Abstract
Although established smokers have a very regular pattern of smoking behavior, converging lines of evidence suggest that the escalation of smoking behavior is a critical factor in the development of dependence. However, the neurobiological mechanisms that underlie the escalation of smoking are unknown, because there is no animal model of the escalation of nicotine intake. On the basis of the pattern of smoking behavior in humans and presence of monoamine oxidase inhibitors in tobacco smoke, we hypothesized that the escalation of nicotine intake may only occur when animals are given extended-access (21 h per day) self-administration sessions after repeated periods of abstinence (24–48 h), and after chronic inhibition of monoamine oxidase using phenelzine sulfate. Intermittent access (every 24–48 h) to extended nicotine self-administration produced a robust escalation of nicotine intake, associated with increased responding under fixed- and progressive-ratio schedules of reinforcement, and increased somatic signs of withdrawal. The escalation of nicotine intake was not observed in rats with intermittent access to limited (1 h per day) nicotine self-administration or daily access to extended (21 h per day) nicotine self-administration. Moreover, inhibition of monoamine oxidase with daily administration of phenelzine increased nicotine intake by ∼50%. These results demonstrate that the escalation of nicotine intake only occurs in animals given intermittent periods of abstinence with extended access to nicotine, and that inhibition of monoamine oxidase may contribute to the escalation of smoking, thus validating both an animal model of the escalation of smoking behavior and the contribution of monoamine oxidase inhibition to compulsive nicotine-seeking.
Keywords: monoamine oxidase, withdrawal, tobacco, addiction, dependence, phenelzine
INTRODUCTION
Tobacco smoking results in an array of illnesses that collectively represent the largest preventable cause of death in the United States (Peto et al, 1996; Torrijos and Glantz, 2006). The primary psychoactive ingredient responsible for the use of tobacco products is nicotine (Cummings and Mahoney, 2006; US Department of Health and Human Services, 1988), which is known to generate modest positive reinforcing effects (eg, Grunberg, 1994; Pomerleau and Pomerleau, 1992).
Established smokers have a very regular pattern of smoking behavior, but converging lines of evidence suggest that the escalation of smoking is a critical factor in the development of dependence and relapse to nicotine dependence. For example, prospective studies found that ∼30–50% of adolescents and young adults who had initiated non-daily smoking showed an escalation of smoking behavior after 4 years (Doubeni et al, 2010; Kim et al, 2009), and that ∼50% of former smokers who experienced a lapse (eg, smoked one cigarette) progressively escalated their smoking behavior to reach pre-abstinence smoking levels in a few days or weeks (Conklin et al, 2005). Although the neurobiological mechanisms that underlie the maintenance of tobacco/nicotine self-administration in subjects with a history of dependence has been extensively studied, the mechanisms that underlie the escalation of nicotine intake are currently unknown, thus hindering the development of effective pharmacological therapies. One explanation for this gap in the literature is the lack of relevant animal models of the escalation of nicotine intake.
The escalation of drug intake, associated with an increased motivation for drug seeking, has been demonstrated in animals allowed to self-administer heroin, methamphetamine, and cocaine in prolonged daily sessions (eg, Ahmed and Koob, 1998; Ahmed et al, 2000; Greenwell et al, 2009; Kitamura et al, 2006), providing robust animal models of the transition to drug dependence. However, the escalation of nicotine intake was not observed when rats were given extended access (23 h per day) to nicotine (Kenny and Markou, 2006; O'Dell et al, 2007; Paterson and Markou, 2004; Valentine et al, 1997).
Compared with heroin, cocaine, and methamphetamine, the acute reinforcing properties of nicotine are modest, reflected by drug users' self-reports (Kozlowski et al, 1989) and the relatively low rate of responding for nicotine under both fixed-ratio (FR) and progressive-ratio (PR) schedules of reinforcement (Dougherty et al, 1981; Henningfield and Goldberg, 1983; Risner and Goldberg, 1983; Stolerman and Jarvis, 1995). Repeated and prolonged exposure to nicotine self-administration induces powerful somatic and emotional withdrawal symptoms (O'Dell et al, 2007; Paterson and Markou, 2004), and these effects may enhance the incentive value of nicotine as a negative reinforcer and help drive excessive nicotine intake. Indeed, we have shown that rats that self-administer nicotine 23 h per day increase their nicotine intake after 2–3 days of forced abstinence. However, nicotine intake returned to near-baseline levels within 4 days of nicotine self-administration (O'Dell and Koob, 2007; George et al, 2007), demonstrating that extended access to nicotine by itself, in contrast to other drugs of abuse, is not sufficient to produce a robust escalation of nicotine intake.
