Abstract
D-serine is an endogenous neurotransmitter that binds to the NMDA receptor, thereby increasing the affinity for glutamate, and the potential for excitotoxicity. The primary source of D-serine in vivo is enzymatic racemization by serine racemase (SR). Regulation of D-serine in vivo is poorly understood, but is thought to involve a combination of controlled production, synaptic reuptake by transporters, and intracellular degradation by D-amino acid oxidase (DAO). However, SR itself possesses a well-characterized eliminase activity which effectively degrades D-serine as well. D-serine is increased two-fold in spinal cords of G93A SOD1 mice – the standard model of amyotrophic lateral sclerosis (ALS). ALS mice with SR disruption show earlier symptom onset, but survive longer (progression phase is slowed), in an SR-dependent manner. Paradoxically, administration of D-serine to ALS mice dramatically lowers cord levels of D-serine, leading to changes in onset and survival very similar to SR deletion. D-serine treatment also increases cord levels of the transporter Asc-1. Although the mechanism by which SOD1 mutations increases D-serine is not known, these results strongly suggest that SR and D-serine are fundamentally involved in both the presymptomatic and progression phases of disease, and offer a direct link between mutant SOD1 and a glial-derived toxic mediator.
Keywords: ALS, D-serine, serine racemase, excitotoxicity, SOD1, DAO, ASC-1
Introduction
One of the most significant biological roles identified for a dextro-rotatory amino acid in recent years is the neurotransmitter function of D-serine. Initially, it was recognized as a ligand for the “glycineB“ site of the NMDA receptor (Kleckner et al., 1988), but the physiological relevance of such agonism was unclear until documentation that the mammalian CNS contains significant levels of D-serine (Hashimoto 1995). Indeed, D-serine exists in higher concentration than does glycine in the areas of the brain where NMDA receptors are most numerous (Schell et al., 1995; Hashimoto et al., 1995). Serine racemase (SR), an enzyme which makes D-serine from L-serine, was originally reported to be expressed constitutively in protoplasmic astrocytes. For this reason, D-serine has been termed a “gliotransmitter.” Control of D-serine release from astrocytes is not well understood, but can be triggered by activation of astrocytic glutamate receptors of the AMPA/kainate classes (Schell et al, 1995) and appears to occur through vesicular mechanisms (Mothet et al 2005). We have documented a dramatic increase in the expression of SR and the production of D-serine in microglia activated by pro-inflammatory stimuli (Wu et al, 2004).
Binding of D-serine to the NMDA receptor greatly enhances the affinity for glutamate (Fadda et al 1988) and also prevents receptor desensitization (Lerma et al., 1990). Thus, any exacerbation of glutamate excitotoxicity by D-serine could be prolonged. Neuronal excitotoxicity is generally considered to result from excess synaptic glutamate, resulting in NMDA receptor over-stimulation and excessive Ca2+ influx that damages the mitochondria, ultimately leading to caspase-mediated cell death (Stout et al., 1998; Rao and Weiss, 2004). However, D-serine provides an alternative mechanism for producing excitoxicity without any change in glutamate levels per se (Heath and Shaw 2002).
The mechanisms regulating production, release, reuptake, and degradation of D-serine are not well understood. Serine Racemase (SR) is regarded as the primary source of D-serine in vivo, while D-amino acid oxidase (DAO) degrades D-serine and several other D-amino acids (Pollegioni and Sacchi 2010). However, SR has a well-characterized eliminase activity toward both D-serine and L-Serine, yielding deamination products similar to that of DAO (Foltyn et al., 2005). Thus, SR eliminase activity degrades D-serine in an irreversible manner, and is not simply a reverse of the racemase reaction. The ability to both produce and degrade D-serine makes SR a very unusual enzyme. Indeed, SR eliminase appears to be the primary mechanism for D-serine degradation in the absence of DAO (Strisovsky et al., 2005). Asc-1, an alanine-serine-cysteine transporter, is the predominant mediator of D-serine reuptake from the synapse (Rutter et al., 2007).
Amyotrophic lateral sclerosis (ALS) is an adult-onset disease characterized by selective death of motor neurons (MNs) in the spinal cord, leading to voluntary muscle paralysis and ultimately death. Mutation of Cu,Zn superoxide dismutase (SOD1) was the first confirmed cause of ALS, and led to the creation of the first animal model -- the G93A hSOD1 ALS mouse. The G93A ALS mouse has become the standard ALS model, and is the best-characterized. Mutant hSOD1 is endowed with a toxic gain of function (Gurney et al., 1994) that initiates non-cell-autonomous degeneration of spinal motor neurons through cross-talk with glial cells (Boillee et al., 2006; Di Giorgio et al., 2007). Glial-mediated excitotoxicity has been implicated as a toxic mechanism in ALS, as evidenced by elevated glutamate in ALS patients (Rothstein et al., 1990; Lin et al 1998), and the ability of EAAT2 (glutamate reuptake transporter) inducers to extend survival in mice and humans (Rothstein et al., 2005; Ganel et al., 2006). Nagai and co-workers (2007) reported on an astrocyte-derived factor that was toxic to motor neurons, and the value of identifying this factor, both in terms of disease pathogenesis and treatment, but did report its identity.
A recent study using microglia isolated from G93A mice showed increased D-serine levels and SR expression, relative to non-transgenic microglia (Sasabe et al., 2007). This suggests that motor neuron death may occur as a result of increased D-serine production by activated glial cells, possibly producing excitotoxicity without any change in glutamate. Interestingly, a recent study by Mitchell et al. (2010) involving one family suggests that mutations to DAO may be a cause ALS. Using the G93A ALS mice, we examined the role of D-serine in disease pathogenesis via two main approaches: 1) a null mutation of the SR gene (Srr−/−) and 2) administration of D-serine. Our results indicate that changes in SR expression and in D-serine impact both the development and progression of ALS-like symptomology in mSOD1 mice.
Materials and Methods
Animals
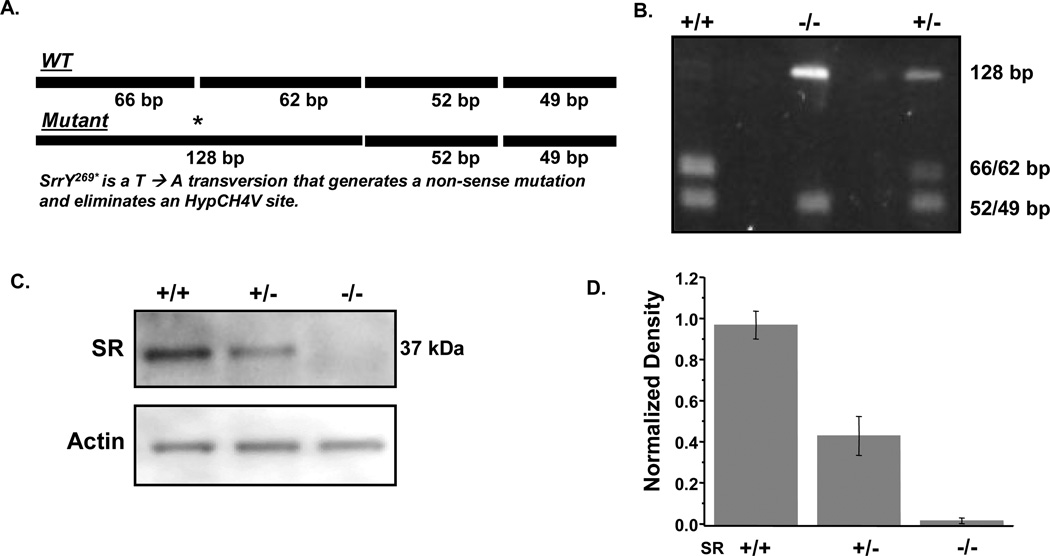
Breeding, care, and treatment of animals was governed by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee (IACUC) and complied with all NIH, USDA, and OLAW guidelines. Transgenic mice with the G93A mutation of the human SOD1 gene (G1H/1) mutation (B6SJL-TgN (SOD1-G93A) 1 Gur) were originally obtained from Jackson Laboratories (Bar Harbor, Maine), but have been bred and maintained locally for many generations using female B6SJL mice (Jackson Laboratory), according to protocol from the vendor. Approximately 40–50% of offspring are transgenic and determination of transgenic status was made via native, SOD1 activity gels utilizing whole blood obtained from tail nicks as described previously (Crow et al., 2005). G93A mice overexpress mutant hSOD1 enzyme by approximately eight-fold, thus G93A mice are easily distinguished from non-transgenic littermates. Male G93A mice were crossed with female Srr−/− produced by Labrie et al. (2009). Srr+/− offspring from the first generation (F1) were then crossed with the Srr−/− founders to produce both Srr+/− and Srr−/− offspring (F2). Presence of the SrrY269* mutation in the last exon was determined using a PCR-amplicon restriction endonuclease protocol as previously described (Labrie et al., 2009). Briefly, genomic DNA from tail pieces was PCR-amplified using the forward primer 5’-ACTAGACTCCGGCTCCGTTT-3’ and the reverse primer 5’CACCCAGTTCAGGGAGGTTA-3’ resulting in a 229 bp amplicon (Fig. 1a). A portion of the amplicon was digested overnight at 37 °C with the restriction enzyme HpyCH4V (NEB) and the fragments were analyzed using a 2% agarose gel in 1X TBE. Digestion analysis of the WT allele produced DNA fragments of 49, 52, 62, and 66 base pairs in length. The SrrY269* mutation in the mutant allele disrupted the first enzyme recognition site (as indicated in Fig. 1a) resulting in digestion products of 49, 52, and 128 base pairs. Since heterozygous animals harbor both alleles, digestion of the amplicon with HpyCH4V produced five fragments: 49, 52, 62, 66, and 128 base pairs.
