Abstract
The effect of application mode on polymerization effectiveness of self-etch adhesives with different pHs has rarely been studied. We applied 2 self-etch adhesives—Adper Prompt L-Pop (APLP, pH ~ 0.8) and Adper Easy-Bond (AEB, pH ~ 2.5)—to dentin with or without agitation (dynamic or static application), to investigate photopolymerization efficacy on dentin, and to understand the role of chemical interaction/reaction between adhesives and dentin. Micro-Raman spectra and imaging were acquired across the dentin/adhesive (D/A) interface. The degree of conversion (DC) of each adhesive as a function of position was calculated. SEM-EDS was used to obtain the elemental distribution along the interface. Photopolymerization efficacies of the two self-etch adhesives on dentin were apparently different. APLP exhibited decreasing DCs as the distance from the D/A interface became greater for both application modes, while the DCs for the dynamic mode were much higher than those for the static mode. As for AEB, the DCs remained almost constant across the adhesive layer and showed no significant difference between two modes. Raman spectral analysis disclosed that the chemical interaction between dentin and adhesives was responsible for the observations. We also verified this by tracking the distribution of the elements Ca and P in the adhesive layers.
Keywords: self-etch adhesives, pH, degree of conversion, micro-Raman, demineralization, imaging
Introduction
Self-etch adhesives must permeate the smear layer and penetrate dental hard tissues by dissolution of hydroxylapatite (HAp) crystallites if reliable bonding is to be achieved (Van Landuyt et al., 2007; Dieng-Sarr et al., 2011). Etching capacity thus plays an important role in the evaluation and comparison of self-etch adhesives, which vary in their aggressiveness by virtue of composition and functional monomer concentration (Tay and Pashley, 2001; Moszner et al., 2005). Efforts have been made to classify the aggressiveness of self-etch adhesives as mild (pH > 2), intermediate (1 < pH < 2), and strong (pH < 1) (Van Meerbeek et al., 2003; Maeda et al., 2008). Depending on their pHs, the self-etch adhesives may differ in penetration depth (Van Meerbeek et al., 2011), D/A interfacial morphology (De Munck et al., 2005), and bonding strength (Shirai et al., 2005).
Despite diverse pHs, self-etch adhesive compounds are relatively less acidic in comparison with the etchants used in the etch-and-rinse systems. They may provide insufficient penetration beyond the smear layer into underlying dentin so that bond strengths could be compromised (Carvalho et al., 2005). Therefore, agitation has been recommended to address this issue. Agitation of adhesives on dentin might also help solvent and water to evaporate (Amaral et al., 2010), since these likely impair polymerization of the monomers within the demineralized substrates. Additionally, agitation could contribute to increased hybrid layer thickness (Finger and Tani, 2005), enhancement of the interaction between adhesive and tooth (Miyazaki et al., 1996), and removal of trapped air bubbles (Velasquez et al., 2006).
The unique design of self-etch adhesives incorporates phosphoric or carboxylic acid groups (associated with etching) with unsaturated double bonds (associated with polymerization) into a single molecule (Moszner et al., 2005) to achieve concurrent etching and priming/bonding functions. Therefore, besides monomer composition, factors such as adhesive pH and agitation might also have a pronounced effect on polymerization efficacy (Tay et al., 2003; Salz et al., 2005). However, detailed information about these processes is still unavailable. In this study, we applied 2 self-etch adhesives—Adper Prompt L-Pop (APLP, pH ~ 0.8) and Adper Easy-Bond (AEB, pH ~ 2.5)—to dentin with or without agitation, to investigate photopolymerization efficacy on dentin, and to understand the role of chemical interaction/reaction between self-etch adhesives and dentin in the above processes. The study tested the hypothesis that agitation would affect photopolymerization efficacy on dentin of the self-etch adhesives with different pHs.