Another limitation of previous studies in animals that failed to demonstrate an escalation of nicotine intake is the lack of specific compounds contained in tobacco smoke that are known to increase the reinforcing efficacy of nicotine (Berlin and Anthenelli, 2001; Fowler et al, 2003). Specifically, tobacco smoke contains monoamine oxidase inhibitors (MAOIs), and MAOIs, such as phenelzine sulfate, enhanced nicotine intake on FR and PR schedules of reinforcement (Guillem et al, 2005, 2006), suggesting that chronic MAO inhibition may contribute to the escalation of nicotine intake.
Therefore, we hypothesized that the escalation of tobacco intake compared with other more acutely reinforcing drugs of abuse may be particularly dependent on ability of tobacco to counter withdrawal symptoms during acute abstinence and MAO inhibition. To test this hypothesis, we measured nicotine self-administration and the somatic signs of nicotine withdrawal in rats given either short access (ShA; 1 h) or long access (LgA; 21 h) to nicotine self-administration either daily or under an intermittent schedule of access that included 24–48 h abstinence periods between sessions. To test whether inhibiting MAO would facilitate the escalation of nicotine intake, phenelzine was administered daily to a subset of rats. The results showed that intermittent access to extended (21 h per day) nicotine self-administration produced a robust escalation of nicotine intake and increased somatic signs of withdrawal. Chronic inhibition of MAO with daily administration of phenelzine enhanced nicotine intake in LgA rats, independent of the effects of deprivation.
MATERIALS AND METHODS
Animals
Forty-four male Wistar rats (250–275 g; Charles River, Hollister, CA) were used for this study. The animals were group-housed and maintained on a 12/12 h light/dark cycle with ad libitum access to food and water. All of the animal procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee, and were in accordance with the National Institutes of Health guidelines.
Drugs
Nicotine hydrogen tartrate salt (Sigma, Natick, MA) was dissolved in saline, pH 7.4, and self-administered via indwelling jugular catheters. Phenelzine sulfate (Sigma, St Louis, MO) was dissolved in nanopure water and administered intraperitoneally (i.p.; 1.0 ml/kg) at a dose of 2 mg/kg, a dose previously shown to enhance nicotine self-administration without producing psychostimulant effects of its own (Guillem et al, 2005). Phenelzine was administered 1 h before nicotine self-administration, beginning on the first day of the experiment.
Operant Chambers
The rats were tested in operant self-administration chambers described previously (O'Dell and Koob, 2007). Specifically, the chambers (Med Associates, St Albans, VT) were kept on a regular light/dark cycle (lights on 2200–1000 h) inside sound-attenuated chambers with continuous white noise. The exit port of the catheter fittings was connected to polyethylene tubing contained inside a protective metal spring that was suspended in the chamber from a swivel attached to a balance arm. Nicotine was delivered via a syringe pump (Razel Scientific Research Instruments, St Albans, VT). Operant sessions were conducted using two retractable levers (ie, active and inactive levers) that extended approximately 1 inch into the chamber. Each response on the active lever resulted in the delivery of nicotine in a volume of 0.1 ml over 1 s. A 28 V white cue light was illuminated above the active lever at the onset of the nicotine infusion and ended following a 20-s timeout period, during which responses were recorded but did not result in drug delivery. The chambers were fitted with a pellet dispenser with a swing door mounted between the two levers on the front wall of the chamber, allowing the subjects to obtain 45 mg chow pellets (precision, Formula A/I, Research Diets, Lancaster, NH) upon nosepoke responses on an FR1 schedule of reinforcement. Water (0.1 ml) was delivered into a metal dipper cup upon a nosepoke response (FR1) to a separate hole located on the back of the chamber.
Nicotine Self-Administration
Detailed procedures for intravenous catheterization and nicotine self-administration have been described previously (George et al, 2007). The rats were first trained to nosepoke for food and water in 21-h sessions before and after recovery from the surgical implantation of jugular catheters, but were not food-trained to respond to the lever that was associated with nicotine delivery. Following the acquisition of these operant responses, the active and inactive levers were extended, and the rats were allowed to self-administer nicotine (0.03 mg/kg per 100 μl/1 s, free base, FR1, timeout 20 s) by pressing the active lever, with all sessions starting immediately following the beginning of the dark phase of the light/dark cycle.
