each subject. Any deviations from the instructed procedure were recorded, such as smaller amounts of CSF drawn. CSF was kept at 4 °C and transported within 9 h to the laboratory at UMCU. Each sample was immediately stored in fractions of 0.5 and 1 ml at −80 °C. One fraction of 0.5 ml was used for GABA measurements.
CSF GABA Measurements
The free GABA quantification method employed here is similar to an ultra-performance liquid chromatography – mass spectrometry (UPLC-MS/MS, multiple stage tandem mass spectrometry) based method for quantification of D-amino acids described elsewhere (Visser et al, 2011). Out of a 0.5-ml aliquot, 50 μl was mixed with 25 μl of an internal standard solution containing 80 μM of [13C3]-L-serine (obtained from Cambridge Isotope Laboratories, Andover, MA). Subsequently, the sample was deproteinized by the addition of acetonitrile and derivatized with the chiral reagent (S)-NIFE (purchased from Sigma-Aldrich, Zwijndrecht, The Netherlands). Analyses were carried out with a Waters Acquity UPLC system equipped with an Acquity 1.7 μm BEH-C18 2.1 × 102 mm column and a VanGuard BEH-C18 2.1 × 5 mm pre-column. The UPLC system was coupled to a Waters Xevo MS operated in positive electron spray mode. The following mass reaction monitoring settings were employed for GABA: parent ion=353.35 Da; daughter ion=120.1 Da; cone voltage=20 V, collision energy=24 V; and for [13C3]-L-serine: parent ion=358.3; daughter ion=120.1; cone voltage=18 V; collision energy=26 V. The retention time of GABA was 10.27 min and that of [13C3]-L-serine 9.14 min. A calibration curve covering the concentration range of interest was included in each measurement session. The peaks were integrated using the computer software TargetLynx 4.1 (Waters, Milford, MA, USA).
Genotyping Procedures and Quality Control (QC)
As part of another, larger study whole-genome SNP data were generated at the UCLA Neuroscience Genomic Core facility using the Illumina Human OmniExpress Beadchip and genotype data of rs7598440 were extracted for use in this study. All genetic QC checks were performed using Plink v1.07 in 240 genotyped individuals, 155 of whom had available CSF GABA levels (see Supplementary Methods). Given the prior evidence on rs7598440 and our limited study population size resulting in insufficient power to perform whole-genome analyses, we studied the association of this single SNP with CSF GABA.
Quantitative Trait Locus (QTL) Analyses
Normality of the CSF GABA distribution was checked using SPSS version 17 (SPSS, Chicago, IL) and defined by a Kolmogorov–Smirnov (K–S) test asymptotic two-tailed P-value >0.05. A linear model accounting for all six patient- and procedure-specific factors known to influence CSF GABA levels was performed in Plink v1.07. These patient covariates include age and sex (Epperson et al, 2005; Marenco et al, 2011), whereas procedure-specific covariates include time elapsed before storage (number of hours from LP until storage at −80 °C), storage duration, the rostrocaudal concentration gradient (reflected by subjects' height), and amount of CSF drawn (Hare, 1981; Manyam and Hare, 1983). A maximum of 5% missing data per covariate was allowed and subjects with more than two missing covariates were excluded. Missing covariate data were replaced by the mean (in the event of height, means were computed separately for the two sexes). Two tests of robustness were carried out. First, subjects on psychotropic medication were left out and the same linear model was run. Second, only covariates that showed suggestive (P<0.1) Spearman's ρ correlations with logGABA were included in the model. Given the prior evidence for this locus, the significance threshold of the association analysis of rs7598440 with CSF GABA levels was set at P<0.05. Outcome measures (standardized regression coefficients, β), the minor allele frequency (MAF), the genotyping success rate, and Hardy–Weinberg equilibrium (HWE) of rs7598440 were computed in Plink. Boxplots of genotype–phenotype associations were generated in SigmaPlot 11. The explained variance by rs7498440 was computed by subtracting the R2 in a linear regression model including covariates that showed suggestive Spearman's ρ correlations (P<0.1) with logGABA from the R2 of a linear model additionally including the rs7598440 genotype (logGABA being the dependent variable; α=0.05).
