Abstract
Combustion smoke contains gases and particulates, which act via hypoxia and cytotoxicity producing mechanisms to injure cells and tissues. While carbon monoxide (CO) is the major toxicant in smoke, its toxicity is exacerbated in presence of other compounds. Here, we examined modulations of mitochondrial and cytosolic energy metabolism by inhalation of combustion smoke versus CO, in vivo, in the rat brain. Measurements revealed reduced activities of respiratory complexes, with greater inhibition by smoke than equivalent CO in ambient air. In the case of respiratory complex IV, inhibition by CO and smoke was similar; suggesting that complex IV inhibition is primarily by the action of CO. In contrast, inhibition of complexes I and III was greater by smoke. Increases in cytosolic lactate dehydrogenase and pyruvate kinase activities accompanied inhibition of respiratory complexes, likely reflecting compensatory increases in cytosolic energy production. Together, the data provide new insights into the mechanisms of smoke inhalation-induced perturbations of brain energetics, which impact neuronal function and contribute to the development of neuropathologies in survivors of exposures to CO and combustion smoke.
Keywords: brain, combustion smoke, carbon monoxide, energetics, mitochondria, respiratory complexes
INTRODUCTION
Combustion produces complex toxic environment consisting of gases, organic irritants and particulates, which act in concert via various hypoxia and cytotoxicity producing mechanisms. Generally, the chemical composition of smoke in combustion fires is unpredictable and is not only material- but also combustion conditions-dependent (Hartzell, 1996; Stuhmiller et al., 2006). Since acute exposures to toxic combustion smoke are associated with morbidity and poor neurological outcomes in military as well as civilian scenarios, substantial efforts are devoted to modeling risks and interpreting outcomes of exposures to various gas mixtures (Smith et al., 1996; Speitel, 1996; Stuhmiller et al., 2006). While carbon monoxide (CO) is the major, recognized toxicant in fires (Raub, Benignus, 2002), its toxicity tends to be amplified by presence of other factors. For example, by elevated CO2, possibly due to stimulation of breathing, further increasing inhalation of toxicants and formation of blood carboxyhemoglobin (COHb) (Fukuda et al., 1989; Kou, Lai, 1994; Hartzell, 1996; Gu et al., 2005). For similar reasons, O2 depletion is considered a toxic component of smoke. Cyanide, which is generated via combustion of nitrogen containing materials, is toxic and has an additive effect in presence of CO. In addition to inorganic compounds, incomplete combustion generates a variety of harmful organic compounds. Among the most toxic compounds is acrolein, a highly reactive aldehyde, which leads to formation of cytotoxic lipid and protein modifications, membrane damages (Calingasan et al., 1999; Luo, Shi, 2005; Liu-Snyder et al., 2006), genotoxic acrolein-DNA adducts (Kawai et al., 2003) as well as changes in nasal uptake of vapors compromising natural barriers and body defenses (Morris et al., 1999; Stefanidou et al., 2008).
To discern progression of events contributing to brain pathophysiology after acute exposure to combustion smoke, we developed a rat model of smoke inhalation injury (Lee et al., 2005). We showed that systemic hemodynamic parameters as well as molecular targets in the rat brain were significantly affected by acute inhalation of smoke (Lee et al., 2005). Systemic changes included increases in COHb levels, reduction in oxygen saturation and blood pH, while in the brain tissue changes in gene expression patterns, lipid peroxidation, DNA oxidation, modulations of the nitric oxide system as well as decreased oxygen consumption by brain mitochondria, were detected (Lee et al., 2005; Chen et al., 2007; Lee et al., 2009). Brain mitochondria preferentially use pyruvate from cytoplasmic glycolysis to generate electrons that are transferred via mitochondrial respiratory chain (RC) to molecular oxygen (Kann, Kovacs, 2007). The multi-subunit components of RC include, complex I; NADH ubiquinone dehydrogenase, complex II; succinate dehydrogenase, complex III; ubiquitinol cytochrome c oxioreductase and complex IV; cytochrome c oxidase. Proper function of respiratory complexes is required for adequate energy generation. Here, we investigated effects of exposures to either combustion smoke or CO on respiratory complexes in the rat brain mitochondria. Since CO is the most recognizable toxicant in smoke, we determined the concentration of CO in combustion smoke produced in our experimental setting and exposed rats to an identical concentration of CO in the context of ambient air. Severity of both treatments was assessed at the systemic and brain tissue levels, including mitochondrial respiratory complexes and cytosolic enzymes involved in brain energy metabolism. At the systemic level, hemodynamic measurements revealed higher blood COHb and more severe acidosis in the case of smoke, compared to equivalent exposure to CO. Taken together, our findings revealed that exposures to equivalent levels of CO in the context of air versus smoke differ in lethality, as well as severity of systemic and tissue specific manifestations. Thus, in terms of synergistic/additive physiologic effects our findings for smoke versus CO exposures are consistent with predictions from modeling adverse effects of gas mixtures (Stuhmiller et al., 2006).
