Abstract
Introduction
We previously showed that select cytokine gene polymorphisms are a significant predictor for pain reported at initial presentation in 446 white patients newly diagnosed with non–small cell lung cancer. This follow-up study explores the extent to which polymorphisms in tumor necrosis factor-α (TNF- α-308 G/A), interleukin (IL)-6 −174G/C, and IL-8 −251T/A could explain variability in pain and analgesic response among those patients (n = 140) subsequently referred for pain treatment.
Methods
Pain severity (0, no pain; 10, worst pain) was assessed at initial consultation and at follow-up visit. The total dose of opioids at the time of first-follow up visit (30 days postconsult) was converted to an equivalent dose of parenteral morphine.
Results
Forty-one percent (57 of 140) of the patients reported severe pain (score >7/10) at initial consultation (mean, 5.5), which significantly decreased to 25% (mean, 4) at first follow-up visit (McNemar = P < 0.001). Polymorphisms in TNF and IL-6 were significantly associated with pain severity (for TNF GG, 4.12; GA, 5.38; AA, 5.50; P = 0.04) and with morphine equivalent daily dose (IL-6 GG, 69.61; GC, 73.17; CC, 181.67; P = 0.004), respectively. Adjusting for demographic and clinical variables, variant alleles in TNFα −308 G/A remained significantly associated with pain severity (b = 0.226; P = 0.036) and carriers of the IL-6 −174C/C genotypes required 4.7 times higher dose of opioids for pain relief (odds ratio, 4.7; 95% confidence interval, 1.2;15.0) relative to GG and GC genotypes.
Conclusions
We provide preliminary evidence of the influence of cytokine genes on pain and response to analgesia in lung cancer patients. Additional studies are needed to validate our findings. The long-term application is to tailored pain therapies.
Introduction
Although improvements in the survival rates of patients with cancer have been observed in recent years, the majority of patients still experience distress and suffering. Pain is one of the most prevalent and debilitating symptoms experienced by cancer patients. Pain prevalence rates of 30% to 40% are reported for patients receiving active cancer treatment, which increases to 80% for patients with advanced cancer. Opioids are the drug of choice for cancer pain. However, huge inter-individual variation in opioid dose requirement is commonly observed. High doses of opioids can be neurotoxic, and repeated opioid dose escalation leads to increased side effects and tolerance. Thus, an improved characterization of patients most likely to respond to opioid analgesia will help facilitate early and more effective pain management.
Pain is a complex trait, and the discovery of pain modulatory mechanisms producing both analgesia and antianalgesia suggest the importance of several biological systems in modifying opioid analgesia. Inflammation caused by tumor-induced mediators, such as cytokines, has been suggested as a potential mechanism for cancer-related pain (1, 2) and conversely, in the development of opioid tolerance. Proinflammatory cytokines released by activated glial cells in response to inflammation, nerve trauma, or both, cause hyperexcitability in pain transmission neurons, and the exaggerated release of substance P and excitatory amino acids from presynapatic terminals produce an exaggerated pain response (3-6). Opioids, including morphine, also increase glial production of proinflammatory cytokines, which excite pain-responsive neurons and counterbalance analgesia by creating compensatory pain facilitation. The effect of cytokines on pain can therefore be hypothesized as bidirectional, causing an enhancement and facilitation of pain as well as increased opioid tolerance.
We recently reported that polymorphisms in IL-8 −251T/A (7) were significantly associated with pain severity in 446 newly diagnosed, previously untreated white patients with non–small cell lung cancer. In this study, we assess the extent to which functional variations in the same cytokine genes, TNF-α −308G/A, IL-6 −174G/C, and IL-8 −251T/A, could explain variability in analgesic response among those patients (n = 140) who subsequently received pain treatment and control from Supportive Care specialists during the period 2000 to 2005. Studies that elucidate the contributions of host genetic variability to analgesic response have high clinical significance.
Materials and Methods
Study Subjects
The study sample was drawn from an ongoing previously described case-control study of lung cancer (8). Case patients with histologically confirmed lung cancer were recruited at the time of initial registration at the Cancer Center and before initiation of radiotherapy or chemotherapy. There were no restrictions with regards to age, sex, ethnicity, or disease stage. All cases were residents of the United States. For this study, we restricted our analyses to a subset of lung cancer patients who were referred to, and received pain treatment from, Supportive Care specialists during 2000 to 2005. This study was approved by the institutional review board at M. D. Anderson Cancer Center and all participants provided written informed consent.
