Abstract
A large proportion of pulpal nociceptors are known to contain neuropeptides such as CGRP. However, the projection of non-peptidergic nociceptors to tooth pulp is controversial. Recently, the non- peptidergic subset of nociceptors has been implicated in mechanical pain in the skin. Since mechanical irritation of pulpal nociceptors is critical for evoking tooth pain under pathophysiological conditions, we investigated whether the non-peptidergic afferents project to tooth pulp as potential mechanotransducing afferents. For clear visualization of the non-peptidergic afferents, we took advantage of a recently generated knock-in mouse model in which an axonal tracer, farnesylated green fluorescence protein (GFP), is expressed from the locus of a sensory neuron-specific gene, Mrgprd. In the trigeminal ganglia (TG), we demonstrated that GFP is exclusively expressed in afferents binding to isolectin B4 (IB4), a neurochemical marker of non-peptidergic nociceptors, but is rarely co-localized with CGRP. Retrograde labeling of pulpal afferents demonstrated that a low proportion of pulpal afferents was co-localized with GFP. Immunohistochemical detection of the axonal tracer revealed that GFP-positive afferent terminals were densely projected into the tooth pulp. These results provide convincing evidence that non-peptidergic nociceptors are projected into the tooth pulp and suggest a potential role for these afferents in tooth pain.
Keywords: pain, nociceptor, dental pulp, immunohistochemistry, transgenic mice, Mas-related receptor MrgD
Introduction
Pain is the predominant sensation in teeth, regardless of the types of stimuli, and no spontaneous sensation arises from dental pulp in healthy teeth (Henry and Hargreaves, 2007). The environment of tooth pulp makes mechanosensitive nociceptors critical in the generation of tooth pain, because unique noxious mechanical stimuli are generated within tooth pulp under pathological conditions. A hydrodynamic stimulus applied to exposed dentin can cause dentinal hypersensitivity (Brannström, 1986). Increased intra-pulpal interstitial pressure under inflammation due to the limited compliance of pulpal tissues also evokes or aggravates tooth pain (Hakumaki and Närhi, 1973; Heyeraas and Berggreen, 1999). A cellular and molecular matrix of such mechanisms has been hypothesized (Hermanstyne et al., 2008; Magloire et al., 2010; Fried et al., 2011; Kim et al., 2011) but is still poorly understood.
Earlier studies on the neurochemical characterization of retrogradely labeled pulpal nociceptors in TG revealed their unique composition. Most pulpal afferents range in size from medium to large (Pan et al., 2003; Kvinnsland et al., 2004; Chung et al., 2011). The projection of peptidergic pulpal afferents has been well-characterized (Pan et al., 2003; Henry and Hargreaves, 2007). However, the presence or the extent of pulpal projection of non-peptidergic nociceptors that bind to the plant lectin isolectin B4 (IB4) has not been convincingly demonstrated and remains controversial in rats (Fried et al., 1989; Yang et al., 2002; Kvinnsland et al., 2004; Park et al., 2006). Demonstration of the projection of the two groups of nociceptors is highly significant, because recent studies have suggested that the peptidergic and non-peptidergic nociceptors constitute two parallel pain circuits, each with a unique function (Hunt and Mantyh, 2001; Zylka et al., 2005; Cavanaugh et al., 2009). For instance, recent works using the approach of selective ablation of a certain subset of primary afferents strongly suggest that IB4-positive non-peptidergic afferents play a critical role in the transduction of innocuous and noxious mechanical stimuli in skin (Abrahamsen et al., 2008; Cavanaugh et al., 2009). Therefore, the non-peptidergic nociceptors projecting to tooth pulp may be implicated as potential mechanosensitive pulpal nociceptors.
Recently, a knock-in mouse line exclusively labeling a major subset of non-peptidergic neurons was generated (Zylka et al., 2005). In these mice (Mrgprd∆EGFPf), an axonal tracer, farnesylated enhanced green fluorescent protein (EGFPf), is expressed from the locus of a sensory neuron-specific G-protein-coupled receptor, Mrgprd (Dong et al., 2001). A highly specific antibody against GFP clearly shows the afferent ‘terminals’, which overcome the technical limitation of labeling the non-peptidergic nerve terminals with IB4. In Mrgprd∆EGFPf mice, all GFP-positive afferents are co-labeled with IB4 and cover approximately 75% of IB4-positive afferents in dorsal root ganglia (DRG). Interestingly, GFP-positive fibers are projected exclusively to skin epidermis but not to other tissues, including corneas and visceral organs (Dong et al., 2001; Zylka et al., 2005). However, it is not known whether Mrgprd-expressing afferents are projected to tooth pulp.