MATERIALS AND METHODS
Chemicals
Mannitol, sodium salt of succinic acid, ADP, ATP, Triton X-100, BSA, Acetyl-CoA, oxaloacetic acid, 2,6-dichlorophenolindophenol (DCPIP), NADH, NAD, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, ubiquinone1, ubiquinone2, rotenone, cytochrome c, phosphoenolpyruvate and L-lactic dehydrogenase were purchased from Sigma Chemical Co. (St. Louis, MO).
Experimental design and statistical analyses
The study was designed to compare the outcomes of two treatments, smoke and CO, on activities of four mitochondrial complexes and two cytoplasmic enzymes. Six groups were used for each treatment: sham-controls and rats at 0, 2, 6 and 24 hours and 7 days after exposure to either smoke or CO, fed into exposure chambers containing normal room air. Rats in the sham-control groups were placed in the respective chambers containing room air only (‘mock-exposure’). The sham-control rats were sacrificed immediately following ‘mock-exposures’, ie, at time point corresponding to 0-hour recovery. There were 6 rats per group and 36 rats per treatment. A separate series of rats were used to characterize effects of smoke and CO on hemodynamic parameters and brain lipid peroxidation. There were 4–5 rats per group and 20–25 rats per treatment. Values were calculated as described for the individual assays and expressed as mean ± SEM. Comparisons of groups were performed using One Way ANOVA (SigmaStat) followed by the multiple comparisons Holm-Sidak test (SigmaStat, Jandel, San Rafael, CA). P-value of less than 0.05 was considered significant.
Smoke composition
The profile of wood-smoke gases generated in our setting, was determined by DataChem Labs Inc. (Cincinnati, OH) using a HP 6890 gas chromatograph with thermal conductivity detector & HP MOLSIV and PLotQ capillary column). For determination of volatile organic compounds (VOCs), smoke samples were collected according to instructions in specialty-prepared canisters, shipped to DataChem Labs Inc. (Salt Lake City, UT), and analyzed by Gas Chromatography/Mass Spectrometry (GC/MS) according to the EPA method TO15.
Exposure to combustion smoke
All experiments were conducted in accordance with standards of humane care approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee, Galveston, Texas. Awake, Sprague-Dawley male rats (250–300 g) were exposed to combustion smoke. Smoke was generated by smoldering wood in a chamber connected by a tube with electric fan to a 20-liter transparent chamber that houses the rats. Smoke intensity was controlled by activating the fan intermittently. The average wood-burning rate was 1.3 gram/min as we described previously (Lee et al., 2005). Sham controls were given similar treatment without smoke. Rats were left to recover for 0, 2, 6, 24 h or 7 days. Brains were harvested and dissected for mitochondrial isolation.
Exposure to carbon monoxide
Awake Sprague-Dawley male rats (250–300 g) were exposed to CO at 3,500 ppm for 30 min using 0.8 m3 steel/glass Hinnars-type exposure chambers. CP Grade Carbon monoxide gas (Scott Specialty Gases, Pasadena, TX) was metered through a mass flow controller (~ 480 ml/min) (Scott Specialty Gases, Pasadena, TX) where it was fed into the air stream mixing in the exposure chamber. CO concentration was monitored in real time by a Miran Sapphire UV monitor (Thermo Electron Corporation, Waltham, MA). Rats were placed in exposure chamber and the parameters were set such that target concentration of CO was reached within 15 min. Rats were readily visible throughout treatment, no mortality or labored breathing was noted.
Carboxyhemoglobin (COHb) and blood gases
Blood was anaerobically collected with a heparinized syringe from the jugular vein of control rats and rats recovering after CO exposures (n=4–5) as we described previously (Lee et al., 2005). COHb and oxygen saturation (O2 Sat) were measured with oximeter (482 CO-Oximeter) and gas tensions, pH and base excess (BE) were measured with System 1302 (Instrumentation Laboratory, Lexington, MA).
Malondialdehyde (MDA)
Brain MDA content was measured using the Bioxytech MDA-586 kit (OxisResearch, Portland, OR) according to manufacturer’s instruction and as we described (Chen et al., 2007). Briefly, snap-frozen brain tissues from control and treated rats (n=4) were dissected and homogenized in four volumes of 20 mM Tris, 10 mM EDTA, pH 7.6 buffer containing 5 mM butylated hydroxytoluene; homogenates were incubated 30 min on ice, spun down and supernatants used for measurements. Concentrations were determined from standard curves using known amounts of MDA and calculated per mg protein. Measurements were done in triplicate.
Isolation of mitochondria
Crude mitochondrial pellets were prepared as described by (Anderson, Sims, 2000) with modifications. All procedures were done on ice; cerebral cortices were dissected out (~100 mg), finely cut and homogenized by 4 strokes of loose pestle in 0.65 ml ice-cold mannitol-sucrose-EDTA (MSE) isolation buffer (0.225 M mannitol, 0.075 M sucrose, 0.1 mM EDTA; pH 7.5, fatty acid free BSA 5 mg/ml), followed by 15 strokes of tight-fitting pestle, and centrifuged at 4°C for 8 min at 1000 rpm to pellet nuclei; pellets were washed with MSE buffer and supernatants centrifuged for 12 min at 15,000 rpm at 4°C to obtain crude mitochondrial pellets. Supernatants, i.e., cytosolic fractions were used for measurements of pyruvate kinase and lactate dehydrogenase. Mitochondrial pellets were washed with 0.25 M sucrose and pelleted at 8000 rpm; pellets were re-suspended in small volumes of MSE buffer. Protein concentrations were determined by the method of Lowry, using bovine serum albumin as standard.