Epidemiology and Clinical Data Collection at Presentation to the Cancer Center
Trained M. D. Anderson staff interviewers collected data on demographics and smoking history at patient registration. Clinical data including stage of disease and history of comorbid conditions (heart disease, stroke, diabetes, emphysema, etc.) were abstracted from the patients’ charts. Depressed mood was assessed using an item from the SF-12, “during the past 4 weeks, have you felt downhearted and blue,” a validated measure of quality of life and one extensively used in studies of cancer patients (9-12).
Data Collection at the Supportive Care Center
Pain Assessment
Patients underwent a structured symptom assessment, medication review, and symptom management. Pain was assessed using an 11-point numeric scale (0, “no pain” and 10, “worst pain”) upon initial consultation and at follow-up visits. This pain item was taken from the Edmonton Symptom Assessment System, a tool frequently used in clinical care of palliative care patients (13-16). Patients rated their pain from 0 to 10 (0, no pain; 10, worst pain).
Follow-up Care
As a part of routine practice, nurses at the Supportive Care Clinic conduct follow-up care of patients by contacting the patient or caregiver about pain control and opioid side effects either by phone or during clinic visits. In the case of uncontrolled pain or the development of side effects, medical instructions are given accordingly by the palliative care physician who assessed the patient at the last visit. These instructions are given over the phone or sometimes the patient comes to the clinic or emergency room for evaluation.
Opioid Dose and Clinical Variables
Charts were reviewed for information on opioid dose. All patients received strong μ opioid receptor agonists including morphine, oxycodone, fentanyl, hydromorphone, and methadone. Due to the different types of opioids prescribed, we translated the daily opioid dose to morphine equivalent daily dose (MEDD). We used the conversion factors shown in Table 1 and calculated the total dose of opioids taken over the past 24 h at the time of first follow-up clinic visit.
Table 1.
MEDD conversion factors
| Opioid with route and dose | Conversion factor | MEDD (mg) |
|---|---|---|
| Morphine p.o. 1 mg | 1 | 1 |
| Morphine i.v. 1 mg | 3 | 3 |
| Hydromorphone p.o. 1 mg | 5 | 5 |
| Hydromorphone i.v. 1 mg | 10 | 10 |
| Oxycodone p.o. 1 mg | 1.5 | 1.5 |
| Methadone p.o. 1 mg | 10 | 10 |
| Methadone i.v. 1 mg | 10 | 10 |
| Fentanyl transdermal 1 μg/h | 2 | 2 |
| Fentanyl i.v. 1 μg | 0.3 | 0.3 |
NOTE: The total dose of opioids received over the preceding 24 h at the time of the first follow-up clinic visit was converted to an equivalent oral morphine dose in milligrams using the conversion factors shown above. The conversion factor for methadone is variable, and there is no single consensus conversion factor for this drug. For the purpose of this study, we used a conversion factor of 10.
Medical charts were also reviewed for the following: presence of neuropathic pain, presence of incident pain, presence of psychological distress, level of cognitive function, and addictive behavior, assessed using the CAGE questionnaire (17). These variables, included in the revised Edmonton Staging System (Table 2), have been shown to be prognostic of stable pain control (18). The revised Edmonton Staging System, developed by Fainsinger and colleagues, is a validated and clinically acceptable tool for pain classification and cancer pain populations’ comparison in research studies (19). It has a good predictive value with a moderate to high interrater reliability (from 0.67 to 0.95; refs. 13, 19). For this study, physician fellows in the Supportive Care Department at the Cancer Center (B.E.O. and H.P.) reviewed the medical charts as documented by the physicians in the Supportive Care Center who examined the patients. Neither fellow had participated in the care of these patients.
Table 2.
