Abstract
There is considerable interest in identifying pharmacological compounds that could be used to facilitate fear extinction. Recently, we showed that the modulation of M-type K+ channels regulates the intrinsic excitability of infralimbic (IL) neurons and fear expression. As muscarinic acetylcholine receptors inhibit M-type K+ channels, cholinergic inputs to IL may have an important role in controlling IL excitability and, thereby, fear expression and extinction. To test this model, we combined whole-cell patch-clamp electrophysiology and auditory fear conditioning. In prefrontal brain slices, muscarine enhanced the intrinsic excitability of IL neurons by reducing the M-current and the slow afterhyperpolarization, resulting in an increased number of spikes with shorter inter-spike intervals. Next, we examined the role of endogenous activation of muscarinic receptors in fear extinction. Systemic injected scopolamine (Scop) (muscarinic receptor antagonist) before or immediately after extinction training impaired recall of extinction 24-h later, suggesting that muscarinic receptors are critically involved in consolidation of extinction memory. Similarly, infusion of Scop into IL before extinction training also impaired recall of extinction 24-h later. Finally, we demonstrated that systemic injections of the muscarinic agonist, cevimeline (Cev), given before or immediately after extinction training facilitated recall of extinction the following day. Taken together, these findings suggest that cholinergic inputs to IL have a critical role in modulating consolidation of fear extinction and that muscarinic agonists such as Cev might be useful for facilitating extinction memory in patients suffering from anxiety disorders.
Keywords: amygdale, prefrontal, M-type K+ channels, plasticity, fear conditioning, PTSD
INTRODUCTION
Learning to recognize that environmental cues predict danger is critical to survival. However, it is equally important to realize when a predictor of danger is no longer relevant. In auditory fear extinction, an animal learns that a tone that previously predicted a footshock no longer signals the shock. Although many advances in understanding the role of the infralimbic (IL) cortex in fear extinction have been made (for reviews, see Quirk and Mueller, 2008; Herry et al, 2010), the cellular mechanisms that mediate the acquisition and consolidation of fear extinction in IL are incompletely known. Recently, we showed that increased IL intrinsic excitability was correlated with reduced fear expression 24-h after extinction training, suggesting that the intrinsic excitability of IL neurons regulates fear expression and extinction. In addition, extinction reduced the slow afterhyperpolarizing potentials (sAHP), suggesting that the modulation of K+ currents is important for fear extinction (Santini et al, 2008). Consistent with this, pharmacological inhibition of M-type K+ channels enhances the intrinsic excitability of IL neurons and facilitated fear extinction (Santini and Porter, 2010). As muscarinic acetylcholine receptors can inhibit M-type K+ channels (Wess et al, 2007; Hernandez et al, 2008), it is plausible that cholinergic inputs to the medial prefrontal cortex (mPFC) (Dalley et al, 2004) increase IL excitability to facilitate fear expression and extinction.
Previous work has shown that muscarinic receptors have a critical role in memory formation in other brain structures (Introini-Collison et al, 1996; McGaugh, 2004). For example, blockade of muscarinic receptors with scopolamine (Scop) impaired the acquisition of auditory and contextual fear conditioning (Wallenstein and Vago, 2001; Bang and Brown, 2009; Pang et al, 2010), inhibitory avoidance (Huang et al, 2010), and spatial memory in the water maze task (Huang et al, 2010). In line with these results, stimulation of muscarinic receptors in the amygdala facilitates consolidation of Pavlovian fear conditioning (Vazdarjanova and McGaugh, 1999; Passani et al, 2001), inhibitory avoidance (Power et al, 2003), and extinction of conditioned place preference (Schroeder and Packard, 2004). Despite the well-studied role of muscarinic receptors in various types of memory, surprisingly little is known about the role of the muscarinic receptors in fear extinction (Kaplan and Moore, 2011). A previous study from Boccia et al (2009) showed that infusions of the cholinergic agonist oxotremorine into the amygdala facilitated extinction of contextual fear. However, it remains to be determined whether endogenous activation of muscarinic receptors normally occurs during fear extinction. Furthermore, whether muscarinic receptors contribute to consolidation of auditory fear extinction via activation of IL neurons is also unknown. Therefore, to examine the role of muscarinic receptors in fear extinction, we combined patch-clamp electrophysiology and auditory fear conditioning.
SUBJECTS AND METHODS
Subjects
All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of the Ponce School of Medicine in compliance with NIH guidelines for the care and use of laboratory animals. Male Sprague–Dawley rats (25–28 days postnatal) were transported from the Ponce School of Medicine colony to a satellite facility nearby where they were housed in transparent polyethylene cages inside a negative-pressure Biobubble (Colorado Clean Room, Ft Collins, CO). Rats were maintained on a 12–12 h light–dark schedule with free access to food (standard laboratory rat chow) and water.
Slice Preparation
Naive rats were deeply anesthetized with pentobarbital (150 mg/kg), and were perfused through the heart with ice-cold high-sucrose solution: 252 mM sucrose, 2 mM KCl, 1.25 mM NaH2PO4, 3 mM MgSO4, 26 mM NaHCO3, 20 mM glucose, and 1 mM CaCl2. Brains were quickly removed and placed in ice-cold artificial cerebral spinal fluid (ACSF) containing 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 26 mM NaHCO3, 20 mM glucose, and 2 mM CaCl2 and bubbled with 95% O2 and 5% CO2. Coronal slices of the mPFC were cut at a thickness of 300 μm with a Vibratome 1000 Plus (Vibratome, St Louis, MO). Slices were incubated at room temperature in ACSF for at least an hour before experiments. The NMDA receptor blocker MK-801 (10 μM) was added during the incubation of slices to increase neuronal survival (Schurr et al, 1995).
Slices were transferred to a submersion recording chamber and perfused at 2–3 ml/min with room temperature ACSF. Neurons were visualized with infrared video microscopy using a 40 × water immersion objective on an upright E600FN microscope (Nikon Instruments, Melville, NY). Whole-cell recordings were done with glass pipettes with a resistance of 3–5 MΩ when filled with an internal solution containing (in mM) KCl (20), Kgluconate (115), HEPES (10), sodium phosphocreatine (10), biocytin (10), adenosine triphosphate (2), guanosine triphosphate (3) and ethyleneglycol-bis(2-aminoethylether)-N,N,N', N'-tetra acetic acid (EGTA, 0.5); pH was adjusted to 7.3 with KOH (290 mOsm) and sucrose was added to adjust osmolarity to 300 mOsm.