Respiratory chain complexes activity
Spectrophotometric assays were done using 40 μg mitochondria, as described (Birch-Machin, Turnbull, 2001; Kirby et al., 2007) with some modifications and absorbance measured on a Beckman DU 650 spectrophotometer (Fullerton, CA). Mitochondria from cortices of controls and rats after smoke/CO exposures (n=6) were analyzed. NADH: ubiquinone oxidoreductase activity (complex I) was measured as a decrease in absorbance following the oxidation of NADH at 340 nm. Prior to measurements, mitochondria were frozen/thawed 3-times and preincubated (5 min 30°C) with 25 mM potassium phosphate pH 7.4, 0.1 mM ubiquinone1, 5 mM KCN, 5 μg antimycin A, with/out 2 μg rotenone. The reaction was initiated by adding 0.2 mM NADH; linear decrease in absorbance was measured over 90 sec. Activity was calculated using an extinction coefficient of 6.22 mM−1cm−1. Succinate: ubiquinone1 oxidoreductase (complex II) reaction included, 20 mM succinate, 0.1 mM ubiquinone1, 2 mM KCN in 25 mM potassium phosphate pH 7.4 and mitochondria; after 5 min at 30°C, the reaction was initiated with 0.05 mM 2,6-DCPIP and the reduction of DCPIP followed at 600 nm. An extinction coefficient of 21 mM−1cm−1 was used. Ubiquinol2: cytochrome c oxidoreductase (complex III) activity was determined by measuring the reduction of oxidized cytochrome c at 550 nm. Mitochondria were preincubated with 25 mM potassium phosphate pH 7.4, 2 mM KCN, 0.1% BSA, 2 μg rotenone and 35 μM ubiquinol2 and reaction initiated with 15 μM oxidized cytochrome c. Optical density was recorded for 2 min and activity calculated using an extinction coefficient 18.5 mM−1cm−1. Complex IV-cytochrome c oxidase activity was measured by recordingthe oxidation of reduced cytochrome c at 550 nm; 15 μM reduced cytochrome c in 20 mM potassium phosphate pH 7.0 preincubated (5 min, 30°C) prior to addition of mitochondria; optical density was measured for 5 min at 1-min intervals; extinction coefficient of 18.5 mM−1cm−1 was used. All assays were done in duplicate. Activities were calculated as nmol min−1 mg−1, expressed as mean±SEM (n=6) and presented as percent of the respective control, which was assigned the value of 100%.
Cytosolic lactate dehydrogenase and pyruvate kinase
Activities were determined as described (Bergmeyer et al., 1974). Briefly, cytosolic pyruvate kinase reaction was in 0.1 M Tris-HCl pH 7.6, 17 mM phosphoenolpyruvate, 1.3 mM NADH, 100 mM MgSO4, 44 mM ADP and freshly prepared 10 units of lactate dehydrogenase; after 5 min at 37°C, the reaction was initiated by adding 40 μg of cytosolic fraction and decrease in absorbance measured at 340 nm; activity was calculated using an extinction coefficient of 6.22 mM−1cm−1. Lactate dehydrogenase (LDH) reaction containing 0.1 M Tris-HCl pH 7.0, 10 mM sodium pyruvate and 0.3 mM NADH (Renner et al., 2003) was preincubated (5 min, 30°C) prior to addition of the cytosolic fraction (40 μg); optical density was measured at 340 nm for 2 min at 30-sec intervals. LDH activity was calculated using an extinction coefficient of 6.22 mM−1cm−1. The assays were done in duplicate.
RESULTS
Smoke profile and CO concentration
CO concentration, as well as concentrations of other components of smoke generated in our experimental setting, was determined to enable dissection of effects induced by exposure to CO in the context of ambient air, from those induced by exposure to the complete mixture of gases and particulates in combustion smoke. Analysis of smoke revealed 3,500 parts per million (ppm) of CO, 11,500 ppm of CO2 and 14.5% O2. The identified organic compounds measured in ppm included, propene 57; chloromethane 1.5; 1,3-butadiene 10; 2,3-butanedione 5; acetone 2; 2-butanone 11; benzene 7; acetaldehyde 2.5; furan 6; toluene 1.4 and acrolein at 1.4. Acrolein and 1,3-butadiene are recognized DNA damaging agents (Kawai et al., 2003; Vodicka et al., 2006). Based on these analyses, rats were exposed either to smoke, or to ambient air supplemented with 3,500 ppm CO, a level matching the concentration of CO in smoke.