Fig. 1. SR genotype determination and the expression of SR and hSOD1 in mouse spinal cords.
(a) Schematic representation of the PCR-amplified portion of the mouse Srr gene. Digestion with the restriction enzyme HpyCH4V produces fragments that are 49, 52, 62, and 66 base pairs. The SrrY269* (T-->A) mutation eliminated the first HpyCH4V recognition site resulting in three fragments of 49, 52, and 128 base pairs. (b) Representative agarose gel analysis containing HpyCH4V-digested DNA from wild-type (+/+, lane 1), homozygous mutant (−/−, lane 2) and heterozygous mutant (+/−, lane 3) mice. The 49 and 52 bp fragments appear as a single band, as do the 62 and 66 bp fragments. (c) Representative western blot of SR and actin expression in G93A mouse spinal cord extracts. (d) Densitometric analysis of SR expression from (c) in spinal cords of SR mutant G93A mice. Bars represent mean % integrated density value (IDV, see Methods for further detail).
Determination of symptom onset
Onset of symptoms is defined as first day of altered hind limb gait (overt motor weakness), comparable to the first symptoms seen in most ALS patients. When compared side-by-side with healthy mice, altered gait is readily apparent to even a casual observer, and is documented via videotape. Altered gait is typically associated with hind limb splaying (when suspended by the tail) and whole body tremors, which occurs very predictably at ~ 90 days of age in this model. The change in gait is caused by the mouse’s inability to support its hind quarter weight on its toes. The front limbs are unaffected at this time so the resulting “toe-to-heel” placement of the hind limbs produces a slower, swaggering, asymmetric gait which has proven to be a very reliable and unvarying indicator of ALS onset. It should be noted that, while traditional motor function measures such as rotarod and hanging wire can reveal onset in groups of mice via examination of trends before and after onset, they are not well-suited to measure onset in individual mice in real-time.
Determination of survival endpoints and survival interval (SI)
Mice were sacrificed when any of the following criteria were met: 1) inability to right themselves within 30 seconds when placed on their sides, 2) inability to eat or drink or move toward food and water placed in low-rimmed dishes on cage floor, 3) > 10% loss of body weight in 24 hours, 4) gross loss of grooming behavior, or 5) labored breathing. The age of symptom onset was subtracted from the sacrifice age for each mouse and a mean survival interval (SI) determined from each group. Survival Interval (SI) is a measure of the rate of disease progression, and is defined as the time from symptom onset to endstage disease and sacrifice (Crow et al., 2005). Decisions on when to sacrifice mice, in all treated groups and genotypes, were validated via a second observer who was blinded to the identity of the particular mouse. Because the decision to sacrifice (based on above criteria) is the sole endpoint in a survival study, having a blinded second opinion is tantamount to blinding throughout the duration of the study.
Grip-strength and body weight analysis
Mice were placed on a custom apparatus comprised of individually cordoned areas of wire mesh. Unlike typical grip-strength measurements which involve allowing mice to grip with fore limbs with the investigator pulling mice away to determine the point of release, this apparatus allows mice to grasp with all four limbs, and release is not forced but rather is a function of simple fatigue. Once the mice were allowed to grasp the wire mesh, the entire apparatus was then tilted at approximately 120 degrees for a maximum of 60 seconds per trial. Four trials were performed per mouse, with five minutes rest between each trial, and the average time suspended was recorded for each mouse at each trial. Each point represents the mean grip time ± S.E.M. NOTE that SR mutant non-transgenic littermates yielded a straight line at 60 sec. and, therefore, were omitted from the graph for clarity. Upon sacrifice of an animal, a “zero” measurement was recorded to maintain an equal n value throughout the study. Body weights were also measured at the time of grip-strength analysis, and the last recorded weight upon sacrifice was utilized throughout the remainder of the study to maintain equal n value.
Preparation of spinal cord homogenates
Animals were euthanized according to the protocol guidelines established by the University of Arkansas for Medical Sciences IACUC. Immediately upon sacrifice via guillotine, the spinal cord was dissected out by cutting the vertebral column just above the ilium. A 1-ml syringe was inserted at the lower opening and the intact spinal cord was expulsed with phosphate-buffered saline, pH 7.4, and immediately dropped into liquid nitrogen for storage at −80 °C until use. For biosensor and HPLC analyses, spinal cords were homogenized via an electric microhomogenizer in two volumes (v/wet weight of tissue) of ultrapure Millipore water and sonicated using a probe sonicator. Aliquots were then diluted 10-fold in water and ultrafiltered in 5-kD molecular weight cut-off centrifugal filters to remove proteins and prevent enzymatic degradation of low molecular weight substances. For SR, mSOD1 and actin immunoblots, spinal cords were homogenized in two volumes of ice cold RIPA buffer (Tris-HCl (50 mM) pH 7.6, NaCl (150 mM), EDTA (1 mM), sodium deoxycholate (1%), NP-40 (1%) and SDS (0.1%)) and subjected to 20,000 × g centrifugation at 4 °C for 15 min. For Asc-1 immunoblots, Triton was added to crude homogenates to ensure uniform solubilization. Protein concentrations were determined using the Coomassie Assay kit (Pierce) using BSA as a standard, and homogenates were stored at −70°C until analyzed.
Formulation of diet and treatment of animals
A previous toxicity study in our laboratory involving D-serine feeding to non-transgenic mice had shown evidence of liver pathology at a dose of 1,600 mg/kg/day. However, no pathology was noted at 16 or 160 mg/kg/day, thus 160 mg/kg/day was chosen as a treatment dose for G93A mice. One g of D-serine powder (MP Biomedicals) was dissolved in a minimal volume of water and added to 1 kg of chow (Harlan 7004) while chow was mixed in a food processor. Moist chow was then put into molds to dry, thereby repelleting the chow to the normal consistency. The final dose of D-serine amounted to 0.16 g/kg body weight/day. This dose is based on an average daily intact of 4 g of chow, which had been measured in our laboratory previously. G93A mice were fed ad libitum from time of symptom onset until time of sacrifice unless otherwise specified. Untreated control mice were provided Harlan 7004 chow ad libitum. All mice for a given study were derived from pooled littermates, sired by the same littermate males, and differing in age by 1–2 days. For studies involving onset administration of D-serine, mice were randomized into treatment and control groups based on symptom onset. That is, the first mouse to develop symptoms was placed in the treatment group, the second in a control group, the third in the treatment group, and so on. In this way, the mean age of onset is identical in each group.