Materials & Methods
Adhesive/Dentin Specimen Preparation
Eight extracted non-carious, unerupted human third molars stored at 4oC in phosphate-buffered saline (PBS) containing 0.002% sodium azide were used. The teeth were collected after the patients’ informed consent was obtained under a protocol approved by the University of Missouri Kansas City adult health sciences IRB. The occlusal one-third of the crown was removed by means of a water-cooled low-speed diamond saw (Buehler Ltd, Lake Bluff, IL, USA). A uniform smear layer was created on the dentin surface by the use of wet 600-grit silicon carbide sandpaper for 30 sec. The tooth specimens were then sectioned perpendicular to the abraded surfaces to obtain equal-sized rectangular slabs (10 × 2 × 1.5 mm). Each slab was notched from the middle position of the bottom side opposite the abraded surface for subsequent fracturing. The prepared dentin slabs were then treated with one of the 2 self-etch adhesives, APLP (3M ESPE, Seefeld, Germany) or AEB (3M ESPE) (adhesive composition in the Appendix). Each adhesive was applied to the abraded dentin surface with (15 sec, dynamic application) or without (15 sec, static application) agitation, gently air-dried for 5 sec, and light-cured for 10 sec (600 mW/cm2, Spectrum 800 halogen light, Dentsply, Milford, DE, USA) with glass coverslips on the top. The specimens obtained were abbreviated as APLP-WA, APLP-WOA, AEB-WA, and AEB-WOA for APLP with and without agitation, and AEB with and without agitation, respectively. To avoid water contamination and potential release of unreacted monomers during cutting, the slabs were not cut by the water-cooled saw; instead, they were fractured from the notches. The exposed D/A interfaces and adhesive layers were ready for the subsequent analyses.
Micro-Raman Spectroscopy
A LabRam HR 800 Raman spectrometer (Horiba Jobin Yvon, Edison, NJ, USA) with monochromatic radiation emitted by a He-Ne laser (a wavelength of 632.8 nm and excitation power of 20 mW) was used. The laser was focused through a 100× Olympus objective to obtain a beam diameter of ~1 µm. Raman spectra were acquired starting from the dentin toward the adhesive layer at 1-µm intervals. The area of interest that was mapped was 22 µm by 18 µm. All the spectra were obtained over the spectral region of 200-2000 cm−1 and with an acquisition time of 60 sec. The degrees of conversion (DCs) of the adhesives were calculated based on the band area ratios of 1640 cm−1 (stretching υ of C=C) to 1458 cm−1(deformation δ of CH2) (R1640/1458), according to the following equation (Zhang and Wang, 2012):
The bands at 960 and 1076 cm−1 were used to identify calcium phosphate complexes, which are the result of the reaction of acidic monomers with the dentin mineral (Penel et al., 1999, 2003). The obtained DC and band ratios of 960/1458 were statistically analyzed by a paired t test.
Scanning Electron Microscopy–Energy-dispersive X-ray Spectrometry (SEM-EDX)
The exposed dentin, D/A interfaces, and adhesive layers of the fractured beams were sputter-coated with a 20-nm layer of carbon, and then analyzed in a field emission SEM (Philips XL 30, Eindhoven, Netherlands) with EDX at 15 kV.
Results
Raman images based on R1640/1458 were generated (Figs. 1a-1d) to show the distribution of unconverted C=C bond content in the adhesive layers. The DCs of the adhesives (Eq.) were measured as a function of position (Fig. 1e). R1640/1458 images for APLP-WA (Fig. 1a) and APLP-WOA (Fig. 1b) were apparently different: The image of APLP-WOA showed higher R1640/1458 than that of APLP-WA in the adhesive layer, indicating higher remaining C=C bonds, especially in the adhesive region away from the D/A interface. In contrast, R1640/1458 images of AEB-WA (Fig. 1c) and AEB-WOA (Fig. 1d) were quite similar and uniform: Both showed comparably lower R1640/1458 than those of APLPs, and displayed no significant change of R1640/1458 with position. Within the 15-µm-thick adhesive region (starting from the D/A interface), the DCs of APLP-WA decreased from ~88% to ~83%, while those of APLP-WOA decreased from 78% to 52% (Fig. 1e). The adhesives AEB-WA and AEB-WOA, however, both exhibited higher and constant DCs (~96%) compared with APLPs.
Figure 1.