COHb and blood gases: Smoke versus CO-induced hemodynamic changes
In contrast to exposures to smoke, no mortality occurred during or post exposure to CO at 3,500 ppm in ambient air. Likewise hemodynamic parameters obtained post smoke (Lee et al., 2005) differed from those obtained post exposure to CO. The major differences were in blood COHb and the extent of acidosis, i.e., acid/base balance (base excess, pH, PCO2). Severe acidosis post smoke was reflected in a significantly lower blood pH 6.64±0.05 (Lee et al., 2005) versus 7.27±0.03 post CO alone (Table 1), as well as in base excess at (−32.1±1.9) post smoke (Lee et al., 2005) versus (−8.0±2.5) post CO (Table 1). Blood COHb levels after CO were 58±3% versus 72±1% post smoke (Lee et al., 2005), revealing that a similar CO level (3,500 ppm) in the context of smoke, results in higher blood COHb, which may be associated with faster breathing in presence of high CO2 and reduced ambient O2 (Hartzell, 1996; Smith et al., 1996).
Table 1.
Carbon monoxide-induced changes in hemodynamic parameters
| Parameter | Control | 0 h Post CO | 2.5 h Post CO | 24 h Post CO |
|---|---|---|---|---|
| COHb (%) | 5.0±2.3 | 58.2±3.1* | 9.0±0.9* | 6.2±1.4 |
| PvO2 (mm Hg) | 50.0±1.5 | 32.0±2.2* | 51.4±3.5 | 49.8±2.9 |
| O2 Sat (%) | 75.6±8.1 | 40.1±5.5* | 73.1±3.3 | 75.1±5.5 |
| PvCO2 (mm Hg) | 46.5±9.3 | 39.6±7.8 | 50.3±7.6 | 40.2±10 |
| BE (mEq/L) | 0.78±2.0 | −8.0±2.5* | 0.7±2.8 | 4.4±1.4 |
| pH | 7.39±0.05 | 7.27±0.03* | 7.38±0.05 | 7.42±0.05 |
The mean ± SD is shown;
indicates that the mean is different from control (n=4–5); P<0.05.
BE, base excess; COHb, carboxyhemoglobin.
Smoke versus CO-induced lipid peroxidation in the brain
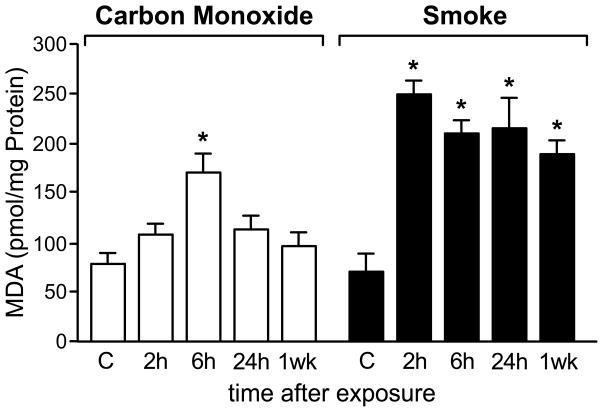
To asses outcomes of smoke and CO inhalation at the tissue level, in the brain, the content of malondialdehyde (MDA), a lipid peroxidation product and biomarker of oxidative stress, was measured. Post smoke, free MDA contents increased 3-fold within 2 h and high levels persisted for at least 1 week (Chen et al., 2007). In contrast, a lesser increase in MDA level was measured after CO exposure, reaching only a 2-fold increase by 6 h with a decline by 24 h and return to control levels by 1 week (Fig. 1). The faster decline in MDA levels after CO exposure indicates that severity of manifestations of CO and smoke inhalation differ at the tissue level. Lipid peroxidation has been documented in the brain and other tissues in ischemia/reperfusion and other pathological conditions (Choi et al., 2004; Garcia et al., 2005; Smith et al., 2005) with a broad range of effects ranging from modulation of gene expression (Marini et al., 2004) to induction of DNA damage (Marnett, 2002).
Figure 1.
MDA levels after exposures to smoke and CO; time course of MDA formation and clearance in the brain; combined 10 groups (n=4). Values represent pmoles of MDA per mg total brain protein expressed as mean±SEM; * indicates different from control, P<0.05.