RESULTS
Subject Characteristics
QC based on genetic data resulted in the exclusion of four subjects with available CSF GABA levels, leaving 151 subjects with measured CSF GABA concentrations for further study (Supplementary Methods). No correlation between CSF logGABA and anxiety state and trait measures (which were normally distributed and filled out by 87% of the subjects) was detected (P=0.9). Although data were complete for three covariates, missing data ranged from 1–5% per covariate for the other three. For one individual multiple covariates were missing, leaving a total of 150 subjects for the linear model. Three of the 151 subjects were on psychotropic medication (SSRIs and an SNRI). An overview of the study population, GABA measurements, covariates, and genotype distributions is shown in Table 1.
Table 1. Subject Characteristics, Means (SD) of Subject Characteristics, and GABA Levels.
| Parameter | Total sample | AA genotype | AG genotype | GG genotype |
|---|---|---|---|---|
| Subjects (N) | 151 | 50 | 79 | 22 |
| Age, mean | 40 | 39 | 42 | 40 |
| Sex (% male) | 70 | 74 | 67 | 73 |
| CSF GABA in μmol/l (SD) | 0.47 (0.28) | 0.54 (0.31) | 0.44 (0.21) | 0.40 (0.36) |
| log CSF GABA in μmol/l (SD) | −0.40 (0.24) | −0.33 (0.23) | −0.40 (0.21) | −0.51 (0.29) |
| Time elapsed before storage in hours (SD) | 5.68 (2.16) | 6.34 (2.19) | 5.48 (2.04) | 4.89 (2.23) |
| Storage time in months (SD) | 9.01 (3.08) | 9.63 (3.46) | 8.61 (2.92) | 9.69 (2.47) |
| Subject height in cm (SD) | 180 (9.5) | 180 (8.3) | 181 (10.0) | 180 (10.7) |
| Amount CSF drawn in ml (SD) | 5.62 (0.60) | 5.57 (0.65) | 5.61 (0.57) | 5.75 (0.61) |
Abbreviations: CSF, cerebrospinal fluid; SD, standard deviation.
Only subjects passing genetic quality control are shown.
CSF GABA
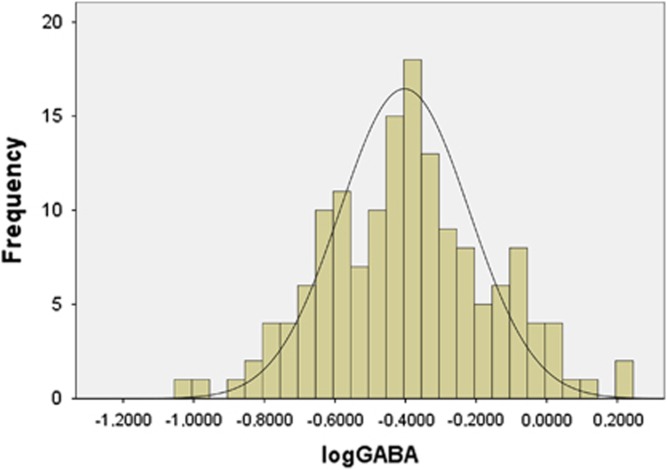
As CSF GABA was not normally distributed (K–S P=0.001), values were logarithm (log) transformed. This resulted in a normal distribution (K–S P=0.84, Figure 1). Mean (SD) CSF GABA and logGABA levels of these subjects were 0.47 (0.28) and −0.40 (0.24) μmol/l, respectively (Table 1).
Figure 1.
Histogram showing the distribution of log transformed CSF GABA levels (μmol/l).
Association of rs7598440 with CSF GABA Levels
Genotyping was successful in all subjects and no departure from HWE was detected (P=0.11); the MAF was 0.42, which is equal to the previously reported frequency based on the 1000 genomes project (CEU population; http://www.ncbi.nlm.nih.gov/snp).
