Abstract
We analyzed entry data from 163 adult hemoglobin SS and Sβ0 thalassemia patients enrolled in the prospective Sickle Cell Pulmonary Hypertension Screening Study and stratified their ECHO-determined tricuspid regurgitant jet velocity (TRV) and serum creatinine concentration according to three blood pressure categories. TRV was ≥2.5 m/sec in 27% of the patients with systolic blood pressure (SBP) <120 mm Hg and diastolic blood pressure (DBP) <70 mm Hg, in 37% with SBP 120–139 mm Hg or DBP 70–89 mm Hg, and in 93% with SBP 140 mm Hg or DBP 90 mm Hg or higher (P <0.0005 for trend). Serum creatinine concentration was 1.0 mg/dL or higher in 7% of patients with SBP <120 mm Hg and DBP <70 mm Hg, in 17% with SBP 120–139 mm Hg or DBP 70–89 mm Hg and 50% with SBP 140 mm Hg or DBP 90 mm Hg or higher (P <0.0005 for trend). Over two years of follow-up, there were trends for more frequent progression to elevated TRV (P = 0.073) or creatinine (P = 0.038) values according to the higher systemic blood pressure categories. Our findings suggest that systolic SBP 120–139 mm Hg or DBP 70–89 mm Hg defines a category of relative systemic hypertension in patients with sickle cell disease that is associated with increased risk for pulmonary hypertension and renal dysfunction. Whether antihypertensive and/or nitric oxide donor therapy in sickle cell disease patients with relative hypertension prevents these and other complications should be determined by clinical trials.
Introduction
A number of studies document that SCD is associated with lower systemic blood pressures than controls [1,2,3,4,5,6,7] and with a lower prevalence of systemic hypertension [1,2,5,7]. Potential mechanisms for the lower blood pressures in SCD patients include obstruction of the vasa recta in the kidney and repeated ischemia to the renal medulla, resulting in a distal renal tubular concentrating defect and hyposthenuria [8], lower body mass index in patients with SCD [4], and lower arterial stiffness [9].
The finding that patients with sickle cell disease have low blood pressure seems to counter recent pathophysiological studies suggesting that these patients develop endothelial dysfunction and systemic vasculopathy associated with reductions in NO bioavailablity [10,11,12,13,14,15,16,17]. However, blood pressures in SCD are higher than in thalassemia patients with a similar degree of anemia [2] and there is an association between cerebrovascular accident and higher blood pressures in SCD, even in a range of systolic and diastolic pressures that would be considered normal by conventional standards [2,3]. These studies raise the possibility that the normal range for blood pressure in patients with SCD should be lower than in healthy controls.
A recent report [7] confirmed that hemoglobin SS patients have lower systolic blood pressures than age-matched African-American controls, but that those with heterozygous sickling hemoglobinopathies (SC, S-βThal) have systolic blood pressures identical to age-matched African-American controls from NHANES III data.
In our recently published study of adult sickle cell disease patients [6], we found significantly higher systolic and mean systemic blood pressures in patients with PAH, as defined by tricuspid regurgitant jet velocity (TRV) ≥2.5 m/sec, than in those without PAH. This finding is consistent with the possibility that similar patho-physiologic pathways underlie both systemic and pulmonary hypertension in SCD. We found that the measures of renal function, BUN and creatinine, were significantly elevated in SCD patients with PAH as well.
Elevated systemic blood pressures also contribute to left ventricular diastolic dysfunction which has been shown to be an independent predictor of death in SCD patients [18]. We found that SCD patients that had systolic BP > 75th percentile (131 mm Hg) had significantly increased left ventricular mass by echocardiography and Doppler evidence of diastolic dysfunction. We also found a good correlation between the E/A ratio and the serum creatinine level suggesting that these were both end-organ effects of relative systemic hypertension. Accordingly, the purpose of the present study was to determine if relative systemic hypertension (i.e. SBP 120–139 mm Hg or DBP 70–89 mm Hg) is associated with complications and adverse outcomes in SCD.