Whole-Cell Recordings
Whole-cell voltage-clamp recordings were obtained from the soma of mPFC pyramidal neurons located in layers II/III and V of IL. Cells were held in current-clamp mode at −60 mV and action potential discharges in response to the injection of depolarizing current pulses were recorded with a patch-clamp amplifier (MultiClamp 700A, Axon Instruments, Union City, CA). Recordings were filtered at 4 kHz, digitized at 10 kHz, and saved to computer using pCLAMP9 (Axon Instruments). Membrane potentials were not corrected for the junction potential of 9 mV. The input resistance was measured from a 5 mV, 50 ms depolarizing pulse in voltage-clamp mode. To measure the M-currents, cells were held in voltage-clamp mode at −30 mV followed by a hyperpolarizing step to −60 mV. In these experiments tetrodotoxin (0.5 μM) was included in the bath to block voltage-gated sodium channels and facilitate the isolation of the M-currents.
To test the effects of stimulating muscarinic acetylcholine receptors in IL pyramidal neurons, muscarine chloride (10 μM, Sigma) was bath applied to mPFC slices for 5 min after establishing a stable baseline recording. In some experiments, the M-type K+ channel blocker, XE-991 (10 μM) was bath applied at the same time as muscarine to test whether stimulating acetylcholine receptors with muscarine occludes the effects of XE-991 on the spike count. We measured the amplitude of the fast afterhyperpolarizing potential (fAHP) for the first spike evoked by the 800 ms depolarizing pulse. The fAHP was measured by subtracting the voltage at the peak of the fAHP from the threshold potential for spike initiation (Santini et al, 2008). The medium afterhyperpolarizing potential (mAHP) and the sAHP were measured after the end of the 800-ms pulse. The mAHP was measured as the peak of the AHP. The sAHP was measured as the average potential during a 200-ms period beginning 800 ms after the end of the 800 ms depolarizing pulse (Santini et al, 2008).
Surgery
Rats were anesthetized with ketamine and xylazine (10 ml/100 g) and placed in the stereotaxic apparatus. After anesthesia, the skin was retracted and holes were drilled in the skull. They were implanted with a single 26 gauge stainless-steel guide cannula (Plastics One, Roanoke, VA) in the mPFC as described previously (Santini et al, 2004). Stereotaxic coordinates aiming toward the IL cortex were 2.8 mm anterior, 1.0 mm lateral, and 4.1 mm ventral from bregma (Paxinos and Watson, 1986), with the cannula angled 11 ° toward the midline in the coronal plane. Rats were allowed 7 days to recover from surgery.
Drugs and Infusion Procedure
The competitive non-selective muscarinic receptor antagonist, Scop hydrobromide (Tocris), was systemically injected (1.5 mg/kg) 30 min before extinction training or immediately after the last extinction trial. Scop was dissolved in 0.9% saline. In a separate set of experiments, the muscarinic agonist cevimeline (Cev) hydrochloride (Tocris) was systemically injected (1 mg/kg, intraperitoneal (i.p.)) 15 min before extinction training or immediately after extinction training. The post-extinction injections of Cev were given for 3 consecutive days. Cev was dissolved in 0.9% saline. In the systemic experiments, the age of the rats ranged between 28 to 34 days old at the time of extinction training.
For the localized infusion experiments, cannula-dummies were removed from guide cannulas and replaced with 33 gauge injectors, which were connected by polyethylene tubing (PE-20; Small Parts, Miami Lakes, FL) to 5 μl syringes mounted in an infusion pump (Harvard Apparatus, Holliston, MA). Rats received infusions of either saline (vehicle) or Scop (10 μg/0.5 μl) into the mPFC 5 min before extinction training. In the intra-IL experiments, the age of the rats ranged between 38 to 42 days old at the time of extinction training. The dose of Scop was based on similar behavioral studies that infused Scop into the prefrontal cortex, perirhinal cortex, amygdala, and/or hippocampal formation regions (Herremans et al, 1996; Barros et al, 2001; Warburton et al, 2003; Winters et al, 2006; Barak and Weiner, 2010). Drugs were infused at a rate of 0.5 μl for 1 min.
Behavioral Apparatus
Rats were fear conditioned, extinguished, and tested in a chamber of 25 × 29 × 28 cm with aluminum and Plexiglas walls (Coulbourn Instruments, Allentown, PA). The floor consisted of stainless steel bars that could be electrified to deliver a mild shock. A speaker was mounted on the outside wall and illumination was provided by a single overhead light. The chamber was situated inside a sound-attenuating box (Med Associates, Burlington, VT) with a ventilating fan, which produced an ambient noise level of 60 dB. The conditioned stimulus (CS) was a 4 kHz tone with duration of 30 s and an intensity of 80 dB. The unconditioned stimulus was a 0.4 mA scrambled footshock, 0.5 s in duration, which co-terminated with the tone during the conditioning phase. Between sessions, floor trays and shock bars were cleaned with soapy water and the chamber walls were wiped with a damp cloth. Behavior was recorded with digital video cameras (Micro Video Products, Ontario, Canada).
Behavioral Procedure
On day 1, rats were fear conditioned to a tone CS (3 tone-shock pairings). After matching for equivalent levels of freezing, conditioned rats were divided into a vehicle (Veh)-treated group and a Scop-treated group. On day 2, rats were systemically injected (30 min before extinction training) or locally infused (5 min before extinction training) with Veh or Scop before extinction training (12 tone-alone trials). On days 3, rats received two tone-alone trials in the same chamber to test for recall of extinction. The same behavioral protocol was used for the post-training experiments with the exception that Veh or Scop were injected immediately after the end of the extinction session on day 2.
For the experiments using the muscarinic agonist Cev, the conditioning phase was exactly as described above. However on day 2, fear conditioned rats received i.p. injections of Veh or Cev 15 min before a partial extinction session that consisted of four tone-alone trials. Both groups received additional partial extinction sessions for 3 consecutive days (days 3–5). For the post-training experiment, the protocol used was the same as described above with the exception that the systemic injection of Cev was given immediately after extinction training. During the subsequent 2 days, rats received a partial extinction session followed by an additional post-training injection. The inter-trial interval was an average of 2 min for all behavioral experiments.
Statistical Analysis
The percentage of time spent freezing (Blanchard and Blanchard, 1972) was used as a measure of conditioned fear. Freezing is the cessation of all movements except respiration. The total time spent freezing during the 30-s tone was measured and converted to percent freezing. The behavioral data were analyzed by observers blinded with respect to experimental group from digital videos using commercial software (FreezeScan, Clever Systems). The electrophysiological data were analyzed using Clampfit (Axon Instruments). Student's t-test, repeated-measures ANOVA, or one-way ANOVA (STATISTICA, Statsoft, Tulsa, OK) were used to analyze the behavioral and electrophysiological data. Following a significant main effect, post-hoc tests were performed with Tukey HSD tests. Values are reported as the mean±SEM.