Edmonton Classification System for cancer pain (ECS-CP) definitions of terms
| Classification | Description |
|---|---|
| 1. Mechanism of pain | |
| No | No pain syndrome |
| Nc | Any nociceptive combination of visceral and/or bone or soft tissue pain |
| Ne | Neuropathic pain syndrome with or without any combination of nociceptive pain |
| Nx | Insufficient information to classify |
| 2. Incident pain | |
| Io | No incident pain |
| Ii | Incident pain present |
| Ix | Insufficient information to classify |
| 3. Psychological distress | |
| Po | No psychological distress |
| Pp | Psychological distress present |
| Px | Insufficient information to classify |
| 4. Addictive behavior | |
| Ao | No addictive behavior |
| Aa | Addictive behavior present |
| Ax | Insufficient information to classify |
| 5. Cognitive function | |
| Co | No impairment |
| Ci | Partial impairment |
| Cu | Total impairment |
| Cx | Insufficient information to classify |
NOTE: Reproduced from http://www.palliative.org/PC/ClinicalInfo/AssessmentTools/AssessmentToolsIDX.html.
Blood Collection and Molecular Analysis
As previously described, genotyping was done using the Taqman platform and the genotype distribution for the whole sample, and the results of the χ2 test for separation from Hardy-Weinberg equilibrium showed that there was no significant departure for any of the polymorphisms (7).
Statistical Analyses
The normality distribution of pain severity and morphine dose was assessed using one-sample Kolmogorov-Smirnov test. ANOVA and χ2 tests were used to assess the relationship between genotypes and pain severity and MEDD.
Multivariable regression analyses were conducted to assess the association between the genotypes (TNF-α −308G/A, IL-6 −174G/C, and IL-8 −251T/A) and pain severity at first follow-up visit and morphine consumption. Candidate variables included demographic variables (age, sex), clinical variables (stage of disease, treatment variables including surgery, chemotherapy and radiotherapy, comorbid conditions) and presence or absence of psychological distress, and level of cognitive function. Variable selection for the final model was conducted by using a backward elimination approach. With the goal of having the most parsimonious model, only variables with P < 0.05 were included in the final multivariable model. A significance level of 5% (two-sided) was used for all the final analyses.
Results
A total of 140 patients were referred to Supportive Care specialists for pain treatment and control. Males made up 54% of the sample, and the mean age was 60 years (SD, 13). Seventy-four percent had metastatic disease, 22% had surgery, 50% received chemotherapy, and 74% received radiotherapy. The majority (90%) of the sample had radiotherapy, surgery, and/or chemotherapy, and 58% reported one or more comorbid conditions (e.g., heart disease, stroke, diabetes). Of these, 20% (28 of 140) reported depressed mood at registration before receiving pain treatment and management at the Supportive Care Center.
The data were most complete during the first follow-up visit, around 30 days from the initial consultation. Mean pain at initial referral for consultation to the Supportive Care Center was 5.5 (SD, 3.0; median, 6; mode, 7) and at first follow-up visit was 4 (SD, 2.9; median, 4; mode, 2; P < 0.001). Using a score of ≥7 for the definition of severe pain, we observed that 41% of the entire sample reported severe pain at consultation and this significantly decreased to 25% at first follow-up clinic visit (Mc Nemar tests <0.01). Mean MEDD reviewed during the first follow-up visit was 89 mg/24 hours (SD, 119) and median was 50 mg/24 hours. The Kolmogorov-Smirnov Z test showed that pain severity at initial consultation (P = 0.103) and first follow-up visit (P = 0.092) were normally distributed but not for the MEDD (P = 0.001). A dose of >120 mg/24 hours was calculated as the 75th percentile for MEDD. Neither the mean nor the median pain was significantly associated with high MEDD, suggesting that stable control of pain was achieved.
We also found that at initial consultation, 42 (30%) patients were documented as having neuropathic pain and 16 (11%) patients had incidental pain. Twenty-six (19%) were identified as having delirium and 34 (24%) were CAGE positive. Sixty (43%) had psychological distress. When we assessed for association with MEDD, only the variable CAGE was associated with MEDD (P < 0.020) and thus was included in the multivariable analyses.
TNF-α, IL-6, IL-8, and Pain Severity at First Follow-up Visit
Polymorphisms in TNF were significantly associated with pain severity at first follow-up visit (TNF GG, 4.12; GA, 5.38; AA, 5.50; P = 0.04; Table 3A) for the total sample. Studies suggest sex differences in pain and analgesic responses (20-23); thus, we also analyzed if the associations between polymorphisms and pain severity and analgesic response vary by sex, recognizing that the population in this study was weighted toward men and the study may be underpowered for analyses of sex differences. When stratified by sex (Table 3B), we found statistically significant differences in pain severity by TNF genotypes for men and by IL-6 genotypes for women (P < 0.05).