Results
163 patients with hemoglobin SS (N = 158) or Sβ0 (N = 3) thalassemia were evaluated with echocardiogram, serum creatinine determination or both, and 86 underwent repeat evaluations a median of 24 months later (interquartile range of 23 to 27 months). The baseline characteristics of the patients are summarized in Table I. Forty-six percent of the patients had systemic blood pressures of <120 mm Hg systolic and <70 mm Hg diastolic (classified as normal), 44% had SBP of 120–139 mm Hg or DBP of 70–89 mm Hg (classified as relative hypertension) and 10% had SBP ≥140 mm Hg or DBP ≥90 mm Hg (classified as hypertension). None of the patients with SBP <120 mm Hg and DBP <70 mm Hg were on hemodialysis, but 3% in the relative systemic hypertension category and 13% with blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic were dialysis dependent. Median values for TRV, creatinine and blood urea nitrogen increased significantly according to higher systemic blood pressure categories. There was a trend to higher lactate dehydrogenase levels with higher blood pressure categories (P = 0.077). In a Spearman correlation of systolic blood pressure and lactate dehydrogenase, a significant positive relationship was found (n = 141, R = 0.172, P = 0.041) There were no significant differences in baseline blood pressure, TRV or creatinine between patients who did and did not have a two-year follow up (P > 0.2).
Table I.
Clinical characteristics of the participants according to systolic blood pressure category. Results are median and interquartile range or number and %.
| SBP <120 and DBP <70 mm Hg (N = 54 to 75) | SBP 120–139 and/or DBP 70–89 mm Hg (N = 51 to 71) | SBP ≥140 and/or DBP ≥90 mm Hg (N = 14 to 17) | P | |
|---|---|---|---|---|
| Age (years) | 33 (26–41) | 31 (26–39) | 43 (28–46) | 0.102 |
| Female sex | 43 (57.3%) | 39 (54.9%) | 9 (52.9%) | 0.699 |
| Hemodialysis | 0 (0%) | 2 (2.9%) | 2 (12.5%) | 0.009 |
| Body mass Index (kg/m2) | 21.7 (19.5–24.0) | 22.4 (20.7–25.2) | 22.8 (19.6–26.8) | 0.405 |
| Systolic blood pressure (mm Hg) | 106 (101–113) | 127 (121–131) | 148 (144–155) | --- |
| Diastolic blood pressure (mm Hg) | 59 (54–64) | 70 (62–76) | 83 (75–87) | --- |
| Tricuspid regurgitant jet velocity (m/sec) | 2.3 (2.0–2.5) | 2.3 (2.0–2.6) | 2.9 (2.7–3.0) | <0.0005 |
| Hemoglobin (g/dL)* | 9.0 (8.1–9.8) | 8.5 (7.7–9.7) | 8.0 (6.4–8.6) | 0.111 |
| Mean corpuscular volume (g/dL)* | 95 (86–101) | 91 (81–99) | 89 (80–96) | 0.063 |
| White blood cells (×10−3/uL) | 10.4 (8.4–11.9) | 10.3 (8.1–13.1) | 10.4 (9.1–13.2) | 0.740 |
| Reticulocytes (×10−3/uL)* | 257 (204–344) | 307 (204–344) | 349 (190–450) | 0.536 |
| Lactate dehydrogenase (U/L) | 324 (250–437) | 372 (295–451) | 411 (292–431) | 0.077 |
| Ferritin (ug/L) | 311 (117–1325) | 408 (140–1291) | 733 (345–1525) | 0.215 |
| Creatinine (mg/dL) | 0.6 (0.5–0.7) | 0.7 (0.5–0.8) | 1.0 (0.7–1.4) | 0.003 |
| Blood urea nitrogen (mg/dL) | 7 (5–10) | 7 (6–11) | 14 (9–21) | 0.005 |
Patients without recent blood transfusion, n = 51, 47, 6.