The objectives of this study were to use immunohistochemical methods, combined with the novel transgenic mouse model, to determine the neurochemical properties of Mrgprd-expressing afferents in trigeminal ganglia (TG) and to examine whether the Mrgprd-expressing afferents project to tooth pulp.
Materials & Methods
Experimental Animals
The Mrgprd∆EGFPf mice were generously provided by Dr. David Anderson (California Institute of Technology, Pasadena, CA, USA). All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under a University of Maryland–approved Institutional Animal Care and Use Committee protocol. Five C57/bl6 and 15 Mrgprd∆EGFPf mice were used for the experiments. All experiments were carried out on adult mice (each weighing from 22 to 30 g).
Retrograde Labeling of Pulpal Afferents
The entire layer of enamel overlying the occlusal surface of the maxillary left first molar was carefully removed without exposure of pulp, following methods described previously (Chung et al., 2011). To remove the dentinal debris, we treated the tooth surface with 10% EDTA for 1 min. For retrograde labeling of pulpal afferents, crystals of Fluorogold (FG; Fluorochrome) were placed on the occlusal surface. To prevent leakage, we sealed the tooth surface with a light-cured resin (Maxcem; KerrUSA). The animals were sacrificed following a week for immunohistochemical studies. Three mice were used for the experiments.
Immunohistochemistry
The mice were transcardially perfused with 4% paraformaldehyde. The maxilla or mandible, including the teeth, was dissected and decalcified with 10% EDTA for 5 to 7 days at 4°C. The tissue was cryoprotected with 30% sucrose and cryosectioned at 30 µm in the tooth and at 12 µm in the TG. The conventional procedures of immumohistochemistry were performed. Since endogenous fluorescence signals from GFP were not detected from afferent terminals in decalcified tooth pulp, we performed immunohistochemical labeling using a specific antibody against GFP (1:2,000, rabbit; Invitrogen, Carlsbad, CA, USA). Although fluorescence from GFP was detected in TG sections, we decided to perform immunohistochemical staining using the anti-GFP antibody to amplify the signals. For double-labeling experiments in TG sections, we used GFP antibody raised in chicken (1:2,000; Aves Lab Inc., Tigard, OR, USA). As a marker of peptidergic afferents, we labeled TG sections with anti-CGRP antibody (1:1,000, rabbit; Millipore, Billerica, MA, USA). To investigate the expression of a nociceptive neuronal marker, we labeled using an antibody against P2X3, an ATP-gated ion channel (1:2,000, guinea pig; Millipore) or Transient Receptor Potential Vanilloid subtype 1 (TRPV1), a capsaicin receptor (1:2,000, goat; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). P2X3 and TRPV1 are expressed in tooth pulpal afferents (Alavi et al., 2001; Ichikawa and Sugimoto, 2004; Chung et al., 2011; Gibbs et al., 2011). We verified the specificity of these primary antibodies either by using genetically engineered mice lacking the expression of the gene of interest or by omitting the primary antibody (Appendix Fig. 1). The sections were incubated with appropriate secondary antibodies conjugated with fluorophores as indicated. For labeling IB4, we used IB4 conjugated with Alexa Fluor 594 (5 µg/mL, Invitrogen). We stained tooth sections with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei of pulp cells.
To count the labeling of neurochemical markers in TG, we used 4 to 5 sections from each ganglion per animal. Each set of markers was quantified in 2 animals. Following retrograde labeling, 4 alternative sets of TG sections were collected, and only 2 non-adjacent slides were counted for FG-labeled neurons and stained for examination of the neurochemical properties of pulpal afferents. FG signal was identified under gold filter (Chroma, Bellows Falls, VT, USA). Images were taken by optical sectioning fluorescence microscopy with appropriate filter sets (Zeiss Axiovert with Apotome, Carl Zeiss Microimaging GmbH, Oberkochen, Germany). We used ImageJ software for analysis. To count the number of TG neurons positively labeled, we determined a threshold intensity in each staining, which was uniformly applied to every section. For classification of neuronal size, we measured the area of the neurons in ImageJ and followed the criteria described elsewhere (small, < 300 µm2; medium, 300 to 600 µm2; large, > 600 µm2) (Ichikawa et al., 2006).