Western Blots
Aliquots of spinal cords (35 µg) were run on 12% SDS PAGE gels and transferred to a PDVF membrane (100 volts for 1 hour). The membranes were then blocked in 5% milk for 30 minutes at 4°C rocking, followed by incubation with primary antibodies. SR antibodies were purchased from Santa Cruz Biotechnology, Inc. (A-17, sc-5751, 1:500), Asc-1 antibody was purchased from Abcam (Asc1:ab70627, 1:5000) and the actin antibody from Sigma-Aldrich (actin:A-3853, 1:10,000). The membranes were washed three times in Tris Buffered Saline containing 0.1% Tween-20 (TBS-Tween) and then incubated with the appropriate secondary antibody in 5% milk at 4°C rocking for one hour, followed by three washes in TBS-Tween. Following development with Lumigen solution A and B (GE Healthcare #RPN 2135), a FluorChem 8900 Alpha Innotech imager was used to visualize each blot, and to perform densitometry. The obtained integrated density value is the sum of all the pixel values after background correction, and %IDV is the percentage that each box contributes to the total density measured, taking background correction into consideration.
D-serine biosensor analysis
D-serine biosensors were manufactured according to procedures developed by Pernot et al (2008). Briefly, the enzyme DAO purified from the yeast Rhodotorula gracilis was immobilized on a platinum wire microelectrode. D-serine in solution (ultrafiltered spinal cord homogenates) diffuses through the probe membrane at the probe tip, and is degraded by yeast DAO into hydroxy-pyruvate, ammonia, and hydrogen peroxide (H2O2), and H2O2 is then oxidized at +500 mV vs. Ag/AgCl. The current (pAmps) produced by H2O2 oxidation is directly proportionally to the D-serine concentration in solution A standard curve of current versus D-serine concentration (using authentic D-serine) is prepared in the same buffer solution used for tissue measurements (PBS). The selectivity of D-serine measurements using these biosensors has been demonstrated previously in rat brain homogenates (Pernot et al., 2008), and confirmed herein.
DAO activity assay
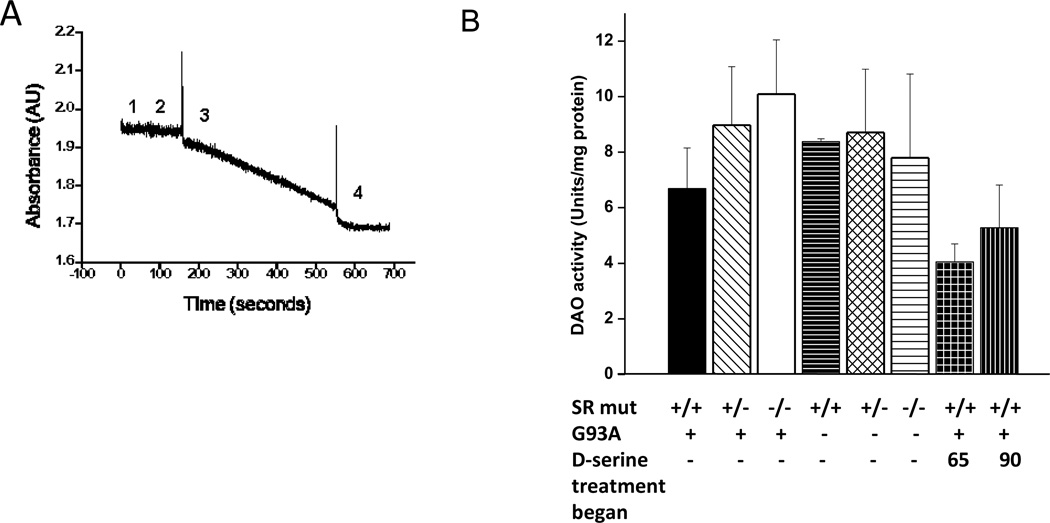
Mouse DAO catalyzes the oxidation of at least seven D-amino acids. However, in diluted tissue homogenates, these amino acids, if present at all, exist at concentrations which are orders of magnitude below their respective KM values and, therefore, produce background rates that are negligible. In vitro assay is accomplished by providing DAO with its primary cofactor, FAD, and a substrate (D-alanine, 30 mM) which yields lactic acid, ammonia, and hydrogen peroxide as products. For real-time monitoring, this reaction is coupled to lactate dehydrogenase (LDH), which uses NADH as a cofactor. Thus, the oxidation of NADH to NAD+ at 340 nm (extinction coefficient = 6,300 M−1cm−1) is followed by UV-visible spectrophotometry, and provides a quantitative measurement of DAO activity (Dawson et al., 1986). A premix of Tris-HCl, pH 8.0, NADH (0.3 mM), FAD (0.042 mg/mL), LDH (8.3 U/mL), and catalase (8.3 U/mL) was added to the cuvette at 37 °C. D-alanine (30 mM) was then added to initiate the reaction, and the rate was monitored. Once initial reaction rates were established, the DAO inhibitor benzoate (31 mM) was added so that only the NADH oxidation related to DAO activity was considered for calculation of enzyme activity. Extensive controls and order-of-addition reactions were carried out (using purified DAO) to validate this coupled in vitro enzyme reaction.
Statistical Analysis
All values are shown as the mean ± SEM unless otherwise indicated. Data were analyzed using OriginPro (Version 7, OriginLabs) software, and comparisons between D-serine treated and untreated littermate controls including symptom onset, survival intervals, and D-serine levels, were made using the unpaired Student’s t-test with each individual D-serine treated group analyzed separately. Comparisons between the six different SR mutant genotypes including symptom onset, survival intervals, D-serine levels, SR expression, and DAO activity, were made using one-way ANOVA tests with Bonferroni and Tukey post hoc pairwise comparisons. In all cases, P < 0.05 was considered to be statistically significant. Statistical significance and individual P-values are indicated in each graph.
Results
G93A mice with SR deletion display earlier symptom but survive longer than G93A controls
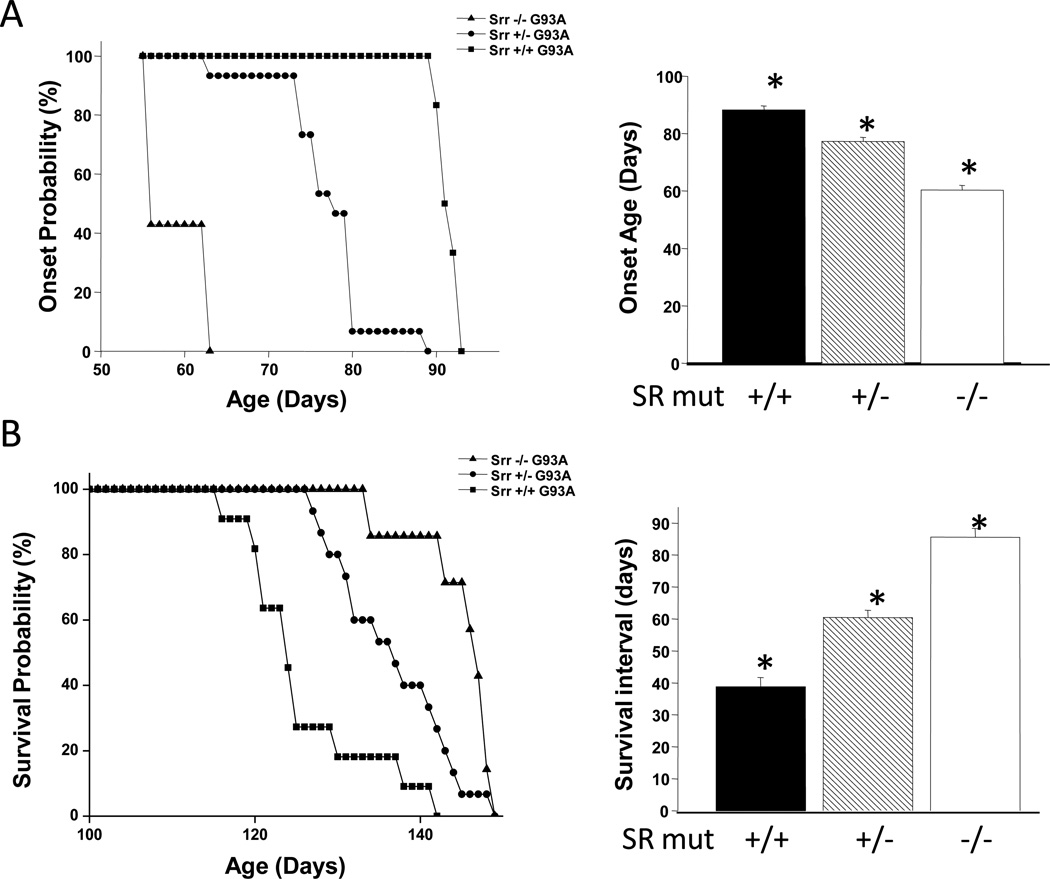
Female mice with a targeted disruption of the SR gene (Srr−/−) were crossed to male mice which were hemizygous for the G93A mutant of SOD1 (G93A+/0). Henceforth the superscripts will be omitted from “G93A+/0”, and all designations will be simply “G93A”. From two litters of offspring (F1), twelve Srr+/−G93A mice were identified by genotyping, and diminshed or absent SR protein expression was confirmed by western blotting and desitometric analysis (Fig. 1b–d). Srr+/−G93A mice were carefully examined at intervals and, based on standardized criteria for symptom onset (overt motor weakness), all twelve mice showed classic symptoms of motor neuron disease (MND) significantly earlier than did Srr+/+G93A. Srr+/−G93A mice (n = 12) showed onset at 77.3 ± 1.4 days, relative to 90.2 ± 1.5 days for Srr+/+G93A mice (n = 11, Fig. 2a). Despite earlier symptom onset, the progression phase was slowed significantly in Srr+/−G93A mice, causing them to live longer than Srr+/+G93A mice by 11 days (Fig. 2b). However, it is important to examine the changes in terms of the rate of disease progression – the more clinically relevant measure. A simple expression of the symptomatic or progression phase of MND is the survival interval (SI), which is defined as the time from overt symptom onset to sacrifice. Using that measure, Srr+/−G93A mice had a mean SI = 60.5 ± 2.2 days, relative to an SI = 38.8 ± 2.9 days for Srr+/+G93A (Fig. 2b). Thus, even a 50% decrease in SR significantly slowed disease progression. As has been reported previously, SR disruption per se produces no overt motor phenotype (Basu et al., 2009; Labrie et al., 2009), and both Srr+/− and Srr−/− mice generated in this study are indistinguishable from wild-type littermates in terms of motor function. Subtle changes in behavior (interpreted as mild “anxiety”) were reported in SR knockout mice considerably older than those used in this study.