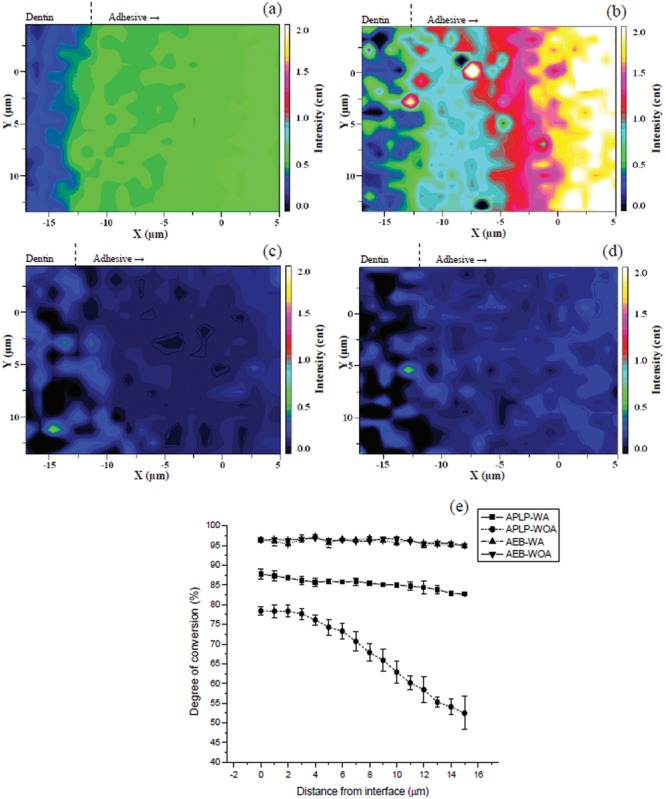
Micro-Raman images of the band area ratios of 1640 cm−1 (stretching υ of C=C) to 1458 cm−1 (deformation δ of CH2) (R1640/1458) as a function of spatial position for (a) Adper Prompt L-Pop applied with agitation (APLP-WA), (b) Adper Prompt L-Pop applied without agitation (APLP-WOA), (c) Adper Easy-Bond applied with agitation (AEB-WA), and (d) Adper Easy-Bond applied without agitation (AEB-WOA). (e) Profiles of degrees of conversion for (a), (b), (c), and (d) adhesives as a function of spatial position. The degrees of conversion of the adhesives were calculated based on the band area ratios of 1640 cm−1 to 1458 cm−1 by use of the Equation.
Representative Raman spectra of the adhesive layers (Fig. 2) conformed that the unpolymerized C=C band at 1640 cm−1 of APLP-WOA was higher than that of APLP-WA. In comparison, the same Raman bands of AEB-WA and AEB-WOA appeared to be similar in intensity, and both were lower than those of APLPs. When the Raman phosphate bands at 1076 and 960 cm−1 were compared, APLP-WA exhibited higher intensities than APLP-WOA, while AEB-WA and AEB-WOA showed relatively comparable, but lower, intensities of both bands than the former two.
Figure 2.
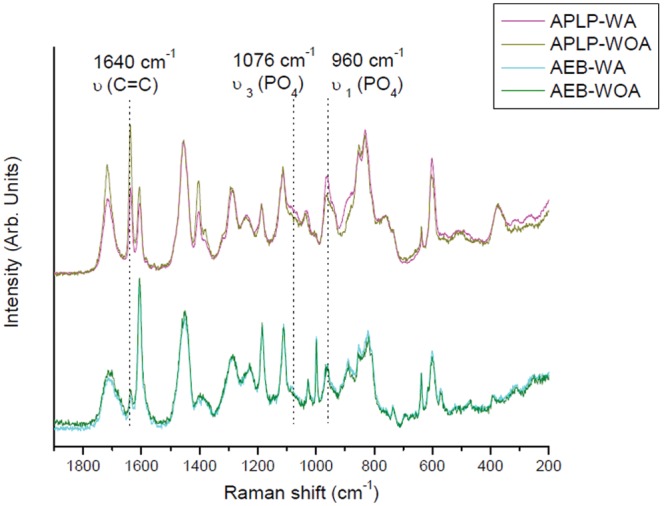
Representative micro-Raman spectra of Adper Prompt L-Pop (with and without agitation, APLP-WA and APLP-WOA) and Adper Easy-Bond (with and without agitation, AEB-WA and AEB-WOA) collected at positions 7 µm away from the dentin/adhesive (D/A) interfaces.