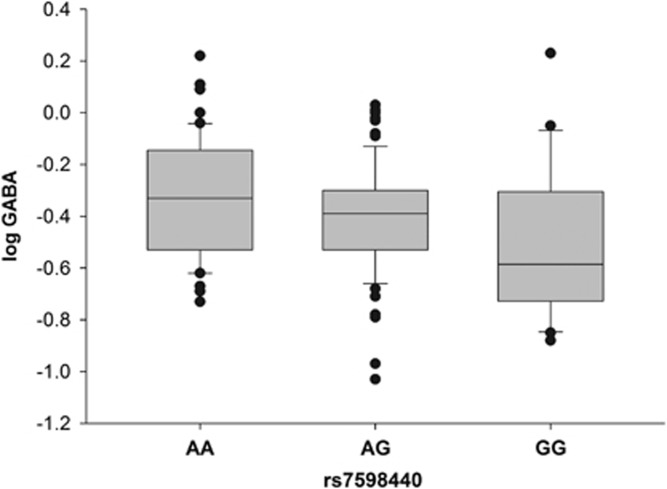
A significant dose-dependent association of the rs7598440 genotype with CSF logGABA levels was detected (β=−0.23; P=0.0066; Figure 2 and Table 2), ie, logGABA (and therefore GABA) concentrations were highest in A homozygous, intermediate in heterozygous, and lowest in G homozygous subjects. The explained variance in logGABA by the rs7598440 genotype in our data set is 5.2% (P=0.004). The two tests of robustness resulted in similar findings. When excluding the three subjects on psychotropic medication, the results did not change (β=−0.23; P=0.0051, Table 2). The only covariate showing suggestive Spearman's ρ correlations with logGABA was time elapsed before storage (Spreaman's ρ=−0.21, P=0.011). The results for the linear model correcting for only this covariate were: β=−0.25; P=0.0031 (Table 2).
Figure 2.
Log transformed GABA levels (μmol/l) by rs7598440 genotype: interquartile ranges (boxes) with medians (lines in boxes), whiskers (10–90 percentiles), and dots (values falling outside the 10–90 percentiles).
Table 2. Summary Statistics of the Linear Models: on All Subjects for whom GABA, Genotypes, and Covariates Were Measured (n=150); on Those Who Were Not on Psychotropic Medication (n=147); and for the Analysis Including Only Covariates that Correlated with GABA (n=150).
| N | β | SE | 95% CI | t-statistic | P-value | |
|---|---|---|---|---|---|---|
| All subjects | 150 | −0.225 | 0.082 | −0.39 to −0.07 | −2.76 | 0.0066 |
| No psychotropic medication | 147 | −0.234 | 0.082 | −0.40 to −0.07 | −2.85 | 0.0051 |
| Only relevant covariates | 150 | −0.245 | 0.081 | −0.40 to −0.09 | −3.01 | 0.0031 |
Abbreviations: β, standardized regression coefficient; SE, standard error; 95% CI, 95% confidence interval.
DISCUSSION
In the first endeavor to identify a QTL associated with CSF GABA levels, a dose-dependent association of the common variant rs7598440 in ERBB4 was detected. These results confirm a 1H-MRS finding that the A-allele of rs7598440 increases GABA concentrations in the human CNS (Marenco et al, 2011) and strengthen the evidence that this variant is implicated in in vivo GABA metabolism in the CNS.
The role of ERBB4 in controlling cortical GABA circuitry development was previously demonstrated (Fazzari et al, 2010). At a general genetic level, it is known that synonymous (Sauna and Kimchi-Sarfaty, 2011) and intronic (Hull et al, 2007) SNPs may contribute to phenotypic variation by means of genotype-specific differences in gene expression levels, RNA stability, RNA splicing, as well as in protein translation rate and protein folding. The demonstrated cis-effect of intronic rs7598440 on human post-mortem ERBB4 expression and splicing in the hippocampus (P=0.009) and DLPFC (P=0.03) may be viewed in light of such phenomena (Law et al, 2007). Our results are in keeping with this ERBB4 expression finding in that the A-allele that increases ERBB4 expression in these tissues (Law et al, 2007) was associated with elevated CSF GABA levels in the current study.