Inhibition of mitochondrial respiratory complexes by exposures to combustion smoke and to CO
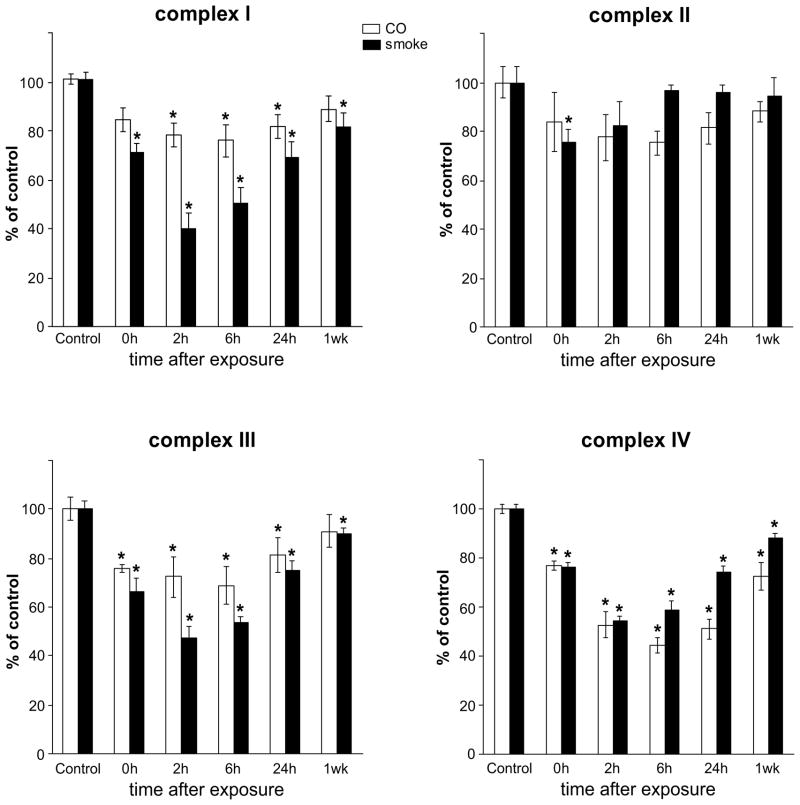
Activities of mitochondrial respiratory complexes (RC) l – IV were measured in cerebral cortices of control rats and rats recovering for 0, 2, 6, 24 h and 7 days after exposure either to smoke or to CO (Fig. 2). Activities of RC were reduced by both exposures, albeit with different timings of onset, durations and magnitudes. Substantial inhibition of complexes I, III and IV was measured following exposures to smoke and CO, however, the onset and duration differed. Complex I activity was more severely affected by smoke than by CO, with a reduction of 30% at 0 h and ~60% at 2 h post smoke; a 20% reduction persisted also at 7-days post smoke. In contrast, a significant inhibition by CO was delayed and first detected at the later, 2-h recovery time with return to normal by 7-days. A similar pattern of inhibition was observed for complex III, where inhibition by smoke exceeded 30% at the 0-h recovery time, reached nearly 60% by 2 h and persisted also by 7-days post smoke, while maximal inhibition by CO reached only 30% with return to near normal by 24 h. In sharp contrast, inhibition of complex IV by CO and smoke, followed a nearly identical pattern with respect to onset, duration and magnitude, with maximal effect seen at the 2- and 6-h recovery times. The effect of CO on complex IV was significantly greater than on complexes I and III, while smoke caused similar inhibition of respiratory complexes I, III and IV Interestingly, in the case of Complex II, although a tendency for reduced activity was observed, statistical significance was reached only in the case of exposure to smoke at a single 2-h, recovery point (Fig. 2).
Figure 2.
Activities of mitochondrial complexes I, II, III and IV from control brains, and brains harvested at 0, 2, 6 and 24 h and 7 days (1 wk) after exposure to either combustion smoke or CO; combined 12 groups (n=6). Significant inhibition of complexes I, III and IV was detected; with most severe inhibition seen at the 2-hour recovery time. Activities are presented as percent of control and expressed as mean±SEM; * indicates different from the respective control; P<0.05.
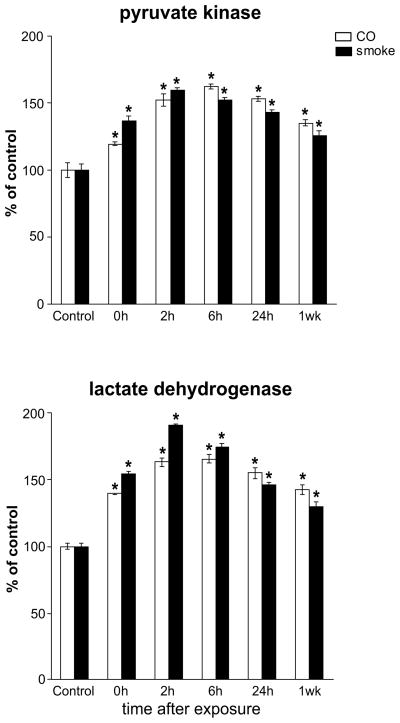
Cytosolic pyruvate kinase and lactate dehydrogenase
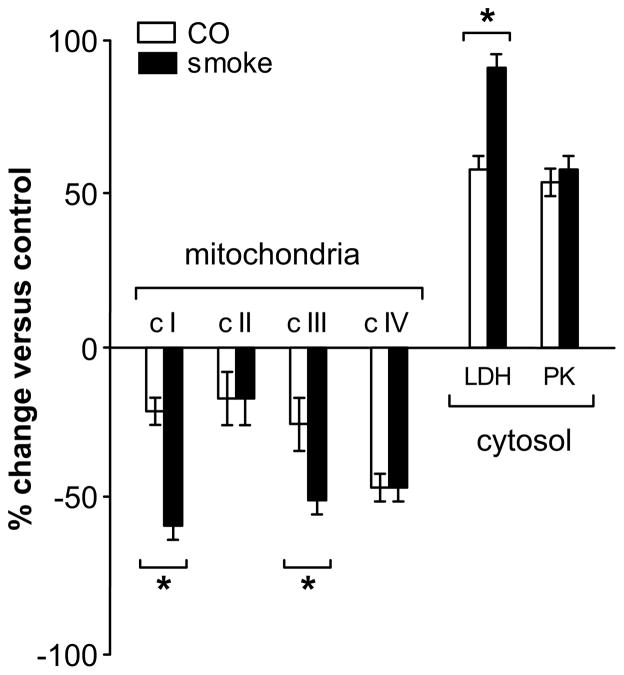
To assess whether cytosolic energy enzymology might be affected under these conditions, cytosolic pyruvate kinase and lactate dehydrogenase activities were measured (Fig. 3). Both smoke and CO exposures caused substantial increases in cytosolic pyruvate kinase and lactate dehydrogenase activities. Increases ranged from 25–90% with an onset immediately after exposures; peak activities were measured by 6 h of recovery and elevated activities persisted beyond the 7-day recovery period. A comparison of the magnitude of changes for the individual respiratory activities and cytosolic enzymes at the 2-h recovery point after exposure to combustion smoke and carbon monoxide is compiled in Figure 4. Interestingly, inhibition of complexes I and III by smoke was significantly greater than by CO, while inhibition of complex IV was identical with both exposures.