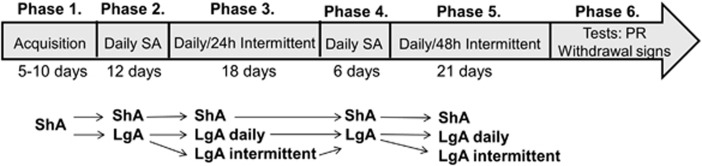
The experimental design is presented in Figure 1. One hour before each self-administration session, the rats received either phenelzine (2 mg/kg, i.p) or vehicle. The study included the following stages:
Figure 1.
Before 1 h of each self-administration (SA) session, the rats received either phenelzine (2 mg/kg, intraperitoneally (i.p.)) or vehicle. The rats were first given access to nicotine for 1 h per day (acquisition) and then separated into two groups given either short access (ShA, 1 h per day) or long access (LgA, 21 h per day) to nicotine for 12 consecutive days. The ShA and LgA rats then self-administered nicotine for an additional 18 days either daily or with a 24-h abstinence period between sessions (ie, intermittent schedule). Subsequently, vehicle-treated rats only were allowed an additional 18 days of nicotine self-administration, with the intermittent groups given a 48-h abstinence period between sessions instead of 24 h. After 48 h of the 18th day, the rats self-administered nicotine on a progressive-ratio (PR) schedule. LgA rats then continued to self-administer nicotine on an fixed-ratio (FR)1 schedule for 21 h. Finally, 48 h later, somatic signs of both spontaneous and mecamylamine-precipitated withdrawal were measured in all of the rats.
Acquisition: the rats were given access to nicotine self-administration for 1 h per day for 5–10 days until responding stabilized.
Daily nicotine self-administration sessions: the rats were divided into an ShA group that self-administered nicotine for 1 h per session and an LgA group that self-administered nicotine for 21 h per session. Both groups self-administered nicotine for 12 consecutive days. The mean nicotine intake on the last 3 days (ie, days 10–12) served as baseline for within-group comparisons with nicotine intake in the next stage of the experiment (ie, daily vs intermittent self-administration).
Daily vs intermittent (24 h abstinence between sessions) nicotine self-administration: ShA and LgA rats self-administered nicotine for an additional 18 days. During this period, self-administration sessions were conducted either daily or with ∼24 h abstinence periods between self-administration sessions (intermittent schedule). Thus, the study at this point included three conditions, each with a vehicle-pretreated group and a phenelzine-pretreated group: LgA with daily access to nicotine, LgA with 24 h intermittent access to nicotine, and ShA with daily access to nicotine. ShA rats with daily access had a 23-h interval between sessions, similar to the LgA rats with 24 h intermittent access. After 18 days under this schedule, all of the rats were given daily access to nicotine until responding returned to near baseline levels and stabilized. The mean nicotine intake on the last 3 days served as baseline for within-group comparisons with nicotine intake in the next stage of the experiment. From this stage onward, only vehicle-pretreated rats were included in the study.
Daily vs intermittent (48 h abstinence between sessions) nicotine self-administration: ShA and LgA rats were then allowed an additional 21 days of nicotine self-administration with ∼48 h abstinence periods between self-administration sessions.
Progressive-ratio: 48 h after the termination of the last self-administration session, the rats were tested on a PR schedule of reinforcement. After the end of PR testing, the rats continued to self-administer nicotine on an FR1 schedule of reinforcement until the end of the 21-h session.
Withdrawal: 48 h after the previous self-administration session, somatic signs of spontaneous and mecamylamine-precipitated withdrawal were measured.
Somatic Signs of Nicotine Withdrawal
Withdrawal signs were measured 1 h before nicotine self-administration (ie, following 2 h or 47 h of abstinence from nicotine). The rats first received a subcutaneous (s.c.) saline injection (1 ml/kg) and were placed into an opaque plastic cylindrical container (30 × 29 cm) 30 min later for 10 min of somatic withdrawal sign observation (ie, spontaneous withdrawal). The subjects then received mecamylamine (1.5 mg/kg, s.c.) and were tested for somatic withdrawal sign (ie, precipitated withdrawal) 30 min later. Somatic signs of nicotine withdrawal were rated according to the method developed by Malin et al (1992). The rats were observed for blinks, body shakes, chews, cheek tremors, escape attempts, foot licks, gasps, writhes, genital licks, hops, head shakes, ptosis, scratches, teeth chattering, and yawns. Multiple successive counts of any sign required a distinct pause between episodes. The total number of somatic signs during the 10-min observation period was defined as the sum of the number of occurrences of all of the aforementioned signs. The observer was blind to the animal's experimental condition.