Results
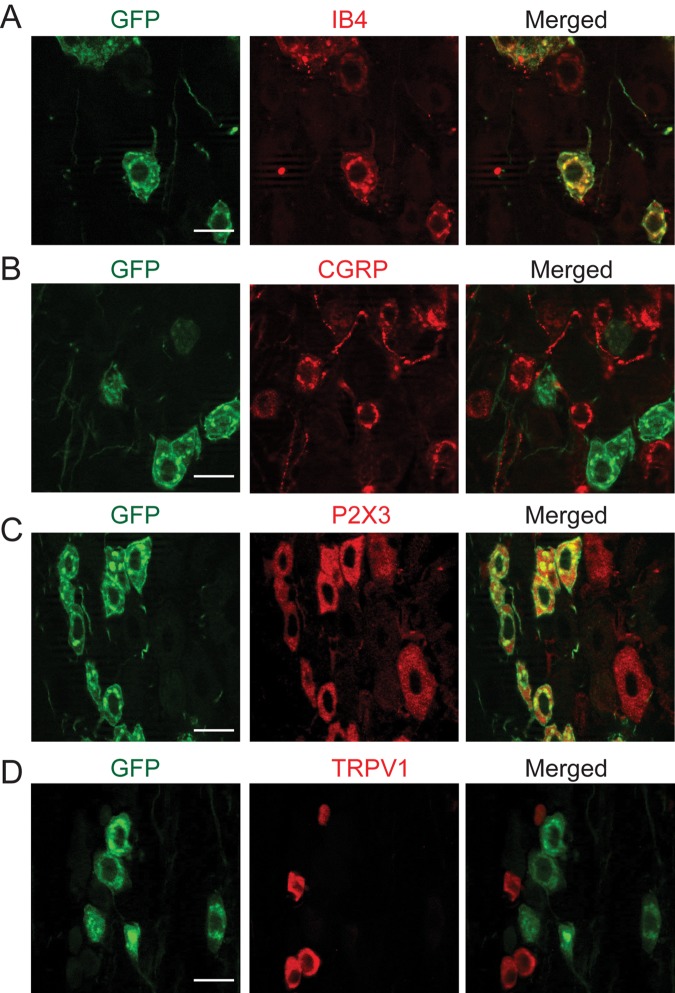
To determine the neurochemical properties of the Mrgprd-expressing afferents in TG, we performed immunohistochemical double-labeling in the TG of Mrgprd∆EGFPf mice. The sizes of GFP-positive neurons ranged from small (68.5%) to medium (31.3%), and the average was 270.4 ± 83.1 µm2 (mean ± standard deviation, n = 489). The entire population of GFP-positive neurons was co-labeled with IB4 (100%, 392/392 neurons from 2 TG; Fig. 1A), and most IB4-positive afferents were co-localized with GFP (75%, 392/525 neurons from 2 TG). In contrast, GFP-positive neurons rarely expressed CGRP (5%, 13/267 neurons from 2 TG; Fig. 1B). GFP-positive afferents were mostly co-localized with nociceptive neuronal marker P2X3 (95%, 132/139 neurons from 2 TG; Fig. 1C). In contrast, GFP-positive afferents were seldom co-localized with TRPV1 (4%, 15/353 neurons from 2 TG; Fig. 1D).
Figure 1.
Co-labeling of GFP with neurochemical markers in TG of Mrgprd∆EGFPf. Double-labeling in TG sections with anti-GFP (left panels in A to D) and IB4 (middle in A), anti-CGRP (B), anti-P2X3 (C), or anti-TRPV1 (D). Right panels show superimposed images. Images were obtained by optical sectioning at 0.5 µm. Scale bar, 20 µm.