Figure 3.
Cytosolic lactate dehydrogenase (LDH) and pyruvate kinase (PK) activities in control brains and brains harvested at 0, 2, 6 and 24 h and 1 week after exposure to either combustion smoke or CO; combined 12 groups (n=6). Activities were significantly elevated following exposures; increased activities persisted beyond the one week time point; activities are presented as percent of control and expressed as mean±SEM; * indicates different from respective control; P<0.05.
Figure 4.
Comparison of the magnitude of change in the individual respiratory activities at the 2-h recovery point after exposure to combustion smoke versus exposure to carbon monoxide. Inhibition of complexes I and III by smoke was greater than by CO, while inhibition of complex IV was identical with both exposures. * Indicates that at the 2-h recovery point, the change induced by exposure to smoke is different than the change induced by exposure to CO; P<0.05.
DISCUSSION
The mechanisms, by which acute inhalation of combustion smoke may lead to neuropathological sequelae in survivors, are incompletely understood. Because CO is the major, toxic component of combustion smoke, which reduces oxygenation and disrupts energy production, it was of interest to assess severity of pathological manifestations of exposure to CO compared to exposure to a similar concentration of CO, in the context of combustion smoke.
To best replicate real scenarios awake rats were used. Rats were exposed smoke or to room air containing CO at concentration matching that of CO present in smoke generated in our experimental setting. Unlike in the case of smoke, lethality was not associated with exposures to CO in air. Furthermore, after exposure to CO, hemodynamic measurements revealed lower blood COHb and lesser acidosis, when compared to those measured after exposure to combustion smoke. It is plausible that the extreme modulations of blood pH and acid/base balance recorded after exposure to smoke, more critically impact the highly metabolic brain tissue. In fact, a faster induction and slower clearance of the lipid peroxidation product, MDA was measured in the brain after smoke when compared to CO, consistent with propagation of greater oxidant mediated tissue damage. Likewise, impairment of mitochondrial respiratory complexes was greater and more persistent after inhalation of smoke compared to CO. Taken together, these findings indicate that exposures to equivalent levels of CO in the context of smoke versus normal air, differ in lethality as well as systemic and tissue specific manifestations. Thus, our results are in agreement with conclusions drawn from modeling of exposures to gas mixtures, which predict additive or even potentially synergistic toxicity (Smith et al., 1996; Speitel, 1996; Stuhmiller et al., 2006).
The specific mechanisms, by which acute exposures to combustion smoke and CO impair mitochondrial activities at the tissue level, are so far incompletely understood. Toxicity of smoke is predominantly attributed to CO via its avid binding to hemoglobin leading to reduction in tissue oxygenation (Ginsburg, 1980; Gu et al., 2005). Additionally, CO interacts with cytochrome c oxidase (Brown, Piantadosi, 1990; Miro et al., 1998), inhibiting its function and causing subsequent respiratory chain impairment via increased generation of reactive oxygen species, which further promote cellular injury. Cytochrome c oxidase activity was significantly reduced in the heart following exposure to moderate levels of CO (Iheagwara et al., 2007). In a setting of smoke exposure, compromise of cytochrome oxidase activity is likely to be even more severe, in part, because the additional components of smoke, hydrogen cyanide (HCN) and hydrogen sulfide (H2S) are also inhibitory (Cooper, Brown, 2008). While inhibition by HCN and H2S is independent of oxygen concentration, inhibition by CO is even greater at reduced oxygen levels. In addition, hyperventilation in response to elevated carbon monoxide (Stuhmiller, Stuhmiller, 2005) or CO2 (Stuhmiller et al., 2006) tends to increase volumes of inhaled toxic gases, thus further exacerbating injury in the setting of smoke. Interestingly, we recorded increased activities of cytosolic lactate dehydrogenase and pyruvate kinase, which are involved in cytosolic energy metabolism. It is plausible that elevated lactate dehydrogenase and pyruvate kinase serve to mitigate reduced mitochondrial energy production and help adapt to compromised respiratory complexes activities. In fact, recent studies report the brain’s ability to utilize lactate as a significant energy source for neurons (Aubert et al., 2005; O’Brien et al., 2007).