Elevated tricuspid regurgitant jet velocity (TRV) values according to systemic blood pressure categories
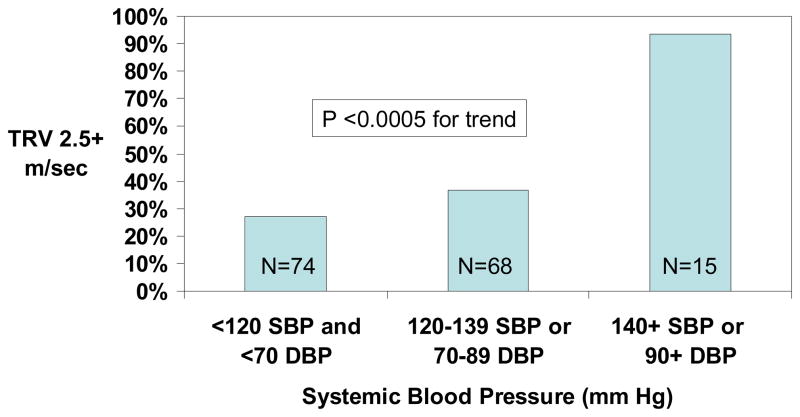
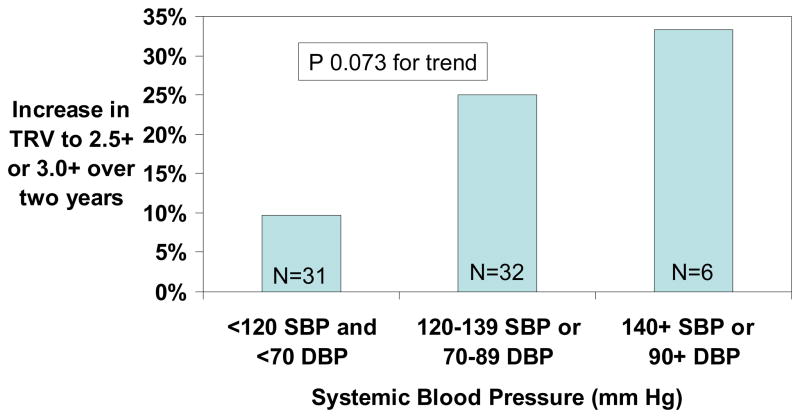
The prevalence of TRV ≥2.5 m/sec was 27% with SBP <120 mm Hg and DBP <70 mm Hg, 37% with SBP 120–139 mm Hg or DBP 70–89 mm Hg and 93% with SBP ≥140 mm Hg or DBP ≥90 mm Hg (Figure 1). Of the 69 patients with initial TRV <3.0 m/sec who were followed-up two years later, TRV increased from <2.5 m/sec to ≥2.5 m/sec or from 2.5–2.9 m/sec to ≥3.0 m/sec in 13 (19%). The progression rate was 10% with normal blood pressure, 25% with relative hypertension and 33% with elevated blood pressure (Figure 2).
Figure 1.
Prevalence of tricuspid regurgitant jet velocity (TRV) ≥2.5 m/sec according to blood pressure category in adult patients with hemoglobin SS or Sβ0 thalassemia.
Figure 2.
Progression of TRV from <2.5 m/sec to ≥2.5 m/sec or from 2.5–2.9 m/sec to ≥3.0 m/sec over two years of follow-up among hemoglobin SS or Sβ0 thalassemia participants with baseline TRV <3.0 m/sec.