RESULTS
Stimulation of Muscarinic Receptors Increased the Number of Evoked Spikes and Burst Firing in IL Pyramidal Neurons
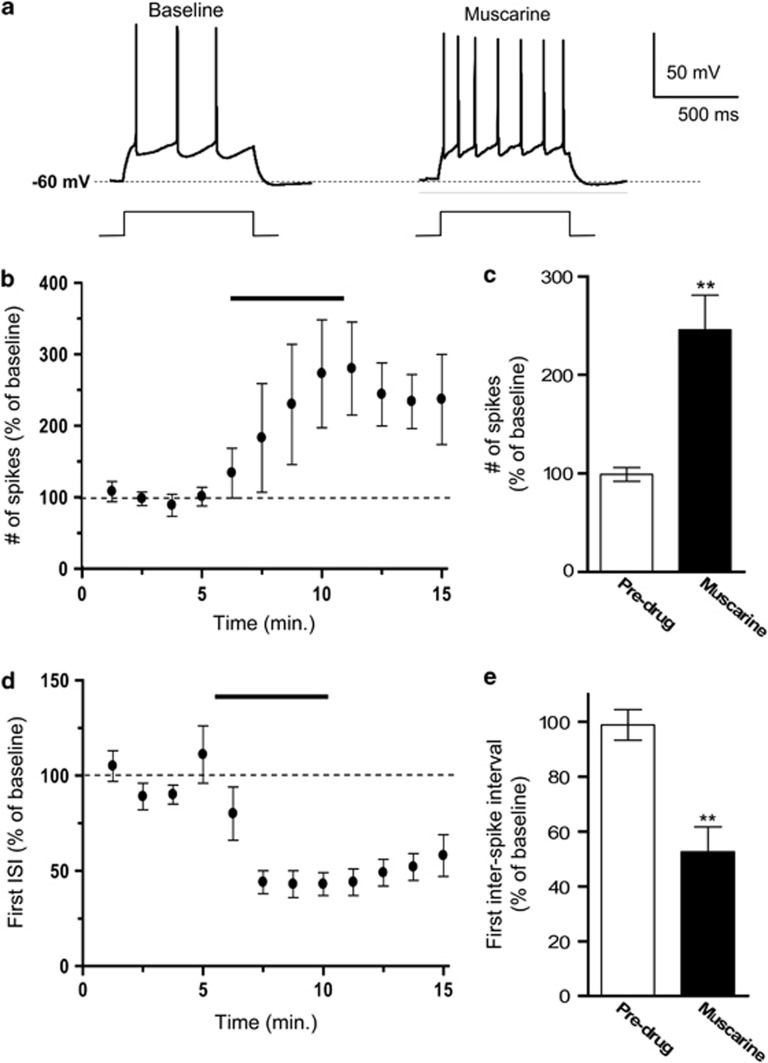
Recently, we showed that pharmacological inhibition of M-type K+ channels enhances IL intrinsic excitability and fear extinction recall (Santini and Porter, 2010). As the activation of muscarinic receptors can inhibit M-type K+ channels (Wess et al, 2007; Hernandez et al, 2008), we hypothesized that stimulation of muscarinic receptors would increase IL excitability and enhance fear extinction recall by blocking M-type K+ channels. To test this, we first assessed the effects of pharmacological stimulation of muscarinic receptors on the intrinsic excitability of IL pyramidal neurons. Coronal slices of the mPFC containing IL were prepared and the intrinsic excitability of IL neurons was assessed with whole-cell patch-clamp recordings. Following baseline recordings, muscarine chloride (10 μM) was bath applied to determine the effects of muscarinic receptor stimulation on the relative excitability of IL neurons. Two measurements of neuronal excitability were examined: the number of evoked spikes and the first inter-spike interval (ISI; Santini and Porter, 2010). First, we measured the number of action potentials elicited by a depolarizing current pulse. To standardize baseline firing across cells, the intensity of the current pulse was adjusted for each cell to evoke between two to three spikes. As shown in Figures 1a–c, bath application of muscarine caused a significant increase in the number of action potentials evoked by a depolarizing current pulse (muscarine=246±35% of baseline; t=3.35, df=4, p=0.005).
Figure 1.
Stimulation of muscarinic receptors increased the intrinsic excitability of IL pyramidal neurons. (a) Representative traces showing the number of spikes evoked by a current pulse during baseline and bath application of muscarine (10 μM). (b, c) Time course and bar graph demonstrating that perfusion of muscarine persistently increased the number of evoked spikes. (d, e) Time course and bar graph demonstrating that muscarine shortened the first inter-spike interval (ISI). **p<0.01.
To determine whether stimulation of muscarinic receptors facilitates bursting firing in IL neurons, we assessed the effects of bath applied muscarine on the first ISI. In the presence of muscarine, the neurons fired spikes with a shorter first ISI (Figure 1d). Muscarine significantly reduced the first ISI (muscarine=53±9% of baseline; t=5.6, df=4, p=0.007) measured at traces showing four evoked spikes (Figure 1e) indicating that stimulation of muscarinic receptors enhanced bursting in IL neurons.