Progressive-Ratio Schedule of Reinforcement
In these sessions, the response requirement for reinforcement was increased according to the following sequence: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, and so on. (Richardson and Roberts, 1996). The PR sessions lasted for either a maximum of 6 h or until 1 h elapsed without a reinforcer delivery. The last ratio completed during the session was defined as the breakpoint.
Statistical Analysis
The data were subjected to analysis of variance (ANOVA) using the SPSS software. In all cases, a normality test and equal variance test were performed before the ANOVA to ensure its validity. Fisher's protected least significant difference (PLSD) post-hoc test and t-tests were used when necessary. The data are expressed as mean±SEM.
RESULTS
Escalation of Nicotine Self-Administration
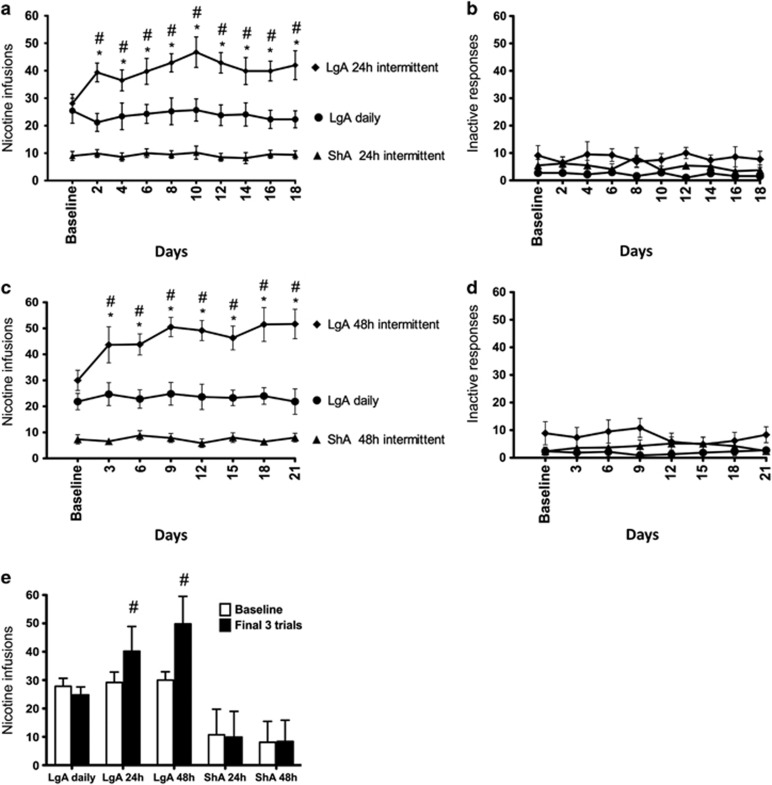
Following acquisition, the rats were given 12 consecutive days of nicotine self-administration for either 1 h per day (ShA) or 21 h per day (LgA). Self-administration stabilized, and the final 3 days of self-administration served as baseline for within-group comparisons. ShA and LgA rats then continued nicotine self-administration for an additional 18 days with either daily access to the drug or 24 h of abstinence between sessions (ie, 24 h intermittent schedule). A repeated-measures ANOVA revealed a significant group × session interaction for total nicotine intake (F18,225=2.086, p<0.05; Figure 2a), with the LgA rats on the intermittent schedule showing a significant increase in total nicotine intake (F9,63=2.629, p<0.02) between sessions. In contrast, the LgA rats that self-administered nicotine daily (F9,81=0.483, p>0.05) and the ShA group (F9,63=0.349, p>0.05) showed no change in nicotine self-administration. All LgA rats tended to self-administer more nicotine during the active (dark) phase compared with the inactive (light) phase of their daily cycle. The escalation of nicotine intake was in general because of increased intake during the active phase, particularly during the first 3 h.
Figure 2.
Nicotine intake (mean±SEM) in rats that self-administered nicotine under a fixed-ratio (FR)1 schedule in either 21 h (long access (LgA)) or 1 h (short access (ShA)) sessions. LgA rats increased their nicotine intake on an intermittent schedule with 24–48 h breaks between sessions, whereas LgA rats on a daily schedule did not. (a) Total number of nicotine infusions per session when the intermittent schedule included 24 h breaks between sessions. (b) Total number of inactive operant responses per session when the intermittent schedule included 24 h breaks between sessions. (c) Total number of nicotine infusions per session when the intermittent schedule included 48 h breaks between sessions. (d) Total number of inactive operant responses per session when the intermittent schedule included 48 h breaks between sessions. (e) Total number of nicotine infusions during baseline (ie, last 3 days of self-administration before separating the rats into daily and intermittent conditions; see Figure 1) vs the last 3 days of daily/intermittent nicotine self-administration. #p<0.05, compared with baseline; *p<0.05, compared with daily self-administration group. n=8–10 per group.