Fig. 2. Phenotypic effects of SR disruption on G93A mice; hastening of onset, but slowing of progression.
SR deletion hastens symptom onset but extends overall survival in an SR gene-dependent manner in G93A mSOD1 mice. (a) Onset probability curves for SR mutant × G93A mice, as compared to untreated G93A mice. Mean age of onset was 38.8 ± 2.9 days, With each 50% SR knockdown, the onset probability curve was shifted to the left by approximately 15 days. Inset: Bars represent mean age of onset in days ± S.E.M. for each genotype. (b) Survival probability curves for each genotype listed in part (a). Despite the earlier symptom onset of SR mutant G93A mice, the survival probability curves were shifted to the right representing an extension of survival. Inset: Bars represent the average survival interval ± S.E.M. Survival Interval (SI) is a more direct measure of the second, progression phase of motor neuron disease. Total Srr+/− mice in this figure represents the sum of F1 and F2 offspring. n values = 11 (7 M/4 F), 15 (6 M/9 F), and 8 (2 M/6 F) for Srr+/+, Srr+/−, and Srr−/−, respectively. * P < 0.001.
Male Srr+/−G93A mice were bred to founder Srr−/− females, yielding eight Srr−/−G93A mice, as well as three additional Srr+/−G93A mice. The three Srr+/−G93A mice were carefully tracked and found to have early symptom onset and extended survival, virtually identical to the F1 generation. (Fig. 2a,b shows the combined onset and SI for all 15 Srr+/−G93A mice from F1 and F2 offspring). All eight Srr−/−G93A mice showed symptom onset even earlier (mean onset = 63 ± 1.6 days, Fig. 2a) than Srr+/−G93A. However, the rate of disease progression was slowed even more, such that the net effect was to live longer, with Srr−/−G93A living 20 days longer than Srr+/+G93A.mice. In terms of survival interval, Srr−/−G93A progressed 2.2X slower, with an SI equal to 85.6 days, relative to 38.8 for Srr+/+G93A mice (Fig. 2b) Comparison of survival intervals of the three genotypes strongly indicates that the net effect of SR deletion is to produce earlier symptom onset, but then to markedly slow disease progression, both in a gene dosage-dependent manner.
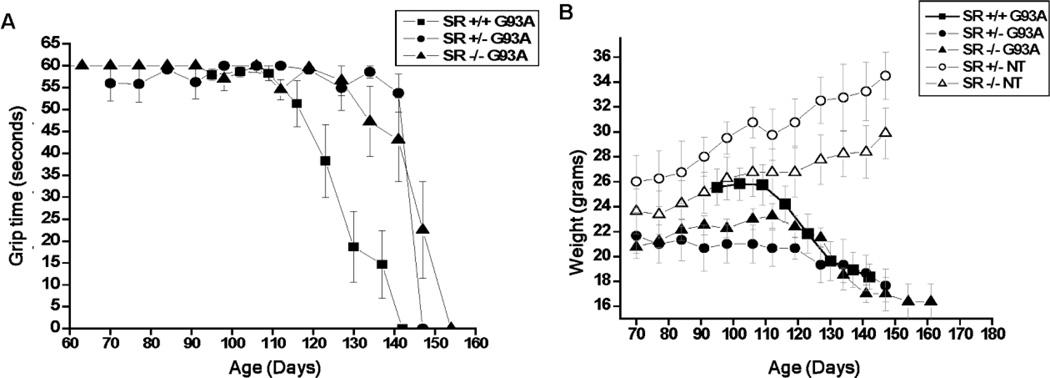
SR deletion slows the rate of motor function loss and body weight in G93A mice
Slowing of the rate of disease progression in both Srr+/−G93A and Srr−/−G93A mice was also evidenced by measurements of both grip strength and body weight (Fig. 3a,b). Despite earlier onset and obvious weakness, Srr+/−G93A and Srr−/−G93A mice retained use of hind limbs much longer, compared to age-matched Srr+/+G93A mice that display partial to complete hind limb paralysis. Body weight measurements revealed that both Srr+/−G93A and Srr−/−G93A lost less total weight and lost it more slowly than did Srr+/+G93A. Both Srr+/− and Srr−/− mice steadily gained weight over this time period (similar to wild-type non-transgenic mice), consistent with SR deletion alone producing no adverse motor or feeding behavior phenotype during the relevant mouse ages. The absence of any overt phenotype has been reported previously with this specific Srr mutation, as well as with two other SR knockout mouse strains created using different approaches (Basu et al., 2009; Labrie et al., 2009; Mustafa et al., 2010; Inoue et al., 2008).
Fig. 3. Grip-strength (four-paw) and body weight comparisons of G93A and non-transgenic (NT) mice with or without SR deletion.
(a) Grip strength was measured at intervals throughout symptom onset and disease progression using a custom wire mesh apparatus that enabled the use of all four paws, as described in methods. Srr+/− and Srr−/− G93A mice maintained grip strength about 20 days longer than the Srr+/+ G93A controls. Each point represents the mean grip time ± S.E.M. NOTE that SR mutant non-transgenic littermates yielded a straight line at 60 sec. and, therefore, were omitted from the graph for clarity. (b) Comparison of body weight changes in SR mutant G93A and non-transgenic littermate mice. SR mutant G93A mice lost weight at a later age despite having an earlier onset. Each point represents average weight ± S.E.M.