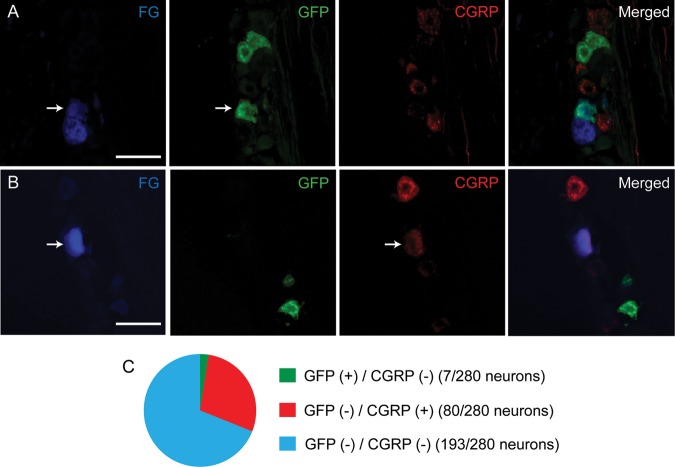
To determine whether Mrgprd-positive afferents are projected to tooth pulp, we performed retrograde labeling of pulpal afferents in Mrgprd∆EGFPf mice using FG. To estimate the proportions of non-peptidergic and peptidergic pulpal afferents, we simultaneously labeled GFP and CGRP in the same sections. We obtained 280 FG-labeled neurons from 3 ganglia from 3 mice. Among them, 2.5% of pulpal afferents were co-localized with GFP (Fig. 2A), whereas 28.6% of pulpal afferents were CGRP-positive (Fig. 2B). The Mrgprd-positive dental afferents were in the small (3/7 neurons) or medium range (4/7 neurons), and the average was 329 ± 108 µm2 (n = 7). Most of the CGRP-positive dental afferents were medium-sized (42.5%, 34/80 neurons), and the proportions of small and large CGRP-positive dental afferents were 31.2% (25/80 neurons) and 26.2% (21/80 neurons), respectively. No pulpal afferent showed co-localization of CGRP and GFP. Most dental afferents were co-localized with neither CGRP nor Mrgprd (68.9%, 193/280 neurons, Fig. 2C).
Figure 2.
GFP- and CGRP-positive pulpal afferents in TG of Mrgprd∆EGFPf mice. Examples of GFP-positive (A) or CGRP-positive (B) pulpal afferents identified by retrograde labeling with fluorogold (FG). Scale bar, 50 µm. Proportions of GFP (+) / CGRP (-) (green), GFP (-) / CGRP (+) (red), GFP (-) / CGRP (-) (blue) FG-labeled afferents are displayed in C.
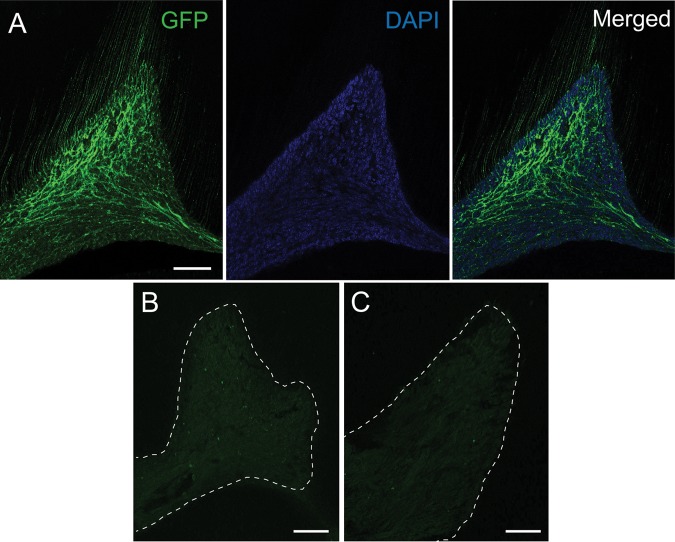
As another approach to demonstrate the projection of Mrgprd-positive afferents to tooth pulp, we examined whether GFP-positive afferent terminals are actually projected to tooth pulp. Immunohistochemical labeling of GFP in the decalcified sections of mouse molars from Mrgprd∆EGFPf mice revealed a dense projection of nerve fibers within pulp (Fig. 3A). The fluorescence signal was not observed when the primary antibody against GFP was omitted (Fig. 3B). The same set of primary and secondary antibodies did not display any signal except faint background in wild-type C57/bl6 mice lacking the expression of GFP (Fig. 3C). The immunohistochemical labeling of GFP was also robust in Mrgprd∆EGFPf mice when another secondary antibody conjugated with Cy3 was used, which was not observed in the absence of primary antibody (Appendix Figs. 1I, 1J).
Figure 3.