Figs. 3a-3d show Raman images of the band area ratios of 960 cm−1 (phosphate) to 1458 cm−1 (CH2) (R960/1458) for the 4 experimental groups. The quantitative plots of R960/1458 as a function of position are given in Fig. 3e. The overall level of R960/1458 for APLP-WA (Fig. 3a) was higher than that of APLP-WOA (Fig. 3b) (p < 0.0001), despite their similar decreasing trend of R960/1458 from the D/A interface into the adhesive layer (Fig. 3e). AEB-WA (Fig. 3c) and AEB-WOA (Fig. 3d) also showed decreasing R960/1458 within the same region as APLPs; however, both AEBs showed relatively lower levels of R960/1458 than APLPs.
Figure 3.
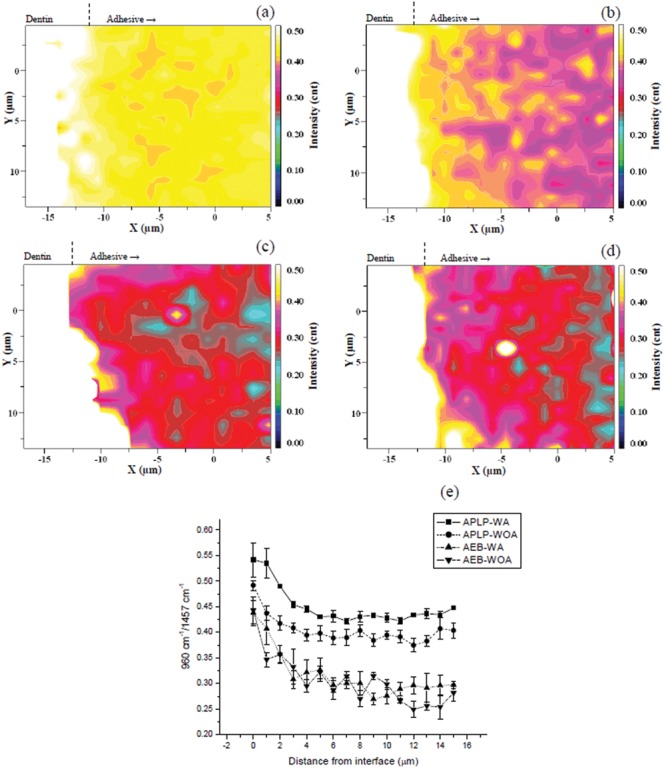
Micro-Raman images of the band area ratios of 960 cm−1 (stretching υ1 of PO4) to 1458 cm−1 (deformation δ of CH2) (R960/1458) as a function of spatial position for (a) Adper Prompt L-Pop applied with agitation (APLP-WA), (b) Adper Prompt L-Pop applied without agitation (APLP-WOA), (c) Adper Easy-Bond applied with agitation (AEB-WA), and (d) Adper Easy-Bond applied without agitation (AEB-WOA). (e) Profiles of band area ratios of 960 cm−1 to 1458 cm−1 (R960/1458) for (a), (b), (c), and (d) adhesives as a function of spatial position.
The elemental distributions of calcium (Ca) and phosphorus (P) along the dentin, D/A interfaces, and adhesive layers (Fig. 4a) indicated that the contents of both elements in the adhesive layers were generally in the order of: APLP-WA > APLP-WOA > AEB-WA > AEB-WOA. Representative EDX spectra collected at positions of ~7 µm away from the D/A interfaces toward the adhesive surface (Figs. 4b, 4c) indicated that the intensities of Ca and P peaks in the spectrum for APLP-WA appeared higher than those of APLP-WOA; the spectrum for AEB-WA also exhibited slightly higher peaks of Ca and P than AEB-WOA. However, the overall levels of both peaks for AEBs were much lower than those of APLPs.
Figure 4.