All of the rats were then allowed to self-administer nicotine for 6 consecutive days to allow a return to baseline levels of intake. The rats were then given 18 additional days of self-administration with either daily access to the drug or 48 h of abstinence between the self-administration sessions. Again, only LgA rats on the intermittent schedule showed a significant increase in nicotine intake compared with the other groups (F7,35=2.604, p<0.03), with a significant group × session interaction (F14,119=2.083, p<0.02; Figure 2c). An analysis of the average intake during the last three sessions compared with baseline showed a significant group × session interaction (F4,33=7.233, p<0.001), with the LgA group showing a significant increase in nicotine intake in both the 24 and 48 h intermittent-access schedules compared with baseline in both the 24 and 48 h abstinence intermittent-access schedules (Figure 2e). No significant differences in the number of inactive operant responses between or within the study groups were observed (p>0.05; Figure 2b and d).
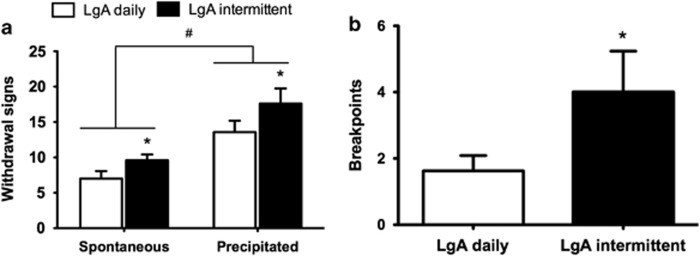
Following the last 48 h abstinence period, self-administration on a PR schedule of reinforcement was examined to assess the motivation for nicotine, compared with rats that self-administered nicotine daily. LgA rats that self-administered nicotine intermittently exhibited higher breakpoints than rats that self-administered nicotine daily (t12=2.002, p<0.05; Figure 3). After the last day of self-administration, the rats were tested for somatic signs of withdrawal at the same time point as the PR test (ie, 48 h into abstinence), with rats first receiving a saline injection (1 ml/kg, s.c.) before a 10-min observation period (ie, spontaneous withdrawal), followed by administration of the nicotine receptor antagonist mecamylamine (1.5 mg/kg, s.c.; ie, precipitated withdrawal). A mixed ANOVA revealed a main effect of treatment (mecamylamine vs saline; F1,10=15.115, p<0.01; Figure 3) and a main effect of the self-administration schedule (daily vs intermittent; F1,10=11.19, p<0.01).
Figure 3.
Withdrawal signs and breakpoints on a progressive-ratio (PR) schedule in long access (LgA) rats that self-administered nicotine either daily or with 48 h abstinence between sessions. (a) Withdrawal signs (mean±SEM) were measured 1 h before nicotine self-administration (ie, following 2 or 47 h of abstinence from nicotine) in rats that received a saline injection (ie, spontaneous withdrawal) or mecamylamine (1.5 mg/kg, subcutaneously (s.c.); ie, precipitated withdrawal). *p<0.05, main effect of intermittent schedule; #p<0.05, main effect of mecamylamine. (b) LgA rats on an intermittent schedule reached significantly higher breakpoints than LgA rats that self-administered nicotine daily. The data are expressed as mean±SEM. *p<0.05. n=7–9 rats per group.
Effect of Phenelzine-Induced MAO Inhibition on Nicotine Self-Administration
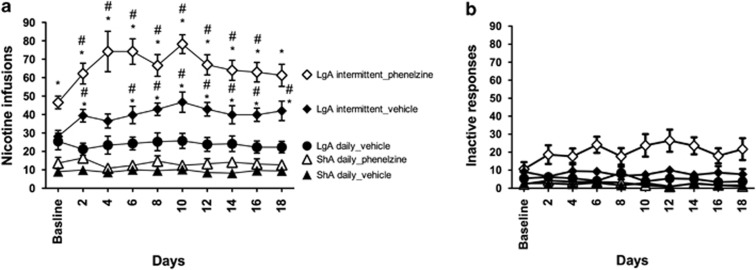
Before 1 h of each self-administration session, all of the rats were injected i.p. with either phenelzine (2 mg/kg) or vehicle (water). During the first 7 days of nicotine self-administration, phenelzine had no significant effect on the acquisition of nicotine self-administration in any of the rats (F6,252=1.67, p>0.05; Supplementary Figure S1). However, daily treatment with phenelzine increased nicotine intake under conditions of extended access to the drug (Figure 4a). Specifically, a two-way ANOVA revealed a significant drug × access interaction (F1,30=5.646, p<0.05), with Fisher's PLSD post-hoc test verifying significantly higher nicotine intake in phenelzine-LgA rats compared with vehicle-LgA rats. No such effect was found for ShA rats, although a trend toward an increase was observed.