Projection of GFP-positive afferents to molar pulp of Mrgprd DEGFPf mice. A pulp horn of a decalcified tooth section stained with (A, C) or without (B) antibody against GFP followed by secondary antibody conjugated to Dylight488. Sections were obtained from either Mrgprd DEGFPf mice (A, B) or wild-type C57/bl6 mice (C). In panel A, labeling of GFP (left), DAPI (middle), and merged image (right) is shown. Control images (B, C) were obtained under identical settings used for taking the image shown in A. The images were obtained by optical sectioning at 2 µm. Dotted lines in B and C represent approximate demarcation between pulp and dentin. Scale bar, 50 µm.
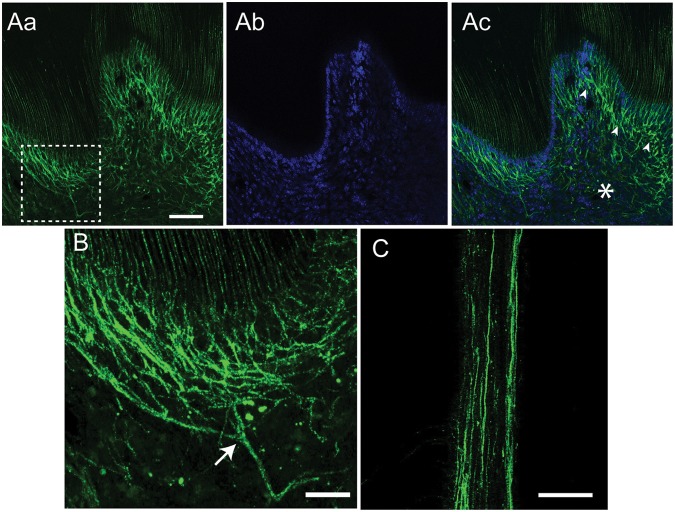
The GFP-positive nerve terminals were located at both coronal (Figs. 4A, 4B) and radicular pulp (Fig. 4C). The density of the GFP-positive terminals was higher in coronal pulp. The GFP-positive terminals formed a nerve plexus (arrowheads in Fig. 4Ac) next to an odontoblast layer, which was determined to be a layer of cells adjacent to dentin in DAPI staining (Fig. 4Ab). The density of GFP-positive nerve terminals was higher in the sub-odontoblastic region than in the core of the coronal pulp (asterisk in Fig. 4Ac). This may be due to the extensive branching of the GFP-positive afferents (Fig. 4B). The GFP-positive nerve terminals were also observed in the pulp of not only maxillary molars but also mandibular molars. Besides tooth pulp, GFP-positive terminals were also observed in intra-oral mucosa covering the gingiva and palate (Appendix Figs. 2A, 2B). However, GFP-positive afferents were absent in salivary glands (Appendix Figs. 2C-2E).
Figure 4.
Dense projection of GFP-positive afferent terminals in a sub-odontoblastic layer. (A, B) Coronal pulp of a decalcified molar was labeled with GFP (Aa) and DAPI (Ab). A merged image is shown in Ac. A dotted square in Aa indicates a region magnified in panel B. Arrowheads in Ac represent sub-odontoblastic plexus of GFP-positive nerve terminals, and an asterisk represents a core region of pulp. The image was obtained by optical sectioning at 2 µm. (B) A stacked image of 5 1-µm optical sections. An arrow represents the main branching point of nerve terminals. (C) A representative image of GFP-positive fibers in radicular pulp obtained by optical sectioning at 2 µm. Scale bar = 50 µm in A and C, 20 µm in B.
Discussion
In this study, we investigated the neurochemical properties of Mrgprd-expressing afferents in the TG. Immunohistochemical staining of the TG from Mrgprd∆EGFPf mice demonstrated that the primary afferents expressing Mrgprd in TG are small to medium-sized and are exclusively co-localized with IB4, but rarely with CGRP. Most of the Mrgprd-positive afferents in the TG were co-localized with P2X3 but not with TRPV1. These properties are consistent with the neurochemical characteristics of Mrgprd-positive afferents in the DRG (Zylka et al., 2005; Cavanaugh et al., 2009). Therefore, we confirmed that the expression of GFP driven by the Mrgprd promoter can be used as a reliable marker for IB4-positive non-peptidergic afferents in the TG.