Table 3. TNFα, IL-6, and IL-8 genotype distribution by pain severity.
| Genotype | n | Panel A
|
Panel B
|
|
|---|---|---|---|---|
| Total
|
Male
|
Female
|
||
| Mean (SD)* | Mean (SD)* | Mean (SD)* | ||
| TNFα −308G/A | ||||
| GG | 65 | 4.12 (2.83) | 4.00 (2.87) | 4.24 (2.82) |
| GA | 32 | 5.38 (3.11) | 5.78 (3.20) | 4.86 (3.0) |
| AA | 2 | 5.50 (0.70) | 5.00 | 6.00 |
| GA+AA | 34 | 5.38 (3.02)† | 5.74 (3.12)† | 4.93 (2.93) |
| IL-6 −174G/C | ||||
| GG | 56 | 5.00 (3.20) | 4.68 (3.21) | 5.40 (3.21) |
| GC | 33 | 4.30 (2.81) | 5.43 (3.18) | 3.47 (2.24) |
| CC | 11 | 3.42 (1.86) | 3.67 (2.5) | 3.40 (0.894)† |
| GC+CC | 44 | 4.11 (2.98) | 4.9 (3.04) | 3.46 (2.02)† |
| IL-8 −251T/A | ||||
| TT | 29 | 4.76 (3.26) | 4.94 (3.60) | 4.54 (2.90) |
| TA | 49 | 4.76 (2.70) | 5.00 (2.88) | 4.52 (2.55) |
| AA | 19 | 3.42 (2.81) | 3.40 (2.41) | 3.44 (3.35) |
| TA+AA | 68 | 4.76 (3.26) | 4.53 (2.82) | 4.24 (2.77) |
ANOVA.
P < 0.05.
We further assessed if the relationship between polymorphisms in TNF and pain severity at first follow-up visit persisted in the multivariable model. Candidate variables included demographic characteristics (age, sex), clinical variables (stage of disease, comorbid conditions), and depressed mood. We found that only polymorphisms in TNF remained as a significant factor for pain severity (b = 0.226; P = 0.036). We did not observe these associations to vary by sex (interaction term, P > 0.05).
TNF-α, IL-6, IL-8, and Differences in Pain Scores at Initial Consultation and First Follow-up Visit
We also assessed the relationship between polymorphisms in TNF-α, IL-6, and IL-8 and differences in pain ratings between the two assessment periods (pain at initial consultation and at first follow-up visit). Improvement in pain scores was observed for carriers of polymorphisms in IL-6 −174GC and IL-8 −251T/A. However, for TNF-α-308GA, the AA genotypes exhibited no improvement in pain scores relative to the GG and GA genotypes (Fig. 1).
Figure 1.
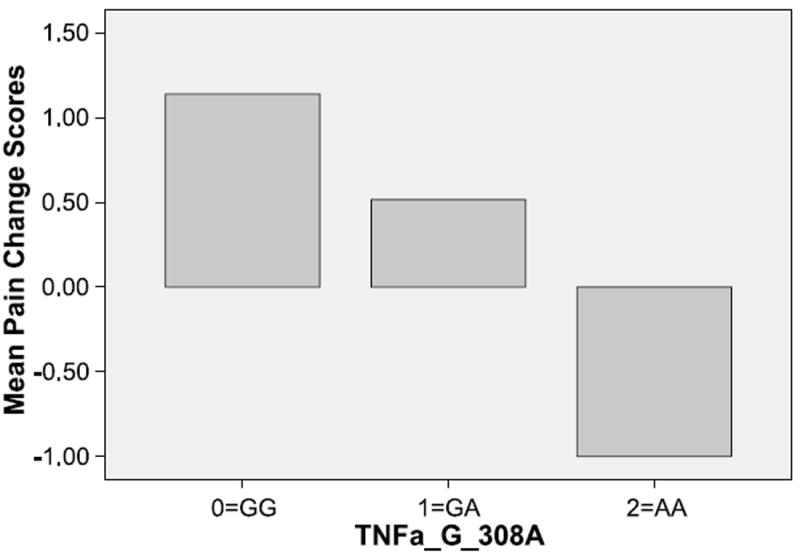
Pain score change (initial consultation and first follow-up visit) by TNF genotypes.