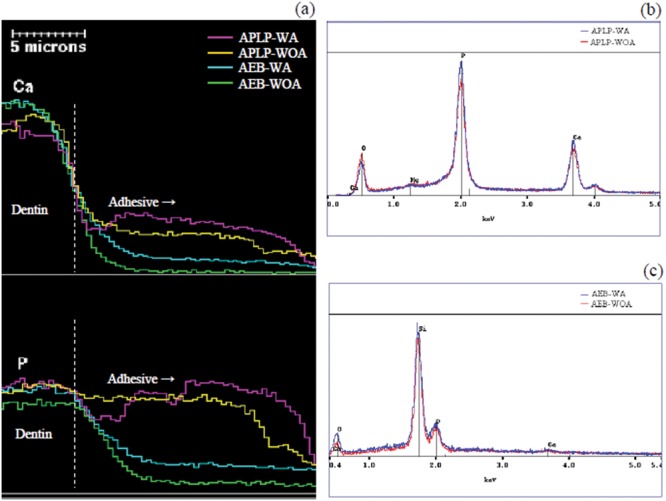
Energy-dispersive x-ray spectrometry (EDX) as a function of spatial position. (a) Profiles of contents of the elements calcium (Ca) and phosphorus (P) as a function of spatial position. EDX spectra of (b) Adper Prompt L-Pop (with and without agitation, APLP-WA and APLP-WOA) and (c) Adper Easy-Bond (with and without agitation, AEB-WA and AEB-WOA) collected at positions 7 µm away from the dentin/adhesive (D/A) interfaces.
Discussion
The results showed distinctly different polymerization performances of the 2 self-etch adhesives APLP and AEB on dentin in terms of their DCs, as well as the DC dependence on the distance from the D/A interface. The hypothesis that agitation affects photopolymerization efficacy of the self-etch adhesives was partially supported. Agitation apparently played different roles in polymerization of these adhesives. The DC of the lower-pH adhesive, APLP, was significantly dependent on agitation, while that of the mild adhesive, AEB, was not. The distinct polymerization of the 2 adhesives as well as the role of agitation may be ascribed to their different chemical reactions with dentin.
Greater chemical reaction with dentin occurred in APLP than in AEB. This could be confirmed by the intensity changes of the 960 and 1076 cm−1 bands. Both bands displayed higher intensities for APLP than for AEB, indicating a higher level of chemical reaction with dentin. The higher Raman intensity of APLP-WA than that of APLP-WOA with these 2 bands further suggested that agitation induced greater chemical reaction. The difference in chemical reactions of the 2 adhesives with dentin, in turn, influenced their respective initiator systems, and therefore their resulting DCs, differently. The negative effect of adhesive pH on initiator systems has been documented (Tay et al., 2003; Moszner et al., 2005). The acid-base reaction with the acidic monomers consumes the amines used in initiator systems, thus causing poor photopolymerization. For example, the lower pH of APLP could barely polymerize the resin: The DC of APLP was only 7.8% when light-cured on an inert glass substrate with a coverslip on the top (data not shown). However, when the self-etch adhesive was applied to dentin, the dentin mineral would buffer the acidic monomers, so that more amine(s) could survive the harsh acidity to initiate photopolymerization. Presumably, more buffering occurred with agitation, producing higher DC than could be achieved without agitation. This explained the polymerization behavior of APLPs. The decreasing gradient of DC for both APLP-WA and APLP-WOA may indicate a reverse pH gradient in the resin, being highest at the dentin and lowest at the adhesive surface. As for AEB, the influence of chemical reaction with dentin on DC was much less significant. We speculate that this could most likely be ascribed to the originally higher pH of AEB (~2.5 compared with ~0.8 of APLP), which already provided less acid-base reaction with the photoinitiators that allowed higher conversion.
EDX data also supplied significant evidence regarding the chemical reaction between the acidic monomers and dentin mineral. Ca and P are two major elements in dentin mineral (HAp) that play active roles in the processes of demineralization and acidic monomer-mineral interaction/reaction at the D/A interface (Fukegawa et al., 2006; Maeda et al., 2008). Tracking Ca and P content along the D/A interface and adhesive layer would enable us to understand these processes. In particular, the element Ca is originally absent in each adhesive and could come only from solubilized dentin apatite. Therefore, the distribution of Ca in the adhesive layer would offer unique information about the degree of dentin demineralization by acidic monomers. The result indicated that different amounts of Ca had diffusion from dentin into each adhesive layer, due to different degrees of demineralization. Moreover, lower pH and agitation favored higher Ca contents in the adhesive layers. The element P was already present in both mineralized dentin and the adhesives (in the form of methacrylated phosphoric esters). As a result, the distribution of P in each adhesive layer implied not only the P brought from dentin, but also the relative content of P in the adhesives. The EDX data were consistent with the Raman results.