Immunohistochemical studies combined with the selective genetic and retrograde labeling strategy revealed clear projection of a previously poorly defined subpopulation of afferents to mouse tooth pulp. The extent of the proportion of IB4-positive pulpal afferents in retrogradely labeled pulpal afferents is controversial. In rats, labeling of IB4 in TG sections showed that less than 5% of retrogradely labeled dental afferents are IB4-positive (Fried et al., 1989; Yang et al., 2002; Kvinnsland et al., 2004; Gibbs et al., 2011). In contrast, the percentages of IB4-positive pulpal afferents in cultured dental afferents were as high as 75% (Park et al., 2006; Kim et al., 2011). The source of such a discrepancy is not clear. It is possible that differences in experimental methods, e.g., labeling of IB4 in fixed tissue sections vs. cultured neurons, or labeling of pulpal afferents with different tracer dyes, might have affected the outcome. In the current study, we labeled pulpal afferents using FG and detected non-peptidergic pulpal afferents through immunohistochemical labeling of the genetically engineered tracer GFP. Using this approach, we found that pulpal afferents were 2.5% GFP-positive in mice. This confirms the results from the studies in rats reporting a rare population of IB4-positive pulpal afferents identified by approaches similar to those used in the current study (Fried et al., 1989; Yang et al., 2002; Kvinnsland et al., 2004; Gibbs et al., 2011).
Despite low proportions of GFP-positive pulpal afferents in the TG, immunohistochemical labeling of the axonal tracer allowed for clear visualization of Mrgprd-positive nerve terminals in tooth pulp. A series of control experiments demonstrated that the fluorescent signals are due not to the non-specific reaction of primary or secondary antibodies, but to specific labeling. So far, actual projection of nerve terminals of IB4-positive afferents to tooth pulp has not been demonstrated in rodents. Considering the low proportion of GFP-positive pulpal afferents, it appears to be contradictory that GFP-positive afferent terminals are densely projected in the tooth pulp. In the molar pulp of rats, however, a subpopulation of individual sensory axonal terminals arborizes extensively in a broad fan shape, and an arbor derived from a single axon innervates as broadly as 80 × 350 µm of the dentinal surface (type C pulpal afferents) (Byers, 1985). Although the neurochemical properties of such afferents are not known, we speculate that the GFP-positive population of neurons might form a large arbor within pulp, based upon the appearance of afferent terminals. Cutaneous afferents expressing MrgB4, another member of the IB4-positive Mrgpr gene family, are another example of such extensively arborizing afferents (Liu et al., 2007).
Our results clearly demonstrated that at least 3 types of afferents project to the mouse molar pulp, each showing distinct neurochemical properties. However, the roles of these afferents in tooth pain remain to be elucidated. Most of the TRPV1-expressing pulpal afferents are CGRP-positive (Gibbs et al., 2011), suggesting the possibility that some peptidergic afferents are transmitting thermal pain. Tooth injury induces branching of peptidergic afferent terminals and release of CGRP (Byers and Närhi, 1999), suggesting the possible involvement of peptidergic pulpal afferents in mechanical nociception as well. The Mrgprd-negative non-peptidergic afferents may constitute the bulk of the medium- to large-diameter pulpal afferents. Some of these may be myelinated afferents that were proposed to transmit mechanical pain within pulp (Fried et al., 2011), which needs to be proven. What could be the role of the Mrgprd-positive pulpal afferents? Mrgprd is expressed in non-peptidergic polymodal c-nociceptors in DRG (Rau et al., 2009). Recent studies have suggested that IB4-positive non-peptidergic afferents play a critical role in mechanical pain in skin (Abrahamsen et al., 2008). The selective destruction of Mrgprd-positive afferents showed impairment in cutaneous mechanical pain but not in heat pain (Cavanaugh et al., 2009). These reports, therefore, suggest the possibility that Mrgprd-positive afferents may contribute to tooth pain evoked by mechanical irritation of pulpal nociceptors. In the future, combination of the Mrgprd∆EGFPf model with other mouse models for selective ablation or activation of the Mrgprd-positive afferents (Cavanaugh et al., 2009; Wang and Zylka, 2009) will help us to determine the contributions of pulpal Mrgprd-positive afferents to nociceptive signal transduction in tooth pulp under pathophysiological conditions.
Acknowledgments
The authors thank Aicha Moutanni, Youping Zhang, and Sen Wang for technical assistance, and Drs. Yong Chul Bae and Jin Ro for helpful discussion.