D-serine treatment of G93A mice produces early onset and slowed progression
A separate study involving administration of D-serine (160 mg/kg/day in chow) to G93A mice was carried out. Quite unexpectedly, mice fed D-serine also exhibited earlier symptom onset and significantly slower disease progression (Fig. 4). When D-serine treatment was begun at 65 days of age, G93A mice became symptomatic only 13 days later, with a mean onset = 78.4 ± 0.5 days, relative to 92 ± 0.5 days for control G93A mice fed the same chow without added D-serine (Fig. 4a,b). Despite early onset, disease progression was slowed such that total lifespan was slightly longer than untreated G93A mice; (SI = 53.5 ± 4.4 days for D-serine treatment, relative to 35.7 ± 4.7 days for untreated). The effect on slowing disease progression was even more apparent when a separate group of G93A mice was treated with D-serine beginning at normal symptom onset (90 days). Mice treated with D-serine at symptom onset (90 days) survived for an additional 55.7 ± 4.2 days, relative to 35.7 days for untreated G93A controls. In terms of slowed progression after onset (SI), the effect of D-serine treatment was virtually identical, regardless of whether mice were treated presymptomatically or at symptom onset (Fig. 4b). These results suggest that the rate of disease progression is slowed to a similar extent, regardless of absolute age of onset. This is significant because most ALS patients can only be treated after symptom onset and differential diagnosis, thus slowing of disease progression is the primary therapeutic goal; early onset become irrelevant in patients who are only treated after onset.
Fig. 4. D-serine treatment of G93A mSOD1 mice presymptomatically and at symptom onset.
(a) D-serine administered in chow (1 gram/kg chow = 160 mg/kg/day) from postnatal day 65 hastens symptom onset by approximately 15 days. Bars represent mean onset day ± S.E.M. (b) Values for untreated G93A mice (Fig. 4c, top bar) represent the means for two groups (controls for SR deletion and controls for D-serine treatment), which were very similar. Initiation of D-serine treatment at the time of normal symptom onset (90 days) demonstrates that it can slow disease progression, independently of any effect on time of onset. Bars represent mean S.I. ± S.E.M. n values = 7 (4 M/3 F), 10 (4 M/6 F), and 9 (4 M/5 F) for controls, presymptomatic D-serine treatment, and D-serine treatment at onset, respectively. * P < 0.001, # P < 0.05. (c) Summary of survival interval effects of SR mutation and D-serine treatment in G93A mSOD1 mice (from figures 3 and 4), illustrating combined effects on symptom onset and ultimate survival.
Biochemical analysis of SR deletion G93A mice and D-serine treated G93A mice
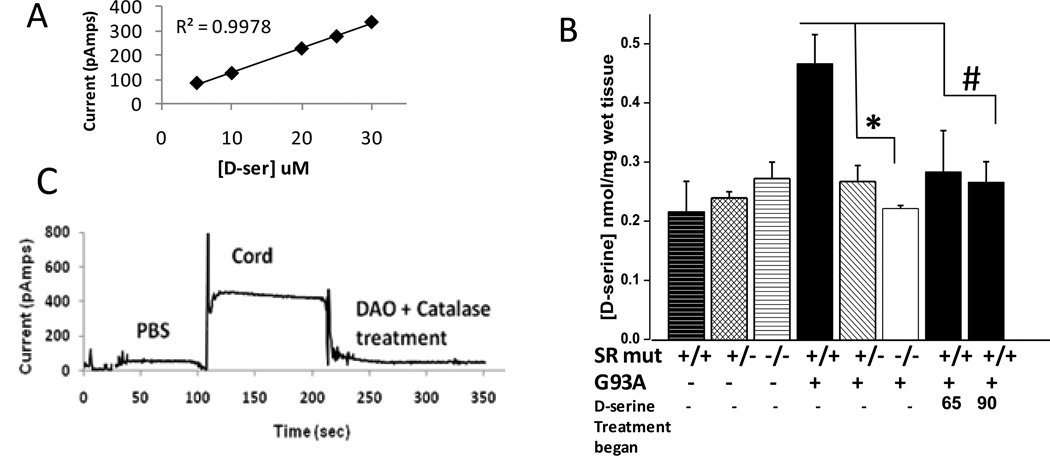
Deletion of SR and D-serine treatment would, logically, be expected to have opposite effects on G93A mice. Thus, in order to provide greater insight into the paradoxically similar effects, D-serine levels were assessed in the spinal cord of all six genotypes of mice, as well as D-serine-treated mice. D-serine concentration in ultrafiltered spinal cord samples was determined using a custom biosensor containing yeast (Rhodotorula gracilis) DAO – an enzyme which has higher specificity for D-serine than DAO isoforms from other species (Pollegioni et al., 1992). D-serine diffusing into the biosensor tip is degraded by yeast DAO, yielding hydrogen peroxide which is, in turn, measured by an electrochemical probe within the sensor tip (Fig. 5a, Pernot et al., 2008). The biosensor utilizes the same principle as commonly used chemiluminescence assays for D-serine, but has the advantage of providing immediate, reproducible, real-time measurements of D-serine.
Fig. 5. D-serine levels in spinal cord of all mice.
(a) Standard curve showing linearity of sensor output (pAmps) in response to different concentrations of authentic D-serine. Representative tracing of a diluted spinal cord ultrafiltrate, with demonstration that addition of porcine kidney DAO and catalase abolishes signal. (b) D-serine values in spinal cords of SR mutant G93A mice and D-serine treated G93A mice. Bars represent average D-serine value ± S.E.M. n values = 4 or greater for all groups, *P<0.01, #P<0.05.
In the absence of the G93A transgene, no significant differences in spinal cord levels of D-serine were seen between Srr+/+, Srr+/−, and Srr−/− mice (Fig. 5b). These findings clearly indicate that additional sources of D-serine exist, consistent with results from SR knockout mice created independently using different approaches (Basu et al., 2009; Labrie et al., 2009; Mustafa et al., 2010). A small amout of free D-serine was present in the chow fed to these mice (not shown), as has also been reported previously, however, it’s not clear that this low-level dietary D-serine is sufficient to produce the levels seen in the Srr−/− mice. D-serine levels in whole cord homogenates from G93A mice were two-fold higher than in wild-type non-transgenic mice (Fig. 5b). Thus, the mere presence of mutated SOD1 produces an increase in cord levels of D-serine. In G93A mice with partial SR deletion (Srr+/−), D-serine was significantly decreased, to levels within 25% of those found in wild-type non-transgenic mice. G93A mice with complete SR deletion (Srr−/−) had D-serine levels equivalent to that in wild-type non-transgenic mice (Fig. 5b).
Interestingly, spinal cord levels of D-serine in G93A mice treated with D-serine at 65 and 90 days were much lower than for untreated G93A mice, and virtually identical to those seen in Srr+/− and Srr−/− mice (Fig. 5b). That is, D-serine treatment produced a net decrease in cord D-serine levels, comparable to that seen with SR deletion and in wild-type animals. Importantly, lower D-serine levels was not a generalized phenomenon in the CNS; in the brains of D-serine treated mice, D-serine values were found to be increased almost 2-fold – 0.97 nmol/mg wet tissue in D-serine treated G93A mice, relative 0.5 nmol/mg tissue for untreated mice (not shown), -- consistent with CNS penetration following oral feeding. An ongoing, extended time course study reveals that D-serine levels in cord decreased within a few days of the initiation of D-serine administration, and remained lower throughout the course of disease (manuscript in preparation).
Induction of metabolism is the most straightforward mechanism to explain how exogenous addition of a small molecule could lead to a net lowering of tissue levels. Thus, D-amino acid oxidase (DAO) activity was measured in spinal cord homogenates of all mice via a coupled enzyme assay (Dawson et al., 1986, Fig. 6a)). Among the six mouse genotypes, DAO activity ranged from 6.6 to 10 units/mg protein, but the differences did not reach the level of statistical significant (Fig. 6b). Values of 4.0 and 5.1 units/mg protein were found for the two groups of D-serine-treated mice, but did not differ significantly from the value of 6.4 units/ mg protein for Srr+/+G93A. Clearly mean DAO activity was not increased in D-serine treated mice, thus lower D-serine in the cords of D-serine treated mice could not be explained on the basis of induction of DAO.
Fig. 6. DAO activity in spinal cords of all mouse groups.
(a) DAO activity assay tracing of porcine kidney DAO used to demonstrate as control reaction. Assay additions are as follows: 1) premix including FAD, NADH, LDH, and catalase, 2) porcine DAO (or spinal cord sample), 3) D-Alanine, and 4) sodium benzoate to inhibit the DAO reaction and control for non-specific NADH oxidation. See Methods for specific concentrations of reactants. (b) DAO activity in cords of all mouse groups. n = 4–6 for all groups.