TNF-α, IL-6, IL-8, and Morphine Equivalent Daily Dose
Polymorphisms in IL-6 −174G/C were significantly associated with MEDD (IL-6 GG, 69.61; GC, 93.6; CC, 181.67; P = 0.004; Table 4A) at the univariate analyses of the whole sample. Although not statistically significant, a trend for higher MEDD by polymorphisms in IL-8 was also observed. Stratified by sex, Table 4B also shows that morphine dose varied by IL-6 genotypes among men but not for women. Men with CC genotypes required the highest morphine dose.
Table 4. TNFα, IL-6, and IL-8 genotype distribution by MEDD.
| Genotype | n | Panel A
|
Panel B
|
|
|---|---|---|---|---|
| Total
|
Male
|
Female
|
||
| Mean (SD)* | Mean (SD)* | Mean (SD)* | ||
| TNF α-308G/A | ||||
| GG | 76 | 85.00 (125.4) | 102.20 (157.81) | 66.76 (72.91) |
| GA | 38 | 79.72 (76.4) | 78.00 (82.05) | 81.88 (73.77) |
| AA | 2 | 37.5 (111.5) | 0.00 | 75.00 |
| GA+AA | 40 | 77 (76.4) | 74.29 (81.76) | 81.47 (71.43) |
| IL-6 −174G/C | ||||
| GG | 37 | 69.61 (77.4) | 72.89 (83.92) | 64.81 (68.06) |
| GC | 53 | 73.17 (93.6) | 75.56 (117.90) | 71.30 (71.97) |
| CC | 24 | 181.67 (228.0)† | 235.71 (107.39) ‡ | 106.00 (98.38) |
| GC+CC | 77 | 97.7 (140.0)† | 120.40 (188.19) | 77.5 (76.40) |
| IL-8 −251T/A | ||||
| TT | 64 | 75.00 (87.5) | 73.64 (95.11) | 77.00 (78.09) |
| TA | 41 | 78.02 (94) | 86.43 (112.49) | 68.60 (68.77) |
| AA | 12 | 102.71 (170.8) | 142.50 (227.68) | 62.92 (75.63) |
| TA+AA | 53 | 85.1 (122.5) | 103.25 (155.11) | 66.76 (70.06) |
ANOVA.
P < 0.005.
P < 0.05.
As mentioned, the Kolmogorov-Smirnov Z test showed that MEDD (P = 0.001) was not normally distributed. Using a dose of >120 mg/24 hours (calculated as the 75th percentile for MEDD as cut-point), multivariable logistic regression analyses showed that those who were carriers of the homozygous variant genotype, IL-6 −174C/C (odds ratio, 4.7; 95% confidence interval, 1.2; 15.0), received higher doses of morphine relative to GG or GC genotypes adjusting for the covariates (CAGE score, depressed mood, pain severity, stage of disease, comorbidities, age, and sex). We did not find any significant sex by genotype interaction.
Discussion
In this study, we found preliminary evidence for the importance of host genetic variability in cytokine genes on pain and analgesic response in patients receiving supportive care treatment. Specifically, we found that polymorphisms in TNF-308G/A and IL6 −174G/C may be important modulators of pain treatment and control. The magnitude of the association was most prominent with IL-6−174CC genotypes, with carriers requiring more than 4× the MEDD relative to heterozygotes and wild-type carriers, even after adjusting for factors known to influence pain relief. The IL-6 gene is localized to chromosome 7p21. The IL-6 −174G/C polymorphism affects transcription, altering serum levels of IL-6, with the C allele associated with significantly lower levels of plasma IL-6 (24, 25). Our findings provide preliminary support for a role of IL-6 in pain severity by showing that patients homozygous for the allele associated with lower plasma IL-6 levels required higher doses of opioids. To our knowledge this is the first study to provide empirical evidence of the important role of IL-6 −174G/C in analgesic response in cancer patients.
IL-6 induces analgesic effects in animal models of inflammation (26). Preclinical data first provided insight on the possible role of IL-6 in differences in opioid analgesia requirement. Bianchi et al. showed that IL-6 knockout mice had a reduced analgesic response to restraint stress. They also showed that the development of tolerance to the analgesic effect of morphine was more rapid in IL-6 –deficient mice and was accompanied by a reduction in the number of opioid receptors in the midbrain (19). Glial activation in response to chronic opioid use has also been suggested to lead to the production of cytokines contributing to the apparent loss of opioid analgesia effectiveness upon repeated opioid administration (tolerance) and leading to the development of opioid dependence (27). Spinal administration of IL-6 to rats with nerve injury has also been shown to result in antinociceptive effects, suggesting its potential as a modulator of pain (28). Clinical studies also show that patients with pain have elevated IL-6 levels (29).