Elevated serum creatinine concentration according to systemic blood pressure categories
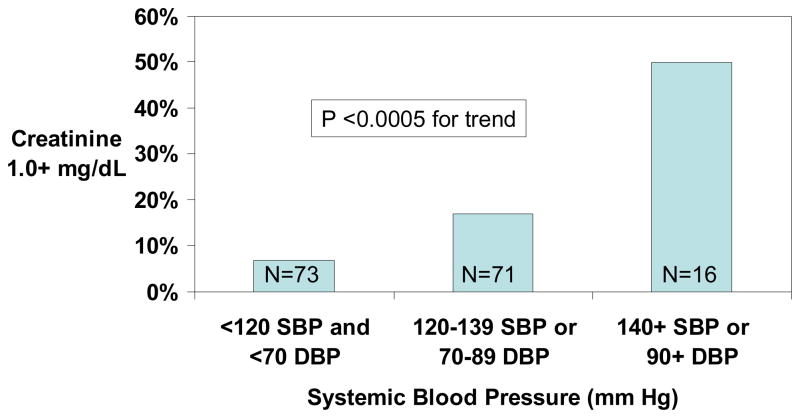
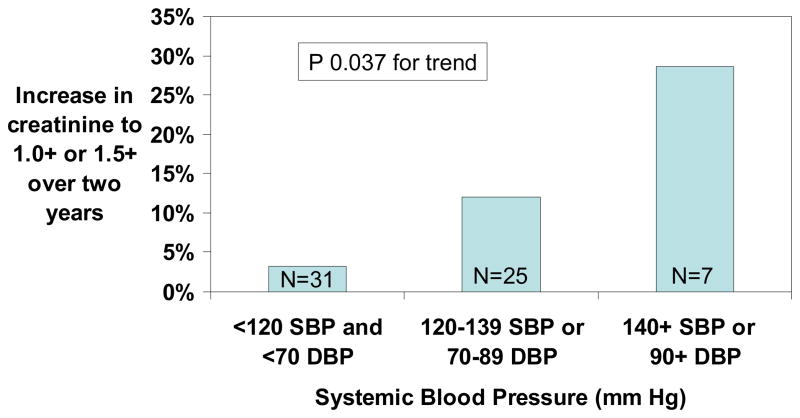
The prevalence of serum creatinine ≥1.0 mg/dL was 7% with SBP <120 mm Hg and DBP <70 mm Hg, 17% with SBP 120–139 mm Hg or DBP 70–89 mm Hg and 50.0% with SBP ≥140 mm Hg or DBP ≥90 mm Hg (Figure 3). Of the 63 patients with initial serum creatinine concentration ≤1.4 mg/dL who were followed-up two years later, serum creatinine increased from <1.0 mg/dL to ≥1.0 mg/dL or from 1.0–1.4 mg/dL to ≥1.5 mg/dL in 6 (9.5%). The progression rate was 3% with normal blood pressure <120 mm Hg, 12% with relative hypertension, and 29% with elevated blood pressure (Figure 4).
Figure 3.
Prevalence of serum creatinine ≥1.0 mg/dL according to systemic blood pressure category in adult patients with hemoglobin SS or Sβ0 thalassemia.
Figure 4.
Increase in serum creatinine concentration from <1.0 mg/dL to ≥1.0 mg/dL or from 1.0–1.4 mg/dL to ≥1.5 mg/dL over two years of follow-up in adult patients with hemoglobin SS or Sβ0 thalassemia.
Progression to hypertension over two years of follow-up
At the two-year follow up evaluation, hypertension was observed in none of 19 patients who initially had SBP <120 mm Hg and DBP <70 mm Hg and in 2 of 19 (16%) of those with relative systemic hypertension at initial evaluation.