The exact genetic mechanisms underlying rs7598440-induced effects on CSF GABA levels are currently unknown. For example, there is no evidence suggesting marked sequence conservation between species at this intronic region of ERBB4 or the presence of a regulatory element in the sequence immediately surrounding rs7598440 (http://genome.ucsc.edu/). In addition, the link between the NRG1-ErbB4 pathway and the pathophysiology of SCZ has yet to be elucidated, although a detected relation between NRG1, ErbB4, glutamate, and dopamine implicates the pathway in neurotransmitter systems relevant to SCZ (Kwon et al, 2008). The agreement in directionality between the 1H-MRS signal (Marenco et al, 2011) and the signal in our study indicates a high validity of both approaches in genetic quantitative analyses of CNS GABA. In this context, our thorough assessment of and correction for all covariates that are known to influence CSF GABA levels are likely to have benefited the reliability of our analyses. The 1H-MRS study corrected for the same patient-related covariates (age and sex) but clearly different procedure-related covariates were accounted for (Marenco et al, 2011).
A limitation of the current study is that no standardized psychiatric interview was employed to screen for psychiatric illness in the study population. Future studies may overcome this caveat by relating psychiatric diagnoses (especially anxiety disorders, alcohol dependence, and abuse) to GABA levels and SNP data. On a similar note, the current design does not allow to parse possible age, sex or other genotype specific modulations of preoperative stress influences on CSF GABA levels. Quantifying perioperative stress in upcoming projects may be a way to address such uncertainties.
To our knowledge, our finding constitutes the first confirmation that CSF can be used to study genotype–phenotype correlations of CNS GABA levels. Such quantitative genetic analyses may be extrapolated to other CSF constituents relevant to SCZ in future studies, eg, amino acids involved in glutamatergic pathways. An interesting question remains whether intra-individual changes in CSF GABA concentrations reflect central GABA activity. This may only be addressed in prospective studies with longitudinal measures of CSF GABA concentrations within the same individuals so that possible changes in these levels can be correlated with genotypes and expression of ERBB4.
Acknowledgments
We thank professor Dr JTA Knape and Dr P Vaessen for their valuable assistance in developing our CSF sampling protocol in the operating rooms.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Bohlen P, Huot S, Palfreyman MG. The relationship between GABA concentrations in brain and cerebrospinal fluid. Brain Res. 1979;167:297–305. doi: 10.1016/0006-8993(79)90824-2. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci USA. 2011;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, O'Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Grove J, Palfreyman MG, Schechter PJ. Cerebrospinal fluid GABA as an index of brain GABA activity. Clin Neuropharmacol. 1983;6:223–229. doi: 10.1097/00002826-198309000-00004. [DOI] [PubMed] [Google Scholar]
- Grove J, Schechter PJ, Hanke NF, de Smet Y, Agid Y, Tell G, et al. Concentration gradients of free and total gamma-aminobutyric acid and homocarnosine in human CSF: comparison of suboccipital and lumbar sampling. J Neurochem. 1982;39:1618–1622. doi: 10.1111/j.1471-4159.1982.tb07995.x. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hare TA. Alterations of central GABAergic activity in neurologic and psychiatric disorders: evaluation through measurements of GABA and GAD activity in cerebrospinal fluid. Mol Cell Biochem. 1981;39:297–304. doi: 10.1007/BF00232581. [DOI] [PubMed] [Google Scholar]
- Hull J, Campino S, Rowlands K, Chan MS, Copley RR, Taylor MS, et al. Identification of common genetic variation that modulates alternative splicing. PLoS Genet. 2007;3:e99. doi: 10.1371/journal.pgen.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci USA. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Manyam BV, Hare TA. Cerebrospinal fluid GABA measurements: basic and clinical considerations. Clin Neuropharmacol. 1983;6:25–36. doi: 10.1097/00002826-198303000-00003. [DOI] [PubMed] [Google Scholar]
- Marenco S, Geramita M, van der Veen JW, Barnett AS, Kolachana B, Shen J, et al. Genetic association of ErbB4 and human cortical GABA levels in vivo. J Neurosci. 2011;31:11628–11632. doi: 10.1523/JNEUROSCI.1529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, et al. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol Psychiatry. 2011;70:636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- Spielberger C. State-Trait Anxiety Inventory:v A Comprehensive Bibliography. Consulting Psychologists Press: Palo Alto; 1989. [Google Scholar]
- Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R, et al. A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of D-amino acids in body fluids. J Chromatogr A. 2011;1218:7130–7136. doi: 10.1016/j.chroma.2011.07.087. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.