Importantly, in our experimental setting deficits in brain mitochondrial complexes activities become evident immediately (0-h recovery) after exposure either to smoke or CO and are further exacerbated at the 2- and 6-h recovery points. Exacerbation is most pronounced in the case of complexes I, III and IV and absent in the case of complex II. Lack of sensitivity of complex II to CO has been reported (Miro et al., 1998). Interestingly, inhibition of complex IV by exposure to CO was significantly greater than the inhibition of complexes I and III. In fact, in the case of complex IV, the magnitude of inhibition by CO was identical to that exerted by smoke (Fig. 4). This suggests that unlike in the case of respiratory complexes I and III, where injurious ROS are known to be produced under compromising conditions (Kussmaul, Hirst, 2006; Drose, Brandt, 2008), inhibition of complex IV by smoke, is mediated primarily by its CO component. One plausible mechanism by which ROS production is induced, is the increased supply of NADH from glycolytic pathway (Kadenbach et al., 2009). In our setting, activation of the glycolytic pathway by smoke is greater than by CO alone (Fig. 3) and may preferentially contribute to the greater inhibition of complexes I & III by smoke when compared to CO.
Notably, in our model despite the fast clearance of blood COHb and restoration of normal systemic parameters, at the brain tissue level, smoke and CO-induced deficits significantly intensify with time after termination of exposure, suggesting that early interventions may be central to prevention of brain damage in survivors. Interestingly, patient data reveal that even mild CO exposures may result in short- and long-term neurophysiological impairments (Weaver, 2009). In fact, following CO poisoning, severely compromised complex IV activities were recorded in lymphocytes of patients with relatively low to moderate COHb levels (11.6%, 19.6% and 22.3%) at admission. Notably, activities of complexes II and III were not significantly affected in that setting, confirming that complex IV is the major target of CO poisoning (Miro et al., 1998). While neuropathology of CO toxicity has been well documented in postmortem analyses (Prockop, Chichkova, 2007), it is not known, whether the differentially affected brain regions differ in their mitochondria susceptibility to CO/smoke.
Our data demonstrate that acute exposures to CO and to combustion smoke directly inhibit mitochondrial respiration and impair activities of mitochondrial respiratory complexes in the rat brain. To what extent impairment of mitochondrial complexes may predict secondary injuries, and by which specific mechanisms the CO/smoke-induced mitochondrial deficits may trigger secondary brain injuries that are associated with long-term neuropathological manifestations, awaits further investigation.
Acknowledgments
This work was supported by National Institutes of Health grants ES014613 and NS039449 to EWE and Shriners Hospitals for Children grant SHG8670 to EWE. We thank Eileen Figueroa and Steve Schuenke for manuscript preparation. The funding organizations played no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Abbreviations
- CO
carbon monoxide
- COHb
carboxyhemoglobin
- DCPIP
dichlorophenolindophenol
- VOCs
volatile organic compounds
- BE
base excess
- MDA
malondialdehyde
- MSE
mannitol-sucrose-EDTA
- RC
respiratory complexes
- HCN
hydrogen cyanide
- H2S
hydrogen sulfide
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anderson MF, Sims NR. Improved recovery of highly enriched mitochondrial fractions from small brain tissue samples. Brain Res Brain Res Protoc. 2000;5:95–101. doi: 10.1016/s1385-299x(99)00060-4. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci U S A. 2005;102:16448–16453. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU, Gawehn K, Grassl M. In: Methods of Enzymatic Analysis. Bergmeyer HU, editor. Academic Press; New York: 1974. pp. 509–510. [Google Scholar]
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- Brown SD, Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J Appl Physiol. 1990;68:604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee HM, Greeley GH, Jr, Englander EW. Accumulation of oxidatively generated DNA damage in the brain: a mechanism of neurotoxicity. Free Radic Biol Med. 2007;42:385–393. doi: 10.1016/j.freeradbiomed.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 2004;1019:47–54. doi: 10.1016/j.brainres.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Sato A, Suzuki A, Trzebski A. Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. J Auton Nerv Syst. 1989;28:61–74. doi: 10.1016/0165-1838(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Garcia YJ, Rodriguez-Malaver AJ, Penaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods. 2005;144:127–135. doi: 10.1016/j.jneumeth.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Ginsburg MD. Carbon monoxide. In: Spencer PS, Schaumburg HH, editors. Experimental and Clinical Toxicology. Williams & Wilkins; London: 1980. [Google Scholar]
- Gu Z, Januszkiewicz AJ, Mayorga MA, Coleman GD, Morrissette CR. Consequences of brief exposure to high concentrations of carbon monoxide in conscious rats. Inhal Toxicol. 2005;17:755–764. doi: 10.1080/08958370500224904. [DOI] [PubMed] [Google Scholar]