Muscarinic Receptors Inhibit M-Currents and the sAHP in IL Pyramidal Neurons
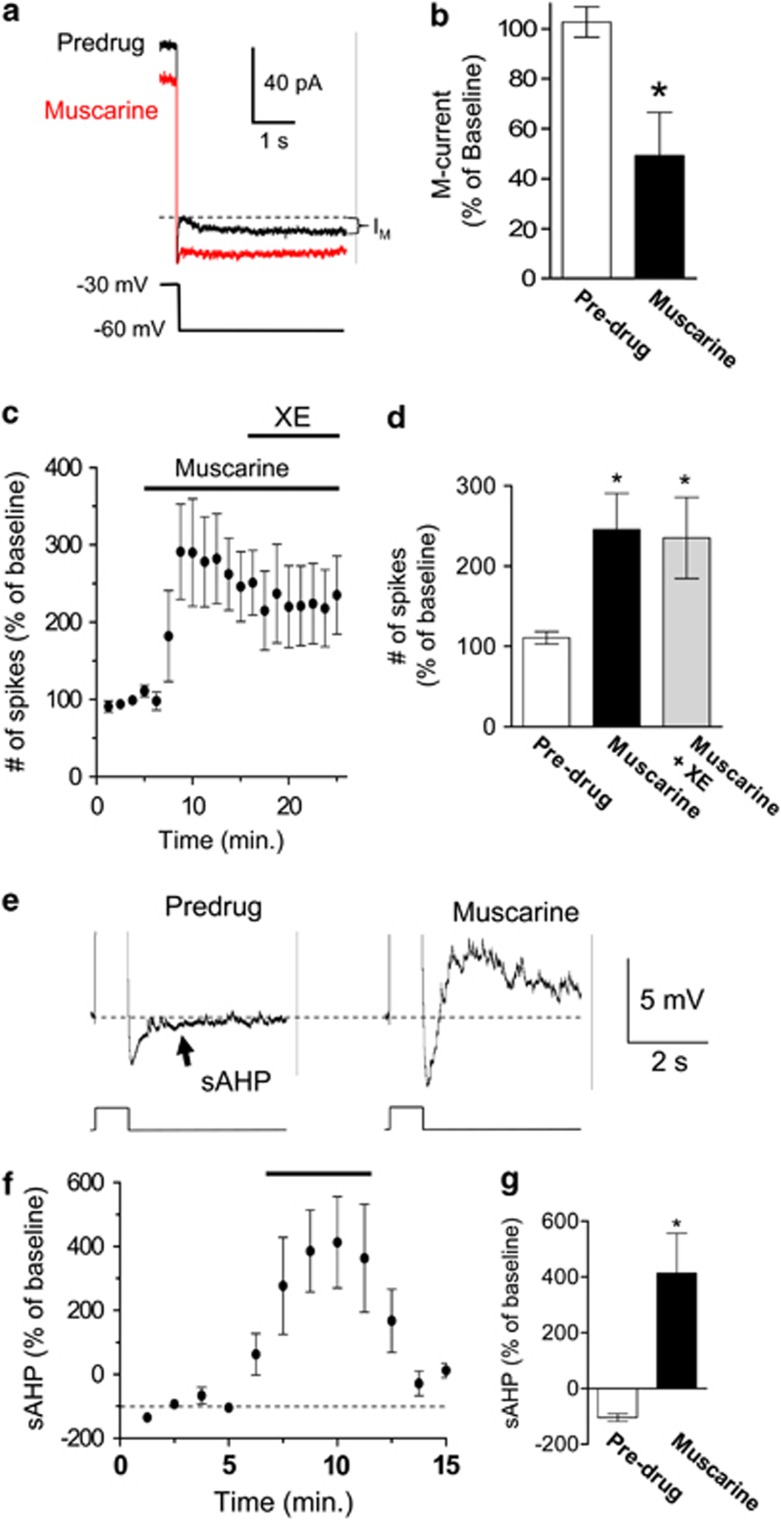
To study the mechanisms by which muscarinic receptors enhance the intrinsic excitability of IL neurons, we examine the effect of muscarinic receptors on the resting membrane potential, input resistance, M-type K+ channels, and the sAHP. There was no effect of muscarine on the resting membrane potential (98±0.6% of baseline; t=0.58, df=4, p=0.59). However, muscarine did increase the input resistance (122±4.2% of baseline; t=5.36, df=4, p=0.006) consistent with the closure of channels that are active at the resting membrane potential of IL neurons such as M-type K+ channels (Santini and Porter, 2010) or Kir channels (Carr and Surmeier, 2007). Since Carr and Surmeier (2007) already showed that stimulation of muscarinic receptors reduces the conductance through Kir channels in IL neurons, we examined the effect of muscarine on M-type K+ channels. As indicated in Figures 2a and b, muscarine reduced the M-current in IL pyramidal neurons (49±17% of baseline; t=4.29, df=3, p=0.023). In addition, after muscarine application, the addition of the M-type K+ channel antagonist XE-991 (10 μM) did not cause a further increase in the number of evoked spikes (Figures 2c and d; muscarine, 246±45% of baseline spikes, muscarine+XE-991, 235±50% of baseline spikes, t=0.63, df=5, p=0.55). As we previously showed that blocking M-type K+ channels with 10 μM XE-991 increases the number of evoked spikes (Santini and Porter, 2010), this suggest that muscarine occluded the effects of XE-991 by blocking M-type K+ channels.
Figure 2.
Stimulation of muscarinic receptors reduced the M-current and blocked the slow afterhyperpolarizing potential (sAHP) in IL pyramidal neurons. (a) Representative traces showing that muscarine (10 μM) reduced the M-currents (IM). (b) Bar graph showing that muscarine significantly reduced the M-currents in IL pyramidal neurons (n=7). (c, d) time course and bar graph showing that muscarine increased the number of evoked spikes while co-application of the M-type K+ channel blocker, XE-991, had no additional effect. (e) Representative traces showing that muscarine blocked the sAHP. (f, g) Time course and bar graph showing that muscarine blocked the sAHP in IL pyramidal neurons (n=3). *p<0.05.
In addition, as shown in Figures 2e–g, muscarine reduced the sAHP evoked by a depolarizing current pulse revealing an afterdepolarizing potential (muscarine, 413±143% of baseline, t=−3, df=4, p=0.02). Blocking M-type K+ channels with 10 μM XE-991 does not affect the sAHP in IL neurons (Santini and Porter, 2010), therefore muscarine also blocks the calcium-dependent potassium channels underlying the sAHP. Consistent with this, modulation of the sAHP by muscarinic receptors has been previously shown in other brain regions (Cox et al, 1994; Zhang et al, 1996; Bond et al, 1999; Egorov et al, 1999; Scroggs et al, 2001; Krause et al, 2002; Sah and Faber, 2002; Ghamari-Langroudi and Bourque, 2004).
We also examined the effects of muscarine on the mAHP and the fAHP. Muscarine's effect on the mAHP was highly variable and therefore not significant (19±58% of baseline, t=1.4, df=4, p=0.22). However, the fAHP was significantly reduced by bath application of muscarine (60±7% of baseline, t=5, df=4, p=0.01). Taken together, these findings indicate that stimulation of muscarinic receptors enhances the intrinsic excitability and burst firing of IL pyramidal neurons by inhibiting several K+ channels.