Discussion
In the emerging paradigm of SCD vasculopathy, increased risk for pulmonary and systemic hypertension, renal dysfunction, proteinuria, stroke and early death may be related to the degree of hemolysis, nitric oxide scavenging and resultant damage to the vasculature [6,19,20,21,22,23]. Other risk factors identified that are associated with pulmonary hypertension and vasculopathy include markers of iron overload, left ventricular diastolic dysfunction, asplenia and cholestatic hepatic dysfunction [6,18]. In this study, we postulated that, similar to patients with diabetes mellitus and renal failure [24], systemic SBP 120–139 mm Hg or DBP 70–89 mm Hg may represent relative hypertension in patients with hemoglobin SS and Sβ0 thalassemia and identify patients at increased risk for pulmonary arterial hypertension and renal dysfunction. Consistent with our hypothesis, we observed increasing prevalences of elevated TRV and creatinine values with the three progressively higher systemic blood pressure categories. We also observed trends to more rapid progression to elevated TRV or creatinine over two years of follow-up according to these increasing blood pressure categories.
Given the high mortality in SCD patients with PAH [6,25,26] and the high progression rate we observed over two years, it is important to find ways to prevent the progression to PAH. Similarly, given the high mortality in patients with end stage renal disease and the progression to elevated serum creatinine concentration over two years, it is important to find ways to prevent progression of renal dysfunction. It is possible that treating relative systemic hypertension (120–139 mm Hg) in patients with hemoglobin SS and Sβ0 thalassemia may inhibit progression to PAH, the advance of renal disease and other vascular complications. In the general population, control of systemic hypertension is essential in preventing cardiovascular complications. It is possible that in SCD control of relative systemic hypertension may be required as well. Even mild increases in pulmonary artery pressure are poorly tolerated in SCD patients [6,25,26] and the same may apply to systemic blood pressures.
Limitations to this study include that only half of the patients enrolled participated in the two year follow up and that the present report on relative systemic hypertension is based on a retrospective analysis.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure describes an independent, continuous risk between BP and risk of cardiovascular disease and recognizes the importance of this relationship by introducing a new classification called prehypertension [24]. Patients in this category (SBP 130–39 or DBP 80–89) are felt to be at significantly increased risk of progressing to hypertension and JNC 7 identifies six compelling conditions for which antihypertensive therapy should be instituted. These conditions include heart failure, postmyocardial infarction, high coronary disease risk, diabetes mellitus, chronic kidney disease and recurrent stroke prevention. Our present study adds to the growing body of evidence associating end-organ effects in the heart, lung, and kidneys from relative systemic hypertension in the SCD population. Thus, SCD may be an additional condition for which antihypertensive therapy should be started at or below the prehypertension level. Clinical trials are needed to guide initiation of therapy and treatment goals in the SCD population.
Methods
The patient population consisted of 163 adults with hemoglobin SS or Sβ0 thalassemia who participated in the Sickle Cell Pulmonary Hypertension Screening Study and who had values recorded for systolic blood pressure and also values for tricuspid regurgitant jet velocity (TRV) by echocardiogram, serum creatinine concentration or both. All patients gave written informed consent and were enrolled between February 2001 and March 2005. Details of the clinical and echocardiographic methods (and study results for most of these patients) were reported previously [6]. For 86 patients in this cohort, follow-up echocardiograms, serum creatinine concentrations and/or systemic blood pressures were determined approximately two years after their initial enrollment. The present report represents a retrospective analysis, although all of the data were collected prospectively.
For the present study, patients were categorized as having normal systemic blood pressure (<120 mm Hg systolic and <70 mm Hg diastolic), relative hypertension (systolic blood pressure 120–139 mm Hg or diastolic blood pressure 70–89 mm Hg), or hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg) based on the baseline blood pressures determined at study entry. Patients with systemic blood pressures >140 mm/Hg systolic or >90 mm Hg diastolic at enrollment were treated with anti-hypertensive agents, but those with lower systemic blood pressures were not.
Elevated TRV was taken to be ≥2.5 m/sec. The relationship of pulmonary blood pressure determined by right heart catheterization with TRV determined by echocardiography has been documented in several studies [6,27]. For example In a study of 41 patients with SCD, TRV correlated with mean pulmonary artery pressure (R = 0.76, P <0.001), pulmonary vascular resistance (R = 0.55, P <0.001), maximal oxygen uptake (R = −0.43, P = 0.011), and the six minute walk test (R = −0.55, P <0.001) [27].