Figure 4.
(a) Phenelzine-induced enhancement of nicotine intake in rats with extended access but not limited access to nicotine. The enhancement of nicotine intake in long-access (LgA) rats was observed during the daily nicotine self-administration stage (ie, baseline) and following the transition to intermittent access. (b) Inactive lever presses. #p<0.05, compared with baseline; *p<0.05, compared with rats that self-administered nicotine daily (analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) post-hoc test). n=7–10 rats per group. The data are expressed as mean±SEM.
When given access to nicotine on a 24-h intermittent schedule, phenelzine-LgA rats, but not phenelzine-ShA rats, further increased their nicotine intake (∼50% increase) compared with vehicle-LgA rats and vehicle-ShA rats, respectively. A repeated-measures ANOVA revealed a significant main effect of treatment (F1,135=21.782, p<0.001), demonstrating that phenelzine increased nicotine self-administration during both daily (baseline) and intermittent nicotine self-administration.
Inactive lever presses were higher during the intermittent phase in phenelzine-LgA rats than in vehicle-LgA rats (F1,135=6.635, p<0.05; Figure 4b), but the proportion of inactive to active lever presses in phenelzine rats remained stable between the daily-access (25.4±5.1%) and intermittent-access (29.6±7.0%) conditions, and was not different from vehicle-treated rats (38.8±8.7% and 20.0±4.1%, respectively). No main effect of phenelzine treatment was found in ShA rats (F1,135=3.094, p>0.05), with no treatment × schedule interaction (F9,135=0.582, p>0.05).
DISCUSSION
The present report demonstrated that repeated cycles of extended access (21 h per day, LgA) to nicotine self-administration followed by 24–48 h of abstinence produced a robust escalation of nicotine intake associated with increased motivational dependence on nicotine, reflected by higher breakpoints under a PR schedule of reinforcement, and with increased physical dependence, reflected by increased somatic signs of withdrawal. No escalation of nicotine intake was observed in LgA rats with daily access to nicotine or ShA rats with 23 h intermittent access to nicotine.
The lack of escalation of nicotine intake in LgA rats with daily access to nicotine or ShA rats with intermittent access to nicotine is consistent with previous studies (O'Dell et al, 2007; Paterson and Markou, 2004). However, repeated periods of 24–48 h of abstinence produced a rapid and stable escalation of nicotine intake in LgA rats (∼50% increase compared with baseline daily access). We previously found a significant increase in nicotine intake following 24–72 h of forced abstinence from extended-access nicotine self-administration, but nicotine intake returned to near baseline levels within 4 days of daily access to nicotine self-administration (O'Dell and Koob, 2007; George et al, 2007). Altogether, these results demonstrate that the robust escalation of nicotine intake requires both extended access to nicotine self-administration and repeated periods of abstinence. These results are consistent with the early course of nicotine dependence in adolescent smokers, in which escalation of tobacco smoking is attributable to the withdrawal symptoms that develop during intermittent smoking (Doubeni et al, 2010). The hypothesis that nicotine withdrawal symptoms during acute abstinence may represent a powerful driving force for the escalation of nicotine intake is also consistent with the observation of increased craving for tobacco smoking following overnight abstinence (Jarvik et al, 2000).
The escalation of drug intake has been hypothesized to involve an upregulation of brain stress systems and downregulation of anti-stress systems, leading to a negative emotional state when access to the drug is prevented. Presumably, the powerful incentive value of nicotine in the dependent user is strongly related to its ability to attenuate this negative emotional state through a negative reinforcement mechanism (Koob and Le Moal, 2001; Koob, 2010; Solomon and Corbit, 1973). Consistent with this possibility, spontaneous and precipitated nicotine withdrawal decreased brain reward function and the efficacy of natural reinforcers (Epping-Jordan et al, 1998; LeSage et al, 2006) and induced anxiety, depression, and irritability (Hughes et al, 1994; Irvine et al, 2001; Engelmann et al, 2009; George et al, 2007).