Systemic Blockade of Muscarinic Receptors Before Extinction Training Impaired Extinction Recall 24-H Later
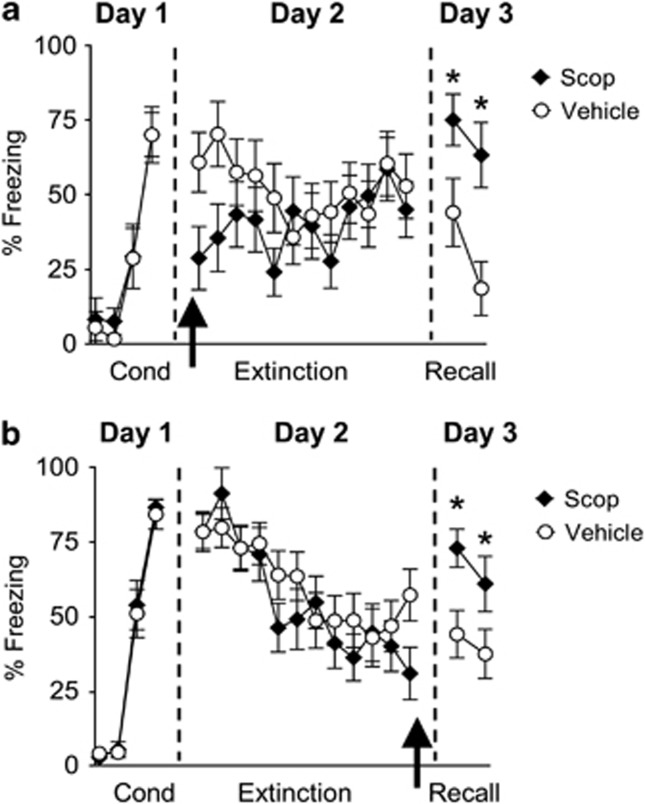
The above experiments raise the possibility that cholinergic input to IL could modulate fear extinction by stimulating muscarinic receptors on IL neurons thereby increasing their excitability and burst firing. If this is the case then blocking muscarinic receptors during extinction should impair extinction recall. To test this, rats were fear conditioned to a tone (Figure 3a). Following fear conditioning on day 1, rats were matched and divided into two groups based on their levels of conditioned freezing during the last conditioning trial (Veh: 70±9% freezing, Scop: 70±7% freezing; t=0.005, df=22, p=0.99). On day 2, rats received i.p. injections of either Veh solution (n=12) or Scop (1.5 mg/kg, n=12) 30 min before extinction training to determine whether muscarinic receptors are activated during extinction of auditory fear conditioning by acetylcholine released from cholinergic terminals. Scop-injected rats showed lower levels of conditioned freezing compared with Veh-infused rats during the first two extinction trials. This suggests that Scop reduced fear expression, although a repeated-measures ANOVA across the entire extinction phase (12 trials) on day 2 missed significance for an effect of the drug (F(1, 22)=1.84, p=0.09). On day 3, Scop-injected rats showed higher levels of conditioned freezing (main effect: F(1, 22)=8.32, p=0.002). Post-hoc comparisons indicate that both test trials were significantly higher in the Scop-injected rats compared with Veh-injected animals (values of p<0.05).
Figure 3.
Systemic injection of Scop before and immediately after extinction training disrupted extinction recall 24-h later. (a) Percent freezing to the tone of Veh-injected rats (empty circles, n=12) and rats injected with Scop (black diamonds, 1.5 mg/kg, n=12) before extinction training. (b) Percent freezing to the tone for Veh-injected rats (n=18) and rats injected with Scop (n=18) immediately after extinction training. Arrows indicate the time of the injection. *p<0.05.
Systemic Blockade of Muscarinic Receptors Immediately After Extinction Training Impaired Extinction Recall 24-H Later
Rats injected with Scop showed impaired recall of extinction 24-h later, suggesting that blockade of muscarinic receptors prevented consolidation of long-term extinction memory. However, the impaired recall of extinction could also be due to impaired learning because the Scop was present during acquisition of extinction as well. To avoid this confound and to test directly whether Scop disrupted consolidation of fear extinction, another group of fear conditioned rats was systemically injected with either Veh (n=18) or Scop (n=18) immediately after extinction training on day 2. Similar to the pre-training injections, blockade of muscarinic receptors immediately after extinction training impaired recall of extinction 24-h later (Figure 3b, F(1, 34)=4.72, p=0.03). Post-hoc comparisons indicate that both test trials were significantly higher in the Scop-injected rats compared with Veh-injected animals (values of p<0.05). Together these findings suggest that muscarinic receptors are critically involved in consolidation of extinction memory.
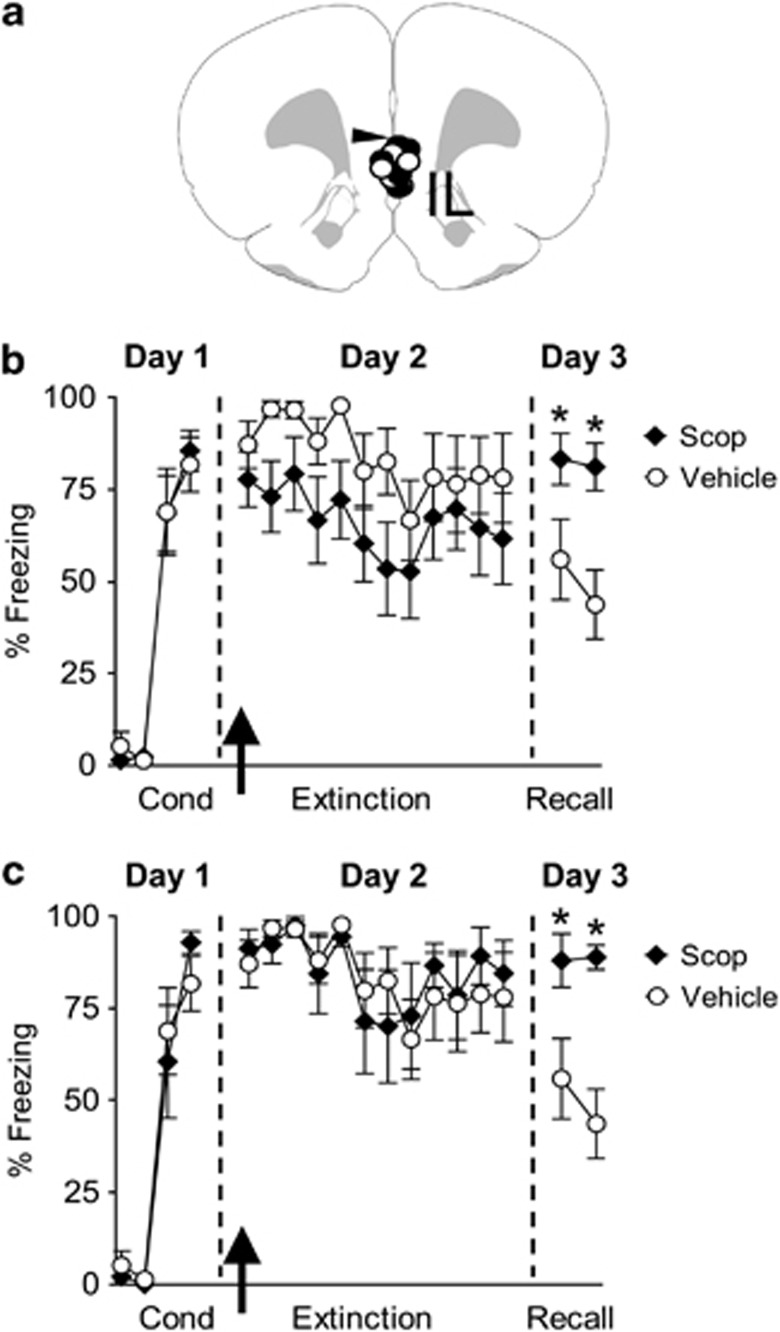
Intra-IL Blockade of Muscarinic Receptors Impaired Extinction Recall 24-H Later
To directly test whether muscarinic acetylcholine receptors in the IL sub-region of the mPFC are necessary for fear extinction, rats implanted with cannulas aimed toward the IL sub-region of the mPFC were fear conditioned (Figure 4a). On day 1, rats showed similar levels of conditioned freezing to the tone (Veh: 81±7% Scop: 85±6% t=0.40, df=19, p=0.68; see Figure 4b). On day 2, rats received intra-IL infusions of either Veh (n=10) or Scop (n=11) 5 min before extinction training. Although Scop-treated rats showed an apparent reduction in freezing during extinction training on day 2, repeated-measures ANOVA showed no effect of Scop on the acquisition of extinction (F(1, 19)=2.54, p=0.12). On day 3, however, Scop-treated rats showed higher levels of conditioned freezing compared with Veh-infused rats, indicating poor recall of extinction memory. Repeated-measures ANOVA revealed a significant main effect (F(1, 19)=11.25, p=0.01) and post-hoc analysis confirmed that during the two test tones the Scop-treated rats froze significantly more than controls (values of p<0.05).