Previous studies have indicated a close relationship between DCs and bonding strengths of adhesives to dental hard tissues (Kanehira et al., 2006; Faria-e-Silva et al., 2010). A high DC may also reduce permeability of the bonding assembly (Cadenaro et al., 2005) and increase interfacial durability and resistance to degradation (Navarra et al., 2009). Therefore, the current observations of the DC variation of self-etch adhesives depending on their pH and application mode are very important. Our results showed that the effectiveness of agitation in improving DC was strongly dependent on the initial pH of the self-etch adhesives used. The DCs of APLP could be raised from 52-78% to 83-88% if applied with agitation, in contrast to the almost-unchanged DC (96%) of AEB with/without agitation. Thus, the results implied that, for lower-pH adhesives such as APLP, application with agitation is especially essential to obtain maximal DCs in the adhesive layers to form reliable dentin bonding.
The DC of AEB was universally greater than that of APLP, and remained almost constant regardless of the application mode or the distance from the D/A interface. Besides pH, factors such as monomer composition (Appendix) also contributed to the higher DC. This characteristic suggested that the less acidic self-etch adhesive AEB was less technique-sensitive compared with the strong APLP adhesive, a trait particularly desirable in clinical applications. In comparison with strong self-etch adhesives, the advantages of mild self-etch adhesives have been well-documented, particularly with regard to bond durability (De Munck et al., 2006), hydrolytic stability (Inoue et al., 2005), and shelf life of storage (Van Meerbeek et al., 2011). In this study, the better polymerization performance of the mild self-etch adhesive AEB was also demonstrated. However, strong self-etch adhesives prevail over mild adhesives in that they can offer rather intense demineralization effects in both enamel and dentin. The intense and deep enamel etch pattern makes low-pH self-etch adhesives more effective for achieving micro-mechanical interlocking in the created porosities (Van Meerbeek et al., 2011). In fact, most of the mild self-etch adhesives required separate phosphoric-acid-etching on uncut enamel prior to adhesive placement. Therefore, proper selection and manipulation of the available self-etch adhesives are especially important in dental practices.
In conclusion, distinct photopolymerization efficacy on dentin of the self-etch adhesives with different pHs was observed. The involved mechanism was related to the chemical reaction/interaction of the acidic functional monomers with the mineral apatite in dentin. Agitation could effectively enhance photopolymerization efficacy of the lower-pH self-etch adhesives by facilitating dentin buffering of the acidity of the adhesives. The dissimilar polymerization behaviors discovered between the strong and mild self-etch adhesives should be considered when the adhesives are applied to dental substrates.
Footnotes
This investigation was supported by Research Grants 5T32DE7294-15 and R15-DE021023 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA.
The authors have no financial interest in the products, equipment, and companies cited in the manuscript.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Amaral RC, Stanislawczuk R, Zander-Grande C, Gagler D, Reis A, Loguercio AD. (2010). Bond strength and quality of the hybrid layer of one-step self-etch adhesives applied with agitation on dentin. Oper Dent 35:211-219 [DOI] [PubMed] [Google Scholar]
- Cadenaro M, Antoniolli F, Sauro S, Tay FR, Di Lenarda R, Prati C, et al. (2005). Degree of conversion and permeability of dental adhesives. Eur J Oral Sci 113:525-530 [DOI] [PubMed] [Google Scholar]
- Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C, Tay FR. (2005). A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials 26:1035-1042 [DOI] [PubMed] [Google Scholar]
- De Munck J, Vargas M, Iracki J, Van Landuyt K, Poitevin A, Lambrechts P, et al. (2005). One-day bonding effectiveness of new self-etch adhesives to bur-cut enamel and dentin. Oper Dent 30:39-49 [PubMed] [Google Scholar]