The role of a repeated withdrawal syndrome in the escalation of intake may be more critical for some drugs of abuse than others. The escalation of heroin, methamphetamine, and cocaine self-administration develops on a simple daily extended-access schedule (eg, Ahmed and Koob, 1998; Ahmed et al, 2000; Ben-Shahar et al, 2004; Greenwell et al, 2009; Kitamura et al, 2006), but nicotine and alcohol (Simms et al, 2008) only show robust escalation of intake under an intermittent LgA schedule. Importantly, the increased self-administration of alcohol and nicotine has been associated with alterations in similar stress systems, most notably corticotropin-releasing factor, presumably in the extended amygdala (George et al, 2007; Heilig and Koob, 2007).
The discrepancy between the escalation of smoking behavior in humans (Conklin et al, 2005; Doubeni et al, 2010; Kim et al, 2009) and difficulties demonstrating the escalation of nicotine intake in animals may also reflect the fact that humans do not abuse nicotine, but rather tobacco. Among other chemicals, tobacco smoke contains both MOA-A and MAO-B inhibitors (Kapelewski et al, 2011), resulting in reduced brain MAO activity in smokers (Fowler et al, 1996). Previous studies showed that rodents pretreated daily with mixed irreversible MAO-A and MAO-B inhibitors, such as tranylcypromine (Villégier et al, 2003, 2006) and phenelzine (Guillem et al, 2005), exhibited increases in nicotine-induced behaviors, including locomotor activity and limited-access self-administration under both FR and PR schedules of reinforcement. In contrast, the present study demonstrated that chronic inhibition of MAO with phenelzine (2 mg/kg) had no effect on the acquisition or maintenance of limited-access nicotine self-administration. However, Guillem et al (2005) reported a phenelzine-induced increase in nicotine self-administration on an FR5 schedule, but not the FR1 schedule. An FR1 schedule was also used in the present study. In the present study, however, phenelzine increased nicotine intake in LgA rats with both daily and intermittent access. Additionally, phenelzine treatment increased nicotine intake during the intermittent phase (ie, escalation) compared with vehicle-treated LgA rats, but it did not increase the relative magnitude of escalation, with phenelzine-LgA rats exhibiting a similar percentage of escalation compared with vehicle-LgA rats. Thus, the enhancing effects of phenelzine on nicotine self-administration were additive with extended-access self-administration. Together with the lack of effect of phenelzine on the magnitude of mecamylamine-precipitated withdrawal signs in LgA rats, these data suggest that the potentiation of nicotine escalation by MAO inhibition did not involve the motivational effect of nicotine withdrawal through a negative reinforcement mechanism, but instead increased the acute rewarding effect of nicotine through a positive reinforcement mechanism.
The dose of phenelzine used in our study was based on previous studies, a dose that inhibits both MAO-A and MAO-B without affecting locomotor behavior (McManus and Greenshaw, 1991), the acute psychostimulant effects of nicotine, or food-maintained responding (Guillem et al, 2005). Inactive lever presses in the present study were higher during the intermittent phase in phenelzine-LgA rats than in vehicle-LgA rats, but this difference was proportional to the increase in active lever presses. In fact, the proportion of inactive to active lever presses in phenelzine-treated rats remained stable between the daily and intermittent conditions and was similar to that of the vehicle-treated rats.
A potential limitation of the present study is that the activity of phenelzine is not limited to MAO inhibition. Lotfipour et al (2011) recently presented findings, which suggested that enhancement of nicotine self-administration by the MAOI tranylcypromine may not be attributable only to its suppression of MAO activity. For example, both the (±)tranylcypromine and (+)tranylcypromine stereoisomers induced nearly 100% MAO inhibition, but had differential effects on the rate of acquisition of nicotine self-administration. Lotfipour et al (2011) found a lack of effect of MAO inhibition by measuring the acquisition of nicotine self-administration under limited-access conditions for only 5 days. In the present study, phenelzine had no effect on the acquisition of nicotine self-administration. However, we found that phenelzine considerably enhanced self-administration under conditions of extended access after weeks of self-administration, suggesting MAO inhibition may be more relevant to nicotine self-administration under extended-access conditions than under limited-access conditions. Moreover, various MAOIs with different pharmacological profiles enhance the effects of nicotine. Future studies will need to explore these interactions with other MAOIs.