Figure 4.
Infusion of Scop into IL before extinction training blocked extinction recall 24-h later. (a) Diagram showing the location of the cannula tips in IL for the Veh- (empty circles) and Scop- (black diamonds) infused animals. (b) Freezing to the tone for Veh-infused rats (n=10) and rats infused with Scop (10 μg/0.5 μl, n=11). Scop-infused rats showed higher fear compared with Veh rats on day 3, indicating impaired recall of extinction memory. Arrow indicates the time of the infusion. (c) Freezing to the tone for Veh-infused rats (n=10) and a subset of Scop-infused rats (n=7) that showed similar levels of freezing as Veh animals on day 2. Scop-infused rats showed higher fear compared with Veh rats on day 3, indicating impaired recall of extinction memory. Arrow indicates the time of the infusion. *p<0.05.
As there was an apparent, although not significant, reduction of fear expression during the acquisition of extinction on day 2 by Scop, it remained possible that impaired recall of extinction on day 3 was related to the effect of Scop on fear expression on day 2. To test this possibility, we normalized fear expression on day 2 by removing four animals from the Scop-treated group that showed very low freezing on day 2. As shown in Figure 4c, when the difference in fear expression during acquisition of extinction was eliminated (F(1, 15)=0.37, p=0.96), the Scop-infused group still showed significantly more freezing during recall on day 3 (F(1, 15)=15.50, p=0.003). Together these findings indicate that muscarinic receptors in IL are involved in long-term extinction memory.
Systemic Stimulation of Muscarinic Receptors Facilitated Extinction Recall 24-H Later
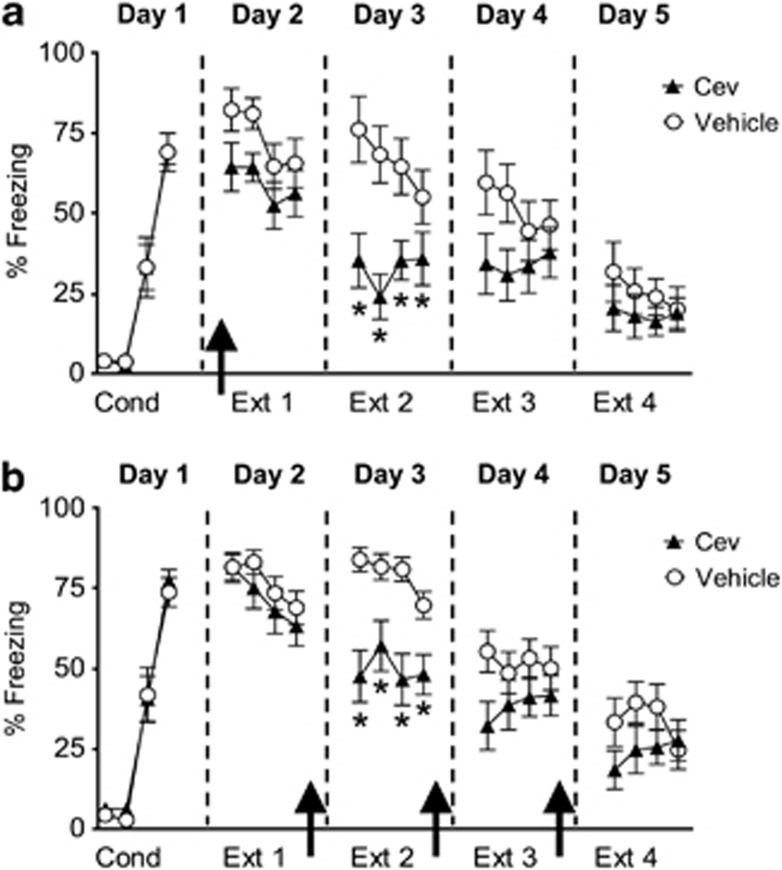
As blocking muscarinic receptors impaired extinction recall, we hypothesized that stimulation of muscarinic receptors would facilitate recall of extinction memory. Given the potential clinical significance of facilitating extinction, we decided to test whether fear extinction could be enhanced with a clinically available muscarinic agonist. We chose to use Cev, because it is approved for use in humans and has been shown to enhance neuronal excitability (Segal and Fisher, 1992) and memory in rodents (Fisher et al, 1991). On day 1, rats were fear conditioned, matched for similar levels of conditioned freezing, and divided into two groups (Veh: 69±6% freezing; Cev: 70±5% freezing; t=0.13, df=22, p=0.89; Figure 5a). On day 2, rats were systemically injected with either Veh (n=12) or Cev (1 mg/kg, n=12) 15 min before a partial extinction session that consisted of four tone-alone trials. During the subsequent 3 days (days 3, 4 and 5), rats received a partial extinction session and were returned to their home cages. Although Cev-injected rats showed slightly lower levels of conditioned freezing compared with Veh-injected rats during the first two extinction trials on day 2, a repeated-measures ANOVA across the entire extinction phase on day 2 found no significant effect of the drug (group × trial interaction: F(3, 66)=0.21, p=0.88) suggesting that Cev had little effect on fear expression. However on day 3, Cev-injected rats showed significantly lower levels of conditioned fear as compared with Veh-injected rats (F(1, 22)=13.02, p=0.001) and post-hoc comparisons indicated that during the four extinction tones the Cev-treated rats froze significantly less than controls (values of p<0.05) suggesting that Cev enhanced consolidation of extinction.
Figure 5.
Systemic injection of the muscarinic agonist Cev given before or immediately after extinction training facilitated extinction recall 24-h later. (a) Freezing to the tone for Veh-injected rats (empty circles; n=12) and rats injected with Cev (black triangles; 1 mg/kg, n=12) 15 min before extinction training. Rats injected with Cev before extinction training showed reduced fear compared with Veh rats on day 3. (b) Freezing to the tone for Veh-injected rats (empty circles; n=16) and rats injected with Cev (black triangles; n=15) immediately after extinction training. Rats injected with Cev immediately after extinction training showed reduced fear compared with Veh rats on day 3, suggesting enhanced consolidation of extinction memory. Arrows indicate the time of the injections. *p<0.05.