We also observed a significant association with polymorphisms in TNF-308G/A and pain severity. The −308 polymorphism is a G → A substitution and reportedly affects gene expression, the rare A allele resulting in higher TNF production (30). TNF-α has been suggested to be critical for the development of inflammatory pain behavior in animal models. Blocking TNF signaling inhibits the development of mechanical allodynia and inflammatory pain. The novel therapeutic potential of TNF inhibitors has also been suggested for conditions such as brain cancer, epilepsy, and chronic pain (31-33). Anti-TNF therapy has also been shown to be profoundly analgesic, with an efficacy similar to that of cyclo-oxygenase-2 inhibition and reduced astrocyte activity in collagen-induced arthritis (34).
In the larger cohort whence this sample was drawn, we found that IL-8 −251T/A was significantly associated with pain severity at presentation, before receiving any cancer therapy (7). Although we also noted a trend for higher MEDD by polymorphisms in IL-8 −251T/A in this follow-up study, we found that polymorphisms in TNF-α-308G/A and IL-6 −174G/C were the significant correlates of pain during treatment and analgesic response, respectively. These findings potentially suggest the role of different pathways in the biology of pain and therefore have important implications in the clinical epidemiology of pain and its treatment. Alternatively, these findings may be viewed as arising from differences in methodology, e.g., the present analysis focuses on a subsample of the patients under chronic opioid therapy, who have had large doses of opioids, or the small sample (thus low power) may account for not detecting associations found in the first study. Nevertheless, taken together, these two studies suggest the importance of cytokines in the biology of pain and warrants further studies.
We also observed sex differences in the association between genotype groups and pain as well as for morphine dose. Specifically, we observed statistically significant differences in pain severity by TNF genotypes for men and by IL-6 genotypes for women and also observed that men with IL-6 −174CC genotypes needed the highest morphine dose. However, these differences did not persist in the multivariable analyses. Studies have shown sex differences in pain and analgesic responses (20-22). For example, there are sex differences in μ and κ opioid receptors with greater μ opioid responses in men and greater κ opioid responses in females (23). Examination of sex differences in cytokine polymorphisms and their relations to pain and analgesic responses should be further explored for the development of individualization of pain treatment.
It is important to note that we found that many of our patients who presented with severe pain at initial consultation still reported severe pain at follow-up (from 41% to 21%) despite opioid therapy. Given that opioids have debilitating side effects, finding potential genetic markers of opioid response would help in preventing unnecessary suffering in opioid-resistant patients.
Previous studies have also shown that psychological distress, presence of neuropathic pain, delirium, and addictive behaviors are markers of poor prognosis for pain control. In our sample, we found that the presence of addictive behavior, defined by CAGE positivity, was associated with the need for greater opioid dosages. However, this association did not persist in the multivariable analyses.
Our study has potential limitations. Variants in other cytokine genes that have been shown to modulate pain in other disease conditions (for example, IL-1 and back pain; ref.35) were not assessed in this study. Further, because IL-6 has both proinflammatory and anti-inflammatory effects, further elucidation of the mechanism by which it affects pain and analgesia is needed. It should also be noted that pain and inflammatory response is a complex process and numerous genes are likely involved. In the most likely clinical application, a combination of multiple polymorphisms would be more likely to be useful as predictors of pain and response to analgesia.
Among the strengths of our study is the assessment of several factors shown to be important in guiding pain treatment. Specifically, we evaluated stage of disease, sex, age, and comorbid conditions. In addition, the population we studied was a group of advanced-stage lung cancer patients referred to a Supportive Care program who all underwent structured symptom assessment and medication review. Although we have a small sample, subjects were not selected based on clinical pain criteria, thus reducing the possibility of selection bias. We could also assert that our study population (e.g., more male patients, the mean age of 60 years, and more advanced cancer patients) is similar to the general lung cancer population as reported in other studies (36).