D-serine-mediated induction of Asc-1 in cord but not brain
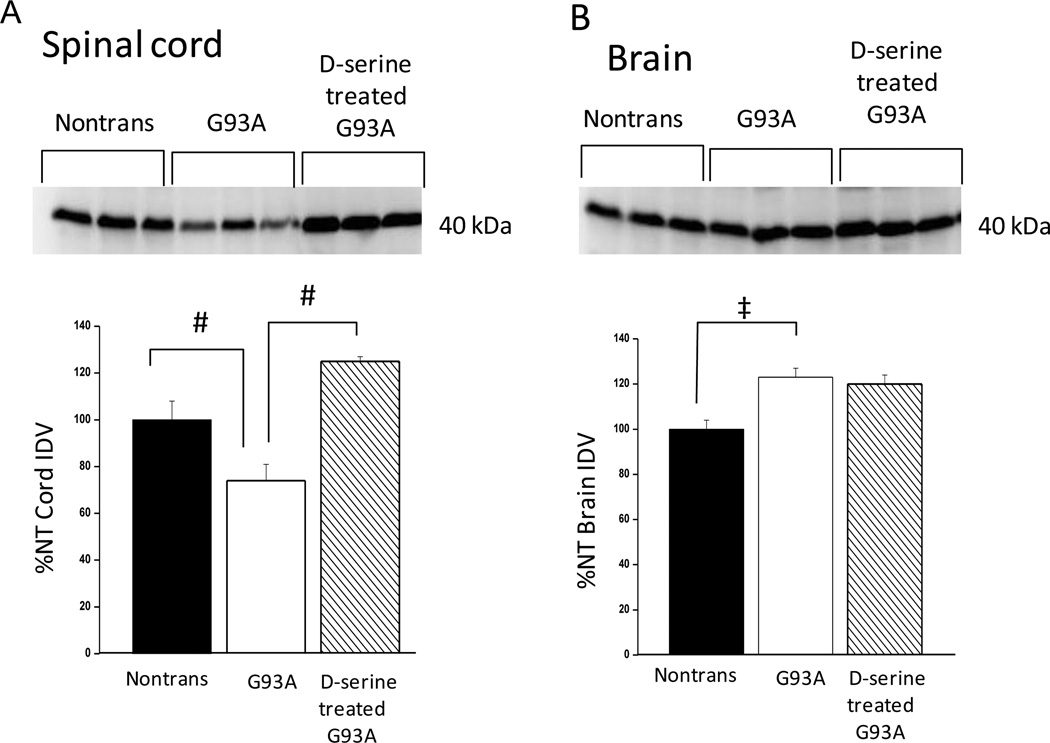
Very little is known regarding regulation of D-serine levels in vivo. The only mechanisms currently known for removing D-serine involve enzymatic degradation by DAO and by SR itself (eliminase activity), as well as synaptic reuptake transporters. Several reports indicate that the transporter Asc-1 plays an important role in modulating D-serine neurotransmitter activity in the CNS (Rutter et al., 2007, Xie et al., 2005). Asc-1 is a high affinity, sodium-independent D-serine transporter (KM for D-serine = 22.8 uM, Nakauchi et al., 2000) which has been reported to be primarily responsible for reuptake of D-serine from the synapse (Rutter et al., 2007; Xie et al., 2005). Asc-1 levels (40 kDa band, Helboe et al., 2003; Rutter et al., 2007) were examined via immunoblots, and were found to be decreased by ~ 25% in G93A mice (relative to non-transgenic littermates) in spinal cord, but increased by a similar amount in whole brain (Fig. 7a,b). However, when D-serine-treated G93A mice were compared to untreated G93A’s, Asc-1 in cord increased by 1.7-fold (70%), but was unchanged in brain. Thus, the increase in Asc-1 was cord-specific. Changes of this magnitude in whole cord homogenates are likely to be very significant, when considering that expression of the Asc-1 transporter is likely limited to a small percentage of the cells present (Xie et al., 2005).
Fig. 7. Expression of D-serine transporter in cord and brain; comparison of non-transgenic, G93A, and D-serine-treated G93A mice.
Western blots with Asc-1 antibody and densitometry were carried out as described in Methods. (a) Spinal cords from the three mouse groups were homogenized as described in Methods. (b) Whole brains from the three mouse groups were prepared as described for spinal cords above. Bars represent average integrated density value (IDV) ± S.E.M.of the 40 kDa band normalized to the nontransgenic (NT) value. #P<0.01, ‡P<0.05, n = 3 for each group.
Discussion
Spinal motor neuron death in ALS SOD1 mice is considered to be a non-cell-autonomous process, whereby glial cells actively promote neuronal death (Boillee et al., 2006). Nagai and co-workers (2007) reported on an astrocyte-derived factor that was toxic to motor neurons, and its importance to both ALS pathogenesis and therapy, yet no specific toxic factor was identified. Sasabe et al. (2007) offered the first evidence for a link between mutated SOD1 and D-serine in both G93A mice and ALS patients. Though compelling, the link between D-serine and ALS was largely correlative. We utilized two different approaches to help establish a more direct cause-and-effect relationship between D-serine and SOD1 mutant-mediated neuronal death in ALS mice. The results provided herein suggest that D-serine may be one possibility for a glial-derived toxic factor, thereby offering a tangible link between mutant SOD1 and non-cell-autonomous motor neuron death, potentially via an excitotoxic mechanism.
The two-fold increase in D-serine in cords of G93A mice (relative to non-transgenic littermates) is consistent with a previous report of D-serine levels in isolated microglia and G93A mouse cord (Sasabe et al., 2007). Thus, expression of the G93A SOD1 mutant, in and of itself, results in increased D-serine levels in the spinal cord of whole animals. No direct link between D-serine and mSOD1 is known to exist; however, it is reasonable to infer that enhanced D-serine production and/or release from glial cells could result from the generalized stress response – so-called glial activation -- that is known to occur in these mice. The abnormally high D-serine level seen in G93A mice appears to be derived from SR, as SR deletion lowers the D-serine value to those of non-transgenic littermates.
Genetic ablation of SR, thought to be the main source of D-serine in vivo, would be expected to lower tissues levels of D-serine, whereas administration of D-serine would be expected to increase tissue levels. The fact that these opposing stimuli both produce strikingly similar effects on disease onset and progression is, at first, paradoxical and counter-intuitive. However, the fact that SR deletion and D-serine treatment both produced a net decrease in cord levels of D-serine – roughly equivalent to those of wild-type non-transgenic mice – offers a possible resolution. The fact that D-serine treatment produced essentially the same phenotypic effects as SR deletion, and actually decreased cord levels of D-serine to a similar extent, argues that the overall effects on motor neuron disease in G93A mice are, indeed, D-serine-mediated. While the overall results, at least in terms of the slowing of disease progression, are consistent with exacerbation of classical glutamate excitotoxicity by D-serine, a direct connection in ALS remains to be determined.
The major decrease in cord levels of D-serine occurred with deletion of the first 50% of SR (from Srr+/+G93A mice to Srr+/−G93A), whereas deletion of the remainder of SR (in Srr−/− mice), produced only a modest (additional) D-serine decrease. Two other SR knockout mouse strains have been created by other investigators, with similar results, i.e., CNS levels of D-serine persisted at significant levels even in the complete absence of SR (Mustafa et al., 2010; Miya et al., 2008). Indeed, in one recent SR knockout mouse, D-serine levels in some brain regions were unaffected (Horio, et. al., 2011). Thus, it would seem that SR is not the sole source of D-serine in vivo. Others have reported D-serine in mouse chow, a finding confirmed in our laboratory, but it is unclear whether that is sufficient to account for all the D-serine that remains in Srr−/− mice. In any case, the results of this study strongly suggest that SR is responsible for the elevation of D-serine under pathologic conditions. Moreover, these results are consistent with mSOD1 stimulating SR-mediated D-serine production via a mechanism that does not involve induction of SR protein per se. Ongoing studies in our laboratory are aimed at understanding how mSOD1 stimulates D-serine production, as well as other possible sources of D-serine in vivo.