To further test whether stimulation of muscarinic receptors enhanced consolidation of extinction memory, another group of fear conditioned rats was systemically injected with either Veh (n=16) or Cev (n=15) immediately after extinction training. On day 1, rats were fear conditioned, matched for similar levels of conditioned freezing and divided into two groups (Veh: 73±4% freezing; Cev: 77±4% freezing; t=0.62, df=29, p=0.54; Figure 5b). On day 2, rats received a partial extinction session that consisted of four tone-alone trials. Immediately after extinction, the rats were systemically injected with either Veh or Cev (1 mg/kg). During the subsequent 2 days (days 3 and 4), rats received a partial extinction session followed by a systemic injection as on day 2. On day 5, both groups received an additional extinction session with no injection. As shown in Figure 5b, on day 2 rats extinguished to a similar degree before any pharmacological manipulation (F(1, 29)=0.83, p=0.37). On day 3, however, Cev-treated rats showed significantly lower levels of conditioned fear as compared with Veh-treated rats (F(1, 29)=24.2, p=0.0003) and post-hoc comparisons indicated that during the four extinction tones the Cev-treated rats froze significantly less than controls (values of p<0.05). Although there was a tendency for the Cev-treated group to freeze less than Veh-treated rats on days 4 and 5, there was no statistical difference (p>0.05). The enhanced extinction recall on day 3 in the Cev-treated group mirrors what was found with the pre-extinction injection indicating that Cev enhanced consolidation of fear extinction.
DISCUSSION
The main findings of our study are (1) the stimulation of muscarinic receptors excites IL neurons allowing them to fire more spikes at a higher frequency, (2) muscarinic receptor stimulation reduces the M-current and the sAHP in IL neurons, (3) blocking muscarinic receptors systemically or locally in IL disrupts recall of fear extinction, and (4) systemic stimulation of muscarinic receptors enhances recall of fear extinction. Together these findings suggest that endogenous stimulation of muscarinic receptors during fear extinction increases IL excitability leading to a long-term memory of fear extinction.
Our results show for the first time that extinction of cued fear conditioning requires muscarinic receptors in IL. The only previous study examining the role of muscarinic receptors in fear extinction showed that contextual fear extinction could be enhanced by stimulating muscarinic receptors in the amygdala (Boccia et al, 2009). Furthermore, the finding that both IL infusion and systemic injection of Scop produced equivalent disruption of extinction recall suggests that muscarinic receptors in IL are necessary for cued fear extinction. Consistent with our findings, Maruki et al (2003) showed that infusions of Scop into the prefrontal cortex before extinction of lever-pressing for food interfered with extinction recall the following day. In addition, it has been shown that following extinction of lever-pressing for food, the levels of acetylcholine in the prefrontal cortex are increased (Izaki et al, 2001). This suggests that stimulation of acetylcholine receptors in the prefrontal cortex is necessary for other forms of reversal learning besides fear extinction.
Although future studies are needed to discern exactly how the stimulation of muscarinic receptors in IL leads to fear extinction, our findings suggest that one mechanism involves increasing the excitability of IL neurons by blocking both M-type K+ channels and the K+ channels underlying the sAHP and fAHP. Furthermore, muscarinic receptors also inhibit inward rectifying Kir2 channels in IL neurons (Carr and Surmeier, 2007). Muscarinic receptor inhibition of various K+ channels would allow IL neurons to fire more readily to tone-induced synaptic inputs and may lead to Hebbian plasticity in IL, which could store the long-term memory of fear extinction. Consistent with this model, directly blocking M-type K+ channels with XE-991 during fear extinction enhances fear extinction (Santini and Porter, 2010) and stimulating muscarinic receptors with a systemic positive allosteric modulator increases the firing of mPFC neurons in vivo (Shirey et al, 2009). The formation of a long-term memory of fear extinction also requires IL stimulation of metabotropic glutamate type 5 receptors and beta adrenergic receptors, both of which also block the sAHP in IL neurons (Mueller et al, 2008; Fontanez-Nuin et al, 2011). Taken together, these findings suggest that interactions between multiple receptors are needed to increase the intrinsic excitability of IL neurons sufficiently to allow the plasticity needed for long-term memory of fear extinction. In support of this possibility, slice experiments have shown that interactions between muscarinic, beta adrenergic, and group 1 metabotropic glutamate receptors can synergistically enhance the intrinsic excitability of neurons (Gereau and Conn, 1994; Moore et al, 2009) and enhance induction of synaptic plasticity (Watabe et al, 2000; Seol et al, 2007).
In conclusion, we have shown that stimulation of acetylcholine muscarinic receptors increases IL neuronal excitability by reducing the M-current, the sAHP, and the fAHP. Furthermore, systemic and intra-IL blockade of muscarinic receptors during extinction impaired recall of extinction 24-h later, suggesting that this muscarinic receptor-mediated increase in IL intrinsic excitability during extinction is necessary for long-term extinction memory. As during extinction the stimulus contingency is suddenly changed from ‘tone-shock' to the novel ‘tone-no shock' association, it is plausible that cholinergic inputs to IL are stimulated to release acetylcholine to mediate attention/alertness processing related to this novel extinction-related association (Hasselmo and McGaughy, 2004; Sarter et al, 2005; Parikh and Sarter, 2008). In line with this, it has been shown that extinction training also increases the levels of other neurotransmitters involved in attention and arousal, such as dopamine and norepinephrine, in IL (Feenstra et al, 2001; Hugues et al, 2007).
Patients suffering from anxiety disorders such as post-traumatic stress disorder (PTSD) commonly exhibit relapse after behavioral psychotherapy, which is thought to be due to deficient consolidation of extinction memory (Charney and Deutch, 1996; Bechara et al, 1999; Milad et al, 2008; Milad et al, 2009). Understanding the cellular mechanisms of fear extinction might lead to the development of novel treatments, which when combined with behavioral therapy might facilitate consolidation of extinction and prevent relapse. Based on our current findings, pharmacological stimulation of muscarinic receptors with cholinergic agonists such as Cev might be useful for facilitating consolidation of extinction memory in PTSD patients.
Acknowledgments
We thank Joel O Nigaglioni and Danny Galarza for their assistance with the behavioral studies, and Dr Gregory J Quirk for his comments on the manuscript. This work was supported by NSF Grant IOS 0842159 to JTP and the RCMI Behavioral Core Facility, which was supported by grants from the National Center for Research Resources (5G12RR003050-26) and the National Institute on Minority Health and Health Disparities (8G12MD007579-27). MS was supported by the PSM RISE Program Grant NIH-NIGMS #GM082406.
The authors declare no conflict of interest.