In conclusion, pain is a complex trait, with several pathways as potential candidates for studying genetic influences. For example, drug metabolizing enzymes, drug transporters, and opioid receptors (37) or cyclo-oxygenases, or pathways involved in the perception and processing of nociceptive information (38), the modulation of the pharmacokinetics or pharmacodynamic effects of analgesics have been explained by genetic factors. We hopefully contributed to this understanding by showing in a preliminary analyses the potential influence of cytokine genes on pain and response to analgesia in lung cancer patients receiving pain treatment and control from Supportive Care specialists. Our findings need to be validated in large prospectively accrued populations and incorporating additional genetic markers in the cytokine pathway.
Acknowledgments
Grant support: CA109043 and CA55769 (M.R. Spitz) from the NIH/National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–31. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- 2.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–55. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 3.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–26. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 5.Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 6.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Gibby CC, Spitz M, Wu X, et al. Cytokine genes and pain severity in lung cancer: exploring the influence of TNF-308 G/A IL6-174G/C and IL8-251T/A. Cancer Epidemiol Biomarkers Prev. 2007;16:2745–51. doi: 10.1158/1055-9965.EPI-07-0651. [DOI] [PubMed] [Google Scholar]
- 8.Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92:1764–72. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 9.Vilaseca I, Chen AY, Backscheider AG. Long-term quality of life after total laryngectomy. Head Neck. 2006;28:313–20. doi: 10.1002/hed.20268. [DOI] [PubMed] [Google Scholar]
- 10.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 12.Wettergren L, Bjorkholm M, Axdorph U, Langius-Eklof A. Determinants of health-related quality of life in long-term survivors of Hodgkin’s lymphoma49. Qual Life Res. 2004;13:1369–79. doi: 10.1023/B:QURE.0000040790.43372.69. [DOI] [PubMed] [Google Scholar]
- 13.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 14.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–71. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Moro C, Brunelli C, Miccinesi G, et al. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–7. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 16.Stromgren AS, Groenvold M, Petersen MA, et al. Pain characteristics and treatment outcome for advanced cancer patients during the first week of specialized palliative care. J Pain Symptom Manage. 2004;27:104–13. doi: 10.1016/j.jpainsymman.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–7. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 18.Fainsinger RL, Nekolaichuk CL, Lawlor PG, Neumann CM, Hanson J, Vigano A. A multicenter study of the revised Edmonton Staging System for classifying cancer pain in advanced cancer patients. J Pain Symptom Manage. 2005;29:224–37. doi: 10.1016/j.jpainsymman.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi M, Maggi R, Pimpinelli F, et al. Presence of a reduced opioid response in interleukin-6 knock out mice. Eur J Neurosci. 1999;11:1501–7. doi: 10.1046/j.1460-9568.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 20.Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–25. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 22.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–7. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 23.Miaskowski C. Gender differences in pain, fatigue, and depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):139–43. doi: 10.1093/jncimonographs/lgh024. [DOI] [PubMed] [Google Scholar]
- 24.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 26.Czlonkowski A, Stein C, Herz A. Peripheral mechanisms of opioid antinociception in inflammation: involvement of cytokines. Eur J Pharmacol. 1993;242:229–35. doi: 10.1016/0014-2999(93)90246-e. [DOI] [PubMed] [Google Scholar]
- 27.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–46. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flatters SJ, Fox AJ, Dickenson AH. Spinal interleukin-6 (IL-6) inhibits nociceptive transmission following neuropathy. Brain Res. 2003;984:54–62. doi: 10.1016/s0006-8993(03)03092-0. [DOI] [PubMed] [Google Scholar]
- 29.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237:65–8. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 30.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor á promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997;94:3195–9. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–60. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7:R807–16. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor α. Br J Pharmacol. 1997;121:417–24. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inglis JJ, Notley CA, Essex D, et al. Collagen-induced arthritis as a model of hyperalgesia: Functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum. 2007;56:4015–23. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- 35.Solovieva S, Leino-Arjas P, Saarela J, Luoma K, Raininko R, Riihimaki H. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109:8–19. doi: 10.1016/j.pain.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Potter J, Higginson IJ. Pain experienced by lung cancer patients: a review of prevalence, causes and pathophysiology. Lung Cancer. 2004;43:247–57. doi: 10.1016/j.lungcan.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 37.Reyes-Gibby CC, Shete S, Rakvag T, et al. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130:25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotsch J, Geisslinger G. Current evidence for a genetic modulation of the response to analgesics. Pain. 2006;121:1–5. doi: 10.1016/j.pain.2006.01.010. [DOI] [PubMed] [Google Scholar]