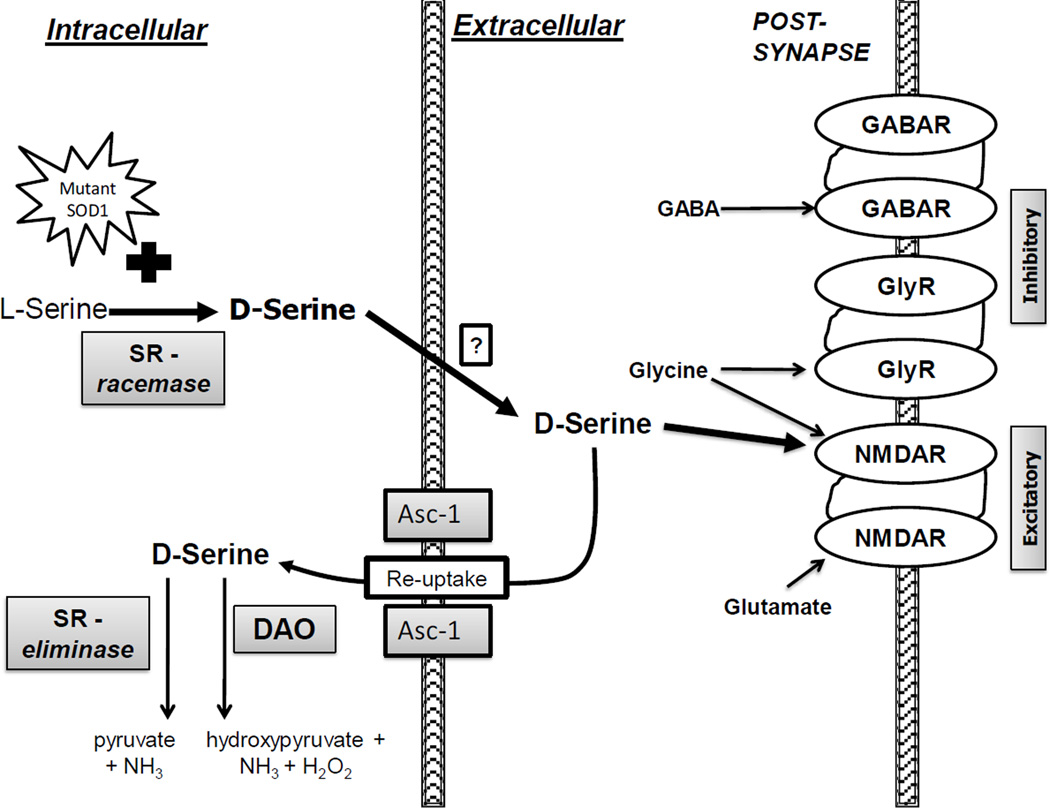
Treatment of G93A mice with D-serine led to a dramatic decrease in cord D-serine (but increased 2-fold in brain) – very similar to the levels seen with SR deletion. For this survival study, D-serine measurements could only be done on mice at endstage disease. Thus, the extent to which D-serine may be dynamically regulated throughout disease is not known, but is the focus of an ongoing extended time course study. The decrease in cord D-serine (and concurrent increase in brain D-serine) indicates that orally administered D-serine is reaching the CNS, but that cord is responding in such a way as to blunt the effect, presumably because D-serine is more toxic to cord than to brain. While compelling evidence from Asc-1 knockout mice point to Asc-1 being the predominant mechanism for D-serine reuptake from the synapse (Rutter et al., 2007), induction of Asc-1 can only alter the location of D-serine, not the absolute amount in the CNS. However, enhanced reuptake would put more D-serine in proximity to SR (Fig. 8), and increase its intracellular concentration relative to L-serine, the other substrate for SR eliminase activity. The elegant work of Foltyn et al. (2005) not only demonstrated that SR possesses constitutive racemase and eliminase activities, but also utilized SR-transfected cells to show that transport of D-serine back into cells resulted in a net decrease in total D-serine content, as a direct result of SR eliminase activity. Thus, the increase in Asc-1 in D-serine-treated G93A mouse cord (but not brain), together with lower overall cord levels of D-serine (but not in brain) is consistent with enhanced reuptake and degradation of D-serine by SR eliminase activity and/or by DAO (Fig. 8). Conversely, decreased expression of Asc-1 in G93A mouse cord (relative to non-trangenic mice, Fig 7a) could help explain higher constitutive D-serine levels in G93A mice, as well as the potential for enhanced toxicity, given that lower Asc-1 would result in more D-serine in the synapse. The fact that genetic deletion of Asc-1 also produces an overt phenotype involving tremors, seizures, and early postnatal death (Xie et al., 2005), is consistent with its physiological importance, presumably via D-serine reuptake from the synapse and lowering the threshold for glutamate excitotoxicity.
Fig. 8. Schematic of D-serine trafficking.
D-serine is synthesized from L-serine by the racemase activity of SR which appears to be enhanced by the presence of mSOD1. Once released into the synaptic cleft (either through vesicles or transporters), D-serine can act as a co-agonist at the NMDA receptor, increasing the affinity of the receptor for glutamate, thereby potentiating excitotoxicity. Excess synaptic D-serine results in excess excitatory transmission which can be opposed by inhibitory transmission at the GABA and glycine receptors. Termination of the neurotransmitter function of D-serine occurs primarily via re-uptake by the D-serine transporter Asc-1 (Rutter et al., 2007). Increased intracellular levels of D-serine (from exogenous administration, diet, other endogenous sources, etc.) would be controlled via degradation by a combination of DAO and the alpha beta eliminase activity of SR. Sites of production, release, binding (NMDA-R), re-uptake, and degradation are all potential therapeutic targets.
The ability of SR to make D-serine, and to simultaneously degrade both D-serine and L-serine, make it a very unusual enzyme. Several cofactors, post-translational modifications, and binding proteins for SR have been described, but very little is known in terms of how they regulate SR physiologically, let alone what effects they may have on racemase versus eliminase activity (Pollegioni and Sacchi, 2010). However, it is clear that eliminase activity is a true degradation pathway, and not simply a reverse racemase reaction. Both racemase and eliminase activities have been measured using recombinant mouse SR, or SR partially purified from tissues (Panizzutti et al., 2001; Strisovsky et al., 2005; Foltyn et al., 2005; Sasabe et al., 2007; Mustafa et al., 2009), suggesting that these activities are constitutive and concurrent. That is, the type of activity measured (racemase or eliminase) appears to be a function of which substrates are added, and the specific in vitro conditions used, rather than any intrinsic property of SR per se. Moreover, Foltyn et al. (2005) determined that the kinetic parameters for both racemase and eliminase activities were similar, and demonstrated that both activities originate from the same active site, utilizing the same cofactors.
The physiological relevance of SR eliminase activity is evidenced by its ability to down-regulate D-serine levels (via degradation) in tissues that lack DAO activity (Martineau et al., 2006). Thus, SR eliminase, together with lower racemase activity, represents what is, arguably, the simplest mechanism for local regulation of D-serine levels in the forebrain, or other regions of the CNS such as the spinal cord (Fig. 8). We saw no evidence of DAO induction in the cord that could account for lowered D-serine levels, although constitutive DAO activity remained and could certainly contribute to the lowering of D-serine (Fig. 8) in D-serine-treated mice, particularly if SR racemase activity was down-regulated in response to exogenous D-serine. Ongoing work in our laboratories is aimed at determining the role of SR in overall D-serine regulation, using the SR knockout mice.
Sasabe et al. (2007) reported a dramatic increase in cord D-serine levels in both familial (A4V, n = 1) and sporadic (n = 3) ALS patients, suggesting that D-serine is fundamental to all forms of human ALS, and not solely a phenomenon associated with SOD1 mutations. Whether or not D-serine is acting exclusively as an NMDA-receptor co-agonist, and thereby exacerbating glutamate excitotoxicity, remains to be determined, but all lines of evidence to date are consistent with that hypothesis. Indeed, Sasabe et al. (2007) offered evidence for D-serine-mediated excitotoxicity in isolated cells. Evidence accumulated over many years implicates glutamate excitotoxicity in motor neuron death, both in patients and in ALS animal models (Rothstein et al., 1990; Lin et al 1998; Heath and Shaw 2002).