References
- Bang SJ, Brown TH. Muscarinic receptors in perirhinal cortex control trace conditioning. J Neurosci. 2009;29:4346–4350. doi: 10.1523/JNEUROSCI.0069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Weiner I. Differential role of muscarinic transmission within the entorhinal cortex and basolateral amygdala in the processing of irrelevant stimuli. Neuropsychopharmacology. 2010;35:1073–1082. doi: 10.1038/npp.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros DM, Mello e Souza T, De David T, Choi H, Aguzzoli A, Madche C, et al. Simultaneous modulation of retrieval by dopaminergic D(1), beta-noradrenergic, serotonergic-1A and cholinergic muscarinic receptors in cortical structures of the rat. Behav Brain Res. 2001;124:1–7. doi: 10.1016/s0166-4328(01)00208-x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;811:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Baratti CM, McGaugh JL. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiol Learn Mem. 2009;91:93–97. doi: 10.1016/j.nlm.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann NY Acad Sci. 1999;868:370–378. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Cox CL, Metherate R, Ashe JH. Modulation of cellular excitability in neocortex: muscarinic receptor and second messenger-mediated actions of acetylcholine. Synapse. 1994;16:123–136. doi: 10.1002/syn.890160206. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Gloveli T, Muller W. Muscarinic control of dendritic excitability and Ca(2+) signaling in CA1 pyramidal neurons in rat hippocampal slice. J Neurophysiol. 1999;82:1909–1915. doi: 10.1152/jn.1999.82.4.1909. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Vogel M, Botterblom MH, Joosten RN, de Bruin JP. Dopamine and noradrenaline efflux in the rat prefrontal cortex after classical aversive conditioning to an auditory cue. Eur J Neurosci. 2001;13:1051–1054. doi: 10.1046/j.0953-816x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- Fisher A, Brandeis R, Karton I, Pittel Z, Gurwitz D, Haring R, et al. (+-)-cis-2-methyl-spiro(1,3-oxathiolane-5,3′) quinuclidine, an M1 selective cholinergic agonist, attenuates cognitive dysfunctions in an animal model of Alzheimer's disease. J Pharmacol Exp Ther. 1991;257:392–403. [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011;21:727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RWT, Conn PJ. A cyclic AMP-dependent form of associative synaptic plasticity induced by coactivation of beta-adrenergic receptors and metabotropic glutamate receptors in rat hippocampus. J Neurosci. 1994;14:3310–3318. doi: 10.1523/JNEUROSCI.14-05-03310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24:7718–7726. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hernandez CC, Zaika O, Tolstykh GP, Shapiro MS. Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol. 2008;586:1811–1821. doi: 10.1113/jphysiol.2007.148304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Huang ZB, Wang H, Rao XR, Zhong GF, Hu WH, Sheng GQ. Different effects of scopolamine on the retrieval of spatial memory and fear memory. Behav Brain Res. 2010;221:604–609. doi: 10.1016/j.bbr.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R, Lena I. Time course of extracellular catecholamine and glutamate levels in the rat medial prefrontal cortex during and after extinction of conditioned fear. Synapse. 2007;61:933–937. doi: 10.1002/syn.20448. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Dalmaz C, McGaugh JL. Amygdala beta-noradrenergic influences on memory storage involve cholinergic activation. Neurobiol Learn Mem. 1996;65:57–64. doi: 10.1006/nlme.1996.0006. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Hori K, Nomura M. Elevation of prefrontal acetylcholine is related to the extinction of learned behavior in rats. Neurosci Lett. 2001;306:33–36. doi: 10.1016/s0304-3940(01)01863-8. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99:217–228. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Krause M, Offermanns S, Stocker M, Pedarzani P. Functional specificity of G alpha q and G alpha 11 in the cholinergic and glutamatergic modulation of potassium currents and excitability in hippocampal neurons. J Neurosci. 2002;22:666–673. doi: 10.1523/JNEUROSCI.22-03-00666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Akema T, Nomura M. Effects of acetylcholine antagonist injection into the prefrontal cortex on the progress of lever-press extinction in rats. Neurosci Lett. 2003;351:95–98. doi: 10.1016/j.neulet.2003.07.012. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Cooper DC, Spruston N. Plasticity of burst firing induced by synergistic activation of metabotropic glutamate and acetylcholine receptors. Neuron. 2009;61:287–300. doi: 10.1016/j.neuron.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MH, Kim NS, Kim IH, Kim H, Kim HT, Choi JS. Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiol Learn Mem. 2010;94:206–213. doi: 10.1016/j.nlm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann NY Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Passani MB, Cangioli I, Baldi E, Bucherelli C, Mannaioni PF, Blandina P. Histamine H3 receptor-mediated impairment of contextual fear conditioning and in-vivo inhibition of cholinergic transmission in the rat basolateral amygdala. Eur J Neurosci. 2001;14:1522–1532. doi: 10.1046/j.0953-816x.2001.01780.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.1986The Rat Brain in Stereotaxic Coordinates2nd edn.Academic Press: San Diego [Google Scholar]
- Power AE, McIntyre CK, Litmanovich A, McGaugh JL. Cholinergic modulation of memory in the basolateral amygdala involves activation of both m1 and m2 receptors. Behav Pharmacol. 2003;14:207–213. doi: 10.1097/00008877-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30:12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophr Bull. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Facilitation of memory for extinction of drug-induced conditioned reward: role of amygdala and acetylcholine. Learn Mem. 2004;11:641–647. doi: 10.1101/lm.78504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Rigor BM. Protection by MK-801 against hypoxia-, excitotoxin-, and depolarization-induced neuronal damage in vitro. Neurochem Int. 1995;26:519–525. doi: 10.1016/0197-0186(94)00148-n. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Cardenas CG, Whittaker JA, Kitai ST. Muscarine reduces calcium-dependent electrical activity in substantia nigra dopaminergic neurons. J Neurophysiol. 2001;86:2966–2972. doi: 10.1152/jn.2001.86.6.2966. [DOI] [PubMed] [Google Scholar]
- Segal M, Fisher A. AF102B, a muscarinic M1 receptor agonist, mimics some effects of acetylcholine on neurons of rat hippocampus slices. Eur J Pharmacol. 1992;220:103–106. doi: 10.1016/0014-2999(92)90019-z. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ. Coactivation of beta-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci. 2000;20:5924–5931. doi: 10.1523/JNEUROSCI.20-16-05924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Paradoxical facilitation of object recognition memory after infusion of scopolamine into perirhinal cortex: implications for cholinergic system function. J Neurosci. 2006;26:9520–9529. doi: 10.1523/JNEUROSCI.2319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Han D, Carlen PL. Temporal specificity of muscarinic synaptic modulation of the Ca(2+)-dependent K+ current (ISAHP) in rat hippocampal neurones. J Physiol. 1996;496 (Part 2:395–405. doi: 10.1113/jphysiol.1996.sp021693. [DOI] [PMC free article] [PubMed] [Google Scholar]