- Hartzell GE. Overview of combustion toxicology. Toxicology. 1996;115:7–23. doi: 10.1016/s0300-483x(96)03492-0. [DOI] [PubMed] [Google Scholar]
- Iheagwara KN, Thom SR, Deutschman CS, Levy RJ. Myocardial cytochrome oxidase activity is decreased following carbon monoxide exposure. Biochim Biophys Acta. 2007;1772:1112–1116. doi: 10.1016/j.bbadis.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B, Ramzan R, Vogt S. Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol Med. 2009;15:139–147. doi: 10.1016/j.molmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Furuhata A, Toyokuni S, Aratani Y, Uchida K. Formation of acrolein-derived 2′-deoxyadenosine adduct in an iron-induced carcinogenesis model. J Biol Chem. 2003;278:50346–50354. doi: 10.1074/jbc.M309057200. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Thorburn DR, Turnbull DM, Taylor RW. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- Kou YR, Lai CJ. Reflex changes in breathing pattern evoked by inhalation of wood smoke in rats. J Appl Physiol. 1994;76:2333–2341. doi: 10.1152/jappl.1994.76.6.2333. [DOI] [PubMed] [Google Scholar]
- Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Greeley GH, Herndon DN, Sinha M, Luxon BA, Englander EW. A rat model of smoke inhalation injury: influence of combustion smoke on gene expression in the brain. Toxicol Appl Pharmacol. 2005;208:255–265. doi: 10.1016/j.taap.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Lee HM, Reed J, Greeley GH, Jr, Englander EW. Impaired mitochondrial respiration and protein nitration in the rat hippocampus after acute inhalation of combustion smoke. Toxicol Appl Pharmacol. 2009;235:208–215. doi: 10.1016/j.taap.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Snyder P, McNally H, Shi R, Borgens RB. Acrolein-mediated mechanisms of neuronal death. J Neurosci Res. 2006;84:209–218. doi: 10.1002/jnr.20863. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46:243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Marini H, Altavilla D, Bellomo M, Adamo EB, Marini R, Laureanti F, Bonaccorso MC, Seminara P, Passaniti M, Minutoli L, Bitto A, Calapai G, Squadrito F. Modulation of IL-1 beta gene expression by lipid peroxidation inhibition after kainic acid-induced rat brain injury. Exp Neurol. 2004;188:178–186. doi: 10.1016/j.expneurol.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- Miro O, Casademont J, Barrientos A, Urbano-Marquez A, Cardellach F. Mitochondrial cytochrome c oxidase inhibition during acute carbon monoxide poisoning. Pharmacol Toxicol. 1998;82:199–202. doi: 10.1111/j.1600-0773.1998.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Morris JB, Stanek J, Gianutsos G. Sensory nerve-mediated immediate nasal responses to inspired acrolein. J Appl Physiol. 1999;87:1877–1886. doi: 10.1152/jappl.1999.87.5.1877. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC. Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res. 2007;32:597–607. doi: 10.1007/s11064-006-9132-9. [DOI] [PubMed] [Google Scholar]
- Prockop LD, Chichkova RI. Carbon monoxide intoxication: an updated review. J Neurol Sci. 2007;262:122–130. doi: 10.1016/j.jns.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev. 2002;26:925–940. doi: 10.1016/s0149-7634(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Renner K, Amberger A, Konwalinka G, Kofler R, Gnaiger E. Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochim Biophys Acta. 2003;1642:115–123. doi: 10.1016/s0167-4889(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeve DR, Grisel JJ, Chen WJ. Neonatal alcohol exposure increases malondialdehyde (MDA) and glutathione (GSH) levels in the developing cerebellum. Brain Res Dev Brain Res. 2005;160:231–238. doi: 10.1016/j.devbrainres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Smith SM, Stuhmiller JH, Januszkiewicz AJ. Evaluation of lethality estimates for combustion gases in military scenarios. Toxicology. 1996;115:157–165. doi: 10.1016/s0300-483x(96)03504-4. [DOI] [PubMed] [Google Scholar]
- Speitel LC. Fractional effective dose model for post-crash aircraft survivability. Toxicology. 1996;115:167–177. doi: 10.1016/s0300-483x(96)03505-6. [DOI] [PubMed] [Google Scholar]
- Stefanidou M, Athanaselis S, Spiliopoulou C. Health impacts of fire smoke inhalation. Inhal Toxicol. 2008;20:761–766. doi: 10.1080/08958370801975311. [DOI] [PubMed] [Google Scholar]
- Stuhmiller JH, Long DW, Stuhmiller LM. An internal dose model of incapacitation and lethality risk from inhalation of fire gases. Inhal Toxicol. 2006;18:347–364. doi: 10.1080/08958370500516010. [DOI] [PubMed] [Google Scholar]
- Stuhmiller JH, Stuhmiller LM. A mathematical model of ventilation response to inhaled carbon monoxide. J Appl Physiol. 2005;98:2033–2044. doi: 10.1152/japplphysiol.00034.2005. [DOI] [PubMed] [Google Scholar]
- Vodicka P, Stetina R, Smerak P, Vodickova L, Naccarati A, Barta I, Hemminki K. Micronuclei, DNA single-strand breaks and DNA-repair activity in mice exposed to 1,3-butadiene by inhalation. Mutat Res. 2006;608:49–57. doi: 10.1016/j.mrgentox.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217–1225. doi: 10.1056/NEJMcp0808891. [DOI] [PubMed] [Google Scholar]