The idea that D-serine may be a key mediator of that toxicity raises several intriguing possibilities, not only for disease pathogenesis, but for therapy as well. The ability of D-serine treatment to slow disease progression is significant, albeit unexpected and apparently due to a paradoxical lowering of cord levels. In most situations, treatment of human ALS can only begin at symptom onset, thereby lessening concerns over early onset with D-serine administration. Nevertheless, if D-serine does exacerbate ALS in any way, and its therapeutic effect is primarily due to a net decrease in cord levels, then D-serine itself may be too risky for consideration as a therapeutic agent. However, lowering cord D-serine levels via specific inhibitors of SR and/or via a D-serine mimic that produces a net decrease in cord levels of D-serine (e.g., inducers of Asc-1), but lack intrinsic toxicity (i.e., ability to bind to NMDA-R) could be realistic therapeutic options. The value of this indirect approach is illustrated by the fact that inducers of the glutamate re-uptake transporter EAAT2 (Rothstein et al., 2005; Ganel et al., 2006) are currently being tested in human clinical trials. In any case, the results presented herein offer evidence for a novel biochemical pathway that may link several disparate pieces of the ALS pathogenesis puzzle. In addition, the notion that an endogenously produced D-amino acid could play a prominent role in a neurodegenerative disease opens new avenues for research into the mammalian biology of other D-amino acids.
Acknowledgements
We hereby acknowledge the following support: Canadian Institutes of Health Research fellowship (VL), CIHR Operating Grant – MOP 111198 (JR), National Institute on Aging/NIH P01AG012411 (SWB), 5R01NS040819-08 (JPC), and the J. Thomas May Center for ALS Research and Translational Medicine (MT, JM, and JPC).
Abbreviations used
- ALS
amyotrophic lateral sclerosis
- AMPA
(2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid
- Asc-1
Asc-type amino acid transporter 1
- CNS
central nervous system
- DAO
D-amino acid oxidase
- EAAT2
excitatory amino acid transporter isoform 2
- mSOD1
mutant superoxide dismutase
- NMDA
N-Methyl-D-aspartic acid
- PAGE
polyacrylamide gel electrophoresis
- PDVF
polyvinylidene fluoride
- SI
survival interval
- SOD1
Cu, Zn-superoxide dismutase
- SR
serine racemase
Footnotes
The authors herein declare no conflict of interest.
References
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Crow JP, Calingasan NY, Chen J, Hill JL, Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann. Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- Dawson RMC, Elliott DC, Elliott WH, Jones KM. Data for biochemical research. 3rd ed. Oxford: Clarendon Press; 1986. p. 122. [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non–cell autonomous effect of glia on motor neurons in an embryonic stem cell–based ALS model. Nat. Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda E, Danysz W, Wroblewski JT, Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988;27:1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, Li P, Toney MD, Kartvelishvily E, Wolosker H. Serine racemase modulates intracellular D-serine levels through an α, β-elimination activity. J. Biol. Chem. 2005;280:1754–1763. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol. Dis. 2006;21:556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hamase K, Konno R, Morikawa A, Zaitsu K. Sensitive determination of D-amino acids in mammals and the effect of D-amino-acid oxidase activity on their amounts. Biol. Pharm. Bull. 2005;28:1578–1584. doi: 10.1248/bpb.28.1578. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T, Nishikawa T. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distributed and aging. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in Amyotrophic lateral Sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- Helboe L, Egebjer J, Moller M, Thomsen C. Distribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur. J. Neurosci. 2003;18:2227–2238. doi: 10.1046/j.1460-9568.2003.02966.x. [DOI] [PubMed] [Google Scholar]
- Horio M Kohno, Fujita Y, Ishima T, Inoue R, Mori H, Hashimoto K. Levels of d-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem.Int. 2011;59:853–859. doi: 10.1016/j.neuint.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Inoue R, Hashimoto K, Harai T, Mori H. NMDA and amyloid 1– 42 induced neurotoxicity is attenuated in serine racemase knock-out mice. J. Neurosci. 2008;28:14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. (N-methyl-D-aspartate) Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH, Roder JC. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum. Mol. Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Prox. Natl. Acad. Sci. U S A. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Martineau M, Baux G, Mothet JP. D-serine signaling in the brain: friend or foe. Trends Neurosci. 2006;29:481–491. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptors activation triggers a calcium-and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Paul P, Chen HJ, Morris A, Payling M, Falchi M, Habgood J, Panoutsou S, Winkler S, Tisato V, Hajitou A, Smith B, Vance C, Shaw C, Mazarakis ND, de Belleroche J. Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc. Natl. Acad. Sci. U S A. 2010;107:7556–7561. doi: 10.1073/pnas.0914128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc.Natl.Acad.Sci.U.S.A. 2009;106(8):2921–2926. doi: 10.1073/pnas.0813105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Ahmad AS, Zeynalov E, Gazi SK, Sikka G, Ehmsen JT, Barrow RK, Coyle JT, Snyder SH, Doré S. Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J. Neurosci. 2010;30:1413–1416. doi: 10.1523/JNEUROSCI.4297-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi J, Matsuo H, Kim DK, Goto A, Chairoungdua A, Cha SH, Inatomi J, Shiokawa Y, Yamaguchi K, Saito I, Endou H, Kanai Y. Cloning and characterization of human brain Na (+)-independent transporter for small neutral amino acids that transports D-serine with high affinity. Neurosci. Lett. 2000;287:231–235. doi: 10.1016/s0304-3940(00)01169-1. [DOI] [PubMed] [Google Scholar]
- Panizzutti R, De Miranda J, Ribeiro CS, Engelender S, Wolosker H. A new strategy to decrease N-methyl-D-aspartate (NMDA) receptor coactivation: inhibition of D-serine synthesis by converting serine racemase into an eliminase. Proc. Natl. Acad. Sci. U S A. 2001;98:5294–5299. doi: 10.1073/pnas.091002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernot P, Mothet JP, Schuvailo O, Soldatkin A, Pollegioni L, Pilone M, Adeline MT, Cespuglio R, Marinesco S. Characterization of a yeast D-amino acid oxidase microbiosensor for D-serine detection in the central nervous system. Anal. Chem. 2008;80:1589–1597. doi: 10.1021/ac702230w. [DOI] [PubMed] [Google Scholar]
- Pollegioni L, Falbo A, Pilone MS. Specificity and kinetics of Rhodotorula gracilis D-amino acid oxidase. Biochim. Biophys. Acta. 1992;1120:11–16. doi: 10.1016/0167-4838(92)90418-d. [DOI] [PubMed] [Google Scholar]
- Pollegioni L, Sacchi S. Metabolism of the neuromodulator D-serine. Cell Mol. Life Sci. 2010;67:2387–2404. doi: 10.1007/s00018-010-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SD, Weiss JH. Excitotoxic and oxidative cross-talk between motor neurons and glia in ALS pathogenesis. Trends Neurosci. 2004;27:17–23. doi: 10.1016/j.tins.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, Patel S. Evidence from gene knockout studies implicates Asc-1 as the primar transporter mediating D-serine uptake in the mouse CNS. Eur. J. Neurosci. 2007;25:1757–1766. doi: 10.1111/j.1460-9568.2007.05446.x. [DOI] [PubMed] [Google Scholar]
- Sasabe J, Chiba T, Yamada M, Okamoto K, Nishimoto I, Matsuoka M, Aiso S. D-serine is a key determinant of glutamate toxicity in Amyotrophic lateral sclerosis. EMBO J. 2007;26:4149–4159. doi: 10.1038/sj.emboj.7601840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc. Natl. Acad. Sci. U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced amyotrophic lateral sclerosis requires mitochondrial calcium uptake. Nat. Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Strisovsky K, Jirásková J, Mikulová A, Rulísek L, Konvalinka J. Dual substrate and reaction specificity in mouse serine racemase: identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the β-eliminase activity. Biochemistry. 2005;44:13091–13100. doi: 10.1021/bi051201o. [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J. Neuroinflammation. 2004;1:2–12. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Dumas T, Tang L, Brennan T, Reeder T, Thomas W, Klein RD, Flores J, O’Hara BF, Heller C, Franken P. Lack of the alanine-serine-cysteine transporter 1 causes tremors, seizures, and early postnatal death in mice. Brain Res. 2005;1052:212–221. doi: 10.1016/j.brainres.2005.06.039. [DOI] [PubMed] [Google Scholar]