- De Munck J, Shirai K, Yoshida Y, Inoue S, Van Landuyt KL, Lambrechts P, et al. (2006). Effect of water storage on the bonding effectiveness of 6 adhesives to class I cavity dentin. Oper Dent 31:456-465 [DOI] [PubMed] [Google Scholar]
- Dieng-Sarr F, Sharrock P, Dabsie F, Gregoire G. (2011). Modifications of the organic and mineral fractions of dental tissues following conditioning by self-etching adhesives. J Dent 39:141-147 [DOI] [PubMed] [Google Scholar]
- Faria-e-Silva AL, Lima AF, Moraes RR, Piva E, Martins LR. (2010). Degree of conversion of etch-and-rinse and self-etch adhesives light-cured using QTH or LED. Oper Dent 35:649-654 [DOI] [PubMed] [Google Scholar]
- Finger WJ, Tani C. (2005). Effect of application mode on bonding performance of self-etching adhesives. Am J Dent 18:41-44 [PubMed] [Google Scholar]
- Fukegawa D, Hayakawa S, Yoshida Y, Suzuki K, Osaka A, Van Meerbeek B. (2006). Chemical interaction of phosphoric acid ester with hydroxyapatite. J Dent Res 85:941-944 [DOI] [PubMed] [Google Scholar]
- Inoue S, Koshiro K, Yoshida Y, De Munck J, Nagakane K, Suzuki K, et al. (2005). Hydrolytic stability of self-etch adhesives bonded to dentin. J Dent Res 84:1160-1164 [DOI] [PubMed] [Google Scholar]
- Kanehira M, Finger WJ, Hoffmann M, Endo T, Komatsu M. (2006). Relationship between degree of polymerization and enamel bonding strength with self-etching adhesives. J Adhes Dent 8:211-216 [PubMed] [Google Scholar]
- Maeda T, Yamaguchi K, Takamizawa T, Rikuta A, Tsubota K, Ando S, et al. (2008). pH changes of self-etching primers mixed with powdered dentine. J Dent 36:606-610 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Platt JA, Onose H, Moore BK. (1996). Influence of dentin primer application methods on dentin bond strength. Oper Dent 21: 167-172 [PubMed] [Google Scholar]
- Moszner N, Salz U, Zimmermann J. (2005). Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater 21:895-910 [DOI] [PubMed] [Google Scholar]
- Navarra CO, Cadenaro M, Codan B, Mazzoni A, Sergo V, De Stefano Dorigo E, et al. (2009). Degree of conversion and interfacial nanoleakage expression of three one-step self-etch adhesives. Eur J Oral Sci 117:463-469 [DOI] [PubMed] [Google Scholar]
- Penel G, Leroy N, Rey C, Lemaître J, Van Landuyt P, Ghanty N, et al. (1999). Qualitative and quantitative investigation of calcium phosphate of biological interest by raman microspectrometry. Rec Res Dev Appl Spectrosc 2:137-146 [Google Scholar]
- Penel G, Delfosse C, Rey C, Hardouin P, Jeanfils J, Delecourt C, et al. (2003). Raman microspectrometry studies of calcified tissues and related biomaterials. Dent Med Probl 40:37-43 [Google Scholar]
- Salz U, Zimmermann J, Salzer T. (2005). Self-curing, self-etching adhesive cement systems. J Adhes Dent 7:7-17 [PubMed] [Google Scholar]
- Shirai K, De Munck J, Yoshida Y, Inoue S, Lambrechts P, Suzuki K, et al. (2005). Effect of cavity configuration and aging on the bonding effectiveness of six adhesives to dentin. Dent Mater 21:110-124 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2001). Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent Mater 17:296-308 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yiu CK, Sanares AM, Wei SH. (2003). Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part I. Single-step self-etching adhesive. J Adhes Dent 5:27-40 [PubMed] [Google Scholar]
- Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. (2007). Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 28:3757-3785 [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. (2003). Adhesion to enamel and dentin: current status and future challenges. Oper Dent 28:215-235 [PubMed] [Google Scholar]
- Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. (2011). State of the art of self-etch adhesives. Dent Mater 27:17-28 [DOI] [PubMed] [Google Scholar]
- Velasquez LM, Sergent RS, Burgess JO, Mercante DE. (2006). Effect of placement agitation and placement time on the shear bond strength of 3 self-etching adhesives. Oper Dent 31:426-430 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y. (2012). Improved degree of conversion of model self-etching adhesives through their interaction with dentine. J Dent 40: 57-63 [DOI] [PMC free article] [PubMed] [Google Scholar]