Altogether, the present results demonstrated that rats escalated their nicotine intake, but the escalation of nicotine intake required extended access to nicotine self-administration with intermittent periods of abstinence and MAO inhibition. These results suggest that the escalation of nicotine intake critically depends on the emergence of withdrawal symptoms that lead to an enhancement of the incentive value of nicotine as a negative reinforcer, and provide a model for testing the neurobiological mechanisms of nicotine escalation and efficacy of possible pharmacological interventions. Moreover, these results directly demonstrated that other compounds present in tobacco smoke, such as MAOIs, may facilitate nicotine intake, suggesting that MAO inhibition may contribute to compulsive nicotine-seeking.
Acknowledgments
This is publication number 21726 from The Scripps Research Institute. This work was supported by the Tobacco-Related Disease Research Program (TRDRP) from the State of California (Grant 17RT-0095), Pearson Center for Alcoholism and Addiction Research, and National Institute on Drug Abuse (DA023597). This work also was based on discussions and support from the Tobacco Etiology Research Network (TERN) of the Robert Wood Johnson foundation. We thank Michael Arends for proofreading the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, Marcus MD. The return to smoking: 1-year relapse trajectories among female smokers. Nicotine Tob Res. 2005;7:533–540. doi: 10.1080/14622200500185371. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Mahoney M. Current and emerging treatment approaches for tobacco dependence. Curr Oncol Rep. 2006;8:475–483. doi: 10.1007/s11912-006-0077-6. [DOI] [PubMed] [Google Scholar]
- Doubeni CA, Reed G, Difranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics. 2010;125:1127–1133. doi: 10.1542/peds.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J, Miller D, Todd G, Kostenbauder HB. Reinforcing and other behavioral effects of nicotine. Neurosci Biobehav Rev. 1981;5:487–495. doi: 10.1016/0149-7634(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Radke AK, Gewirtz JC. Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology (Berl) 2009;207:13–25. doi: 10.1007/s00213-009-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, et al. Low monoamine oxidase B in peripheral organs in smokers. Proc Natl Acad Sci USA. 2003;100:11600–11605. doi: 10.1073/pnas.1833106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE. Overview: biological processes relevant to drugs of dependence. Addiction. 1994;89:1443–1446. doi: 10.1111/j.1360-0443.1994.tb03741.x. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal vs other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and sublective craving for cigarettes. Pharmacol Biochem Behav. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kapelewski CH, Vandenbergh DJ, Klein LC. Effect of the monoamine oxidase inhibition on rewarding effects of nicotine in rodents. Curr Drug Abuse Rev. 2011;4:110–121. doi: 10.2174/1874473711104020110. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Fleming CB, Catalano RF. Individual and social influences on progression to daily smoking during adolescence. Pediatrics. 2009;124:895–902. doi: 10.1542/peds.2008-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Wilkinson DA, Skinner W, Kent C, Franklin T, Pope M. Comparing tobacco cigarette dependence with other drug dependencies: greater or equal ‘difficulty quitting' and ‘urges to use,' but less ‘pleasure' from cigarettes. JAMA. 1989;261:898–901. doi: 10.1001/jama.261.6.898. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Pentel PR. Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav. 2006;83:585–591. doi: 10.1016/j.pbb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Arnold MM, Hogenkamp DJ, Gee KW, Belluzzi JD, Leslie FM. The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology. 2011;61:95–104. doi: 10.1016/j.neuropharm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- McManus DJ, Greenshaw AJ. Differential effects of antidepressants on GABAB and β-adrenergic receptors in rat cerebral cortex. Biochem Pharmacol. 1991;42:1525–1528. doi: 10.1016/0006-2952(91)90420-a. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. ‘Nicotine deprivation effect' in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl) 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ.1995The scientific case that nicotine is addictive Psychopharmacology (Berl) 1172–10.discussion, 14–20. [DOI] [PubMed] [Google Scholar]
- Torrijos RM, Glantz SA. The US Public Health Service ‘treating tobacco use and dependence clinical practice guidelines' as a legal standard of care. Tob Control. 2006;15:447–451. doi: 10.1136/tc.2006.016543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services . The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General, 1988 (DHHS Publication no. [CDC] 88-8406) Centers for Disease Control and Prevention, Office on Smoking and Health: Rockville; 1988. [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav. 2003;76:267–274. doi: 10.1016/s0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, et al. Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology. 2006;31:1704–1713. doi: 10.1038/sj.npp.1300987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.