Footnotes
This project is supported by NIH-NIDCR DE019694 and DE020866 to MKC, and GM087369 and NS054791 to XD.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, et al. (2008). The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321:702-705 [DOI] [PubMed] [Google Scholar]
- Alavi AM, Dubyak GR, Burnstock G. (2001). Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res 80:476-483 [DOI] [PubMed] [Google Scholar]
- Brannström M. (1986). The hydrodynamic theory of dentinal pain: sensation in preparations, caries, and the dentinal crack syndrome. J Endod 12:453-457 [DOI] [PubMed] [Google Scholar]
- Byers MR. (1985). Terminal arborization of individual sensory axons in dentin and pulp of rat molars. Brain Res 345:181-185 [DOI] [PubMed] [Google Scholar]
- Byers MR, Närhi MV. (1999). Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med 10:4-39 [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. (2009). Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA 106:9075-9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee J, Duraes G, Ro JY. (2011). Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res 90:1103-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. (2001). A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106:619-632 [DOI] [PubMed] [Google Scholar]
- Fried K, Arvidsson J, Robertson B, Brodin E, Theodorsson E. (1989). Combined retrograde tracing and enzyme/immunohistochemistry of trigeminal ganglion cell bodies innervating tooth pulps in the rat. Neuroscience 33:101-109 [DOI] [PubMed] [Google Scholar]
- Fried K, Sessle BJ, Devor M. (2011). The paradox of pain from tooth pulp: low-threshold “algoneurons”? Pain 152:2685-2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Melnyk JL, Basbaum AI. (2011). Differential TRPV1 and TRPV2 channel expression in dental pulp. J Dent Res 90:765-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakumaki MO, Närhi MV. (1973). Effect of intrapulpal pressure stimulation on the activity of sensory nerves of dental pulp. Acta Physiol Scand 88:584-586 [DOI] [PubMed] [Google Scholar]
- Henry MA, Hargreaves KM. (2007). Peripheral mechanisms of odontogenic pain. Dent Clin North Am 51:19-44 [DOI] [PubMed] [Google Scholar]
- Hermanstyne TO, Markowitz K, Fan L, Gold MS. (2008). Mechanotransducers in rat pulpal afferents. J Dent Res 87:834-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyeraas KJ, Berggreen E. (1999). Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med 10:328-336 [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. (2001). The molecular dynamics of pain control. Nat Rev Neurosci 2:83-91 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. (2004). The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res 998:130-135 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Matsuo S, Terayama R, Yamaai T, Sugimoto T. (2006). Aspartate-immunoreactive primary sensory neurons in the mouse trigeminal ganglion. Brain Res 1082:67-72 [DOI] [PubMed] [Google Scholar]
- Kim HY, Chung G, Jo HJ, Kim YS, Bae YC, Jung SJ, et al. (2011). Characterization of dental nociceptive neurons. J Dent Res 90:771- 776. [DOI] [PubMed] [Google Scholar]
- Kvinnsland IH, Luukko K, Fristad I, Kettunen P, Jackson DL, Fjeld K, et al. (2004). Glial cell line-derived neurotrophic factor (GDNF) from adult rat tooth serves a distinct population of large-sized trigeminal neurons. Eur J Neurosci 19:2089-2098 [DOI] [PubMed] [Google Scholar]
- Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. (2007). Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci 10:946-948 [DOI] [PubMed] [Google Scholar]
- Magloire H, Maurin JC, Couble ML, Shibukawa Y, Tsumura M, Thivichon-Prince B, et al. (2010). Topical review. Dental pain and odontoblasts: facts and hypotheses. J Orofac Pain 24:335-349 [PubMed] [Google Scholar]
- Pan Y, Wheeler EF, Bernanke JM, Yang H, Naftel JP. (2003). A model experimental system for monitoring changes in sensory neuron phenotype evoked by tooth injury. J Neurosci Methods 126:99-109 [DOI] [PubMed] [Google Scholar]
- Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, et al. (2006). Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem 281:17304-17311 [DOI] [PubMed] [Google Scholar]
- Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, et al. (2009). Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci 29:8612-8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zylka MJ. (2009). Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci 29:13202-13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Bernanke JM, Pan Y, Naftel JP. (2002) Expression of RET immunoreactivity by pulpal afferent neurons of the rat [abstract]. J Dent Res 82(Spec Iss A):291 http://iadr.confex.com/iadr/2002SanDiego/techprogram/abstract_18363.htm [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. (2005). Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45:17-25 [DOI] [PubMed] [Google Scholar]