Elevated serum creatinine concentration was taken to be ≥1.0 mg/dL. We chose this serum creatinine value as indicative of early renal involvement because among the cohort of hemoglobin SS patients <40 years of age in the large CSSCD cohort SS patients, the 75th percentiles for serum creatinine values were at or below 1.0 mg/dL [28]. The mean ± SD body mass index (BMI) in patients with an elevated creatinine was 21.8 ± 4.4 kg/m2 while the BMI in those with a normal creatinine was 23.6 ± 6.1 kg/m2 (P = 0.130).
Continuous variables were compared according to blood pressure category with the Kruskal-Wallis test and categorical variables with Cochran’s linear trend test.
Acknowledgments
Supported in part by grant nos. 2 R25 HL003679-08 and 1 R01 HL079912-02 from NHLBI and by Howard University GCRC grant no 2MOI RR10284-10 from NCRR, NIH, Bethesda, MD.
Contributor Information
Victor R. Gordeuk, Center for Sickle Cell Disease and Department of Medicine, Howard University, Washington, DC.
Vandana Sachdev, Vascular Medicine Branch, NHLBI, Bethesda, MD.
James Taylor, Vascular Medicine Branch, NHLBI, Bethesda, MD.
Mark T. Gladwin, Vascular Medicine Branch, NHLBI, Bethesda, MD.
Gregory Kato, Vascular Medicine Branch, NHLBI, Bethesda, MD.
Oswaldo L. Castro, Center for Sickle Cell Disease and Department of Medicine, Howard University, Washington, DC.
References
- 1.Johnson CS, Giorgio AJ. Arterial blood pressure in adults with sickle cell disease. Arch Intern Med. 1981 Jun;141(7):891–3. [PubMed] [Google Scholar]
- 2.Rodgers GP, Walker EC, Podgor MJ. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993 Mar;305(3):150–6. doi: 10.1097/00000441-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, Vichinsky E. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997 Feb;102(2):171–7. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- 4.Aderibigbe A, Omotoso AB, Awobusuyi JO, Akande TM. Arterial blood pressure in adult Nigerian sickle cell anaemia patients. West Afr J Med. 1999 Apr-Jun;18(2):114–8. [PubMed] [Google Scholar]
- 5.Ernst AA, Weiss SJ, Johnson WD, Takakuwa KM. Blood pressure in acute vaso-occlusive crises of sickle cell disease. South Med J. 2000 Jun;93(6):590–2. [PubMed] [Google Scholar]
- 6.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 7.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17:2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 8.Saborio P, Scheinman JI. Sickle Cell Nephropathy. J Am Soc Nephrol. 1999;10:187–192. doi: 10.1681/ASN.V101187. [DOI] [PubMed] [Google Scholar]
- 9.Lemogoum D, Van Bortel L, Najem B, Dzudie A, Teutcha C, Madu F, et al. Arterial stiffness and wave reflections in patients with sickle cell disease. Hypertension. 2004;44:924–9. doi: 10.1161/01.HYP.0000148506.73622.ba. [DOI] [PubMed] [Google Scholar]
- 10.Aslan M, Ryan TM, Adler B, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98(26):15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberhardt RT, McMahon L, Duffy SJ, et al. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol Oct. 2003;74(2):104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- 12.Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–8. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 13.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278(6):H1799–1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- 14.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest Oct. 2004;114(8):1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nath KA, Shah V, Haggard JJ, et al. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R1949–1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 16.Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation Apr. 2004;11(2):179–193. doi: 10.1080/10739680490278592. [DOI] [PubMed] [Google Scholar]
- 17.Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Curr Opin Hematol Mar. 2003;10(2):99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev V, Machado RF, MD, Shizukuda Y, Rao YN, Sidenko S, Ernst I, StPeter M, RDCS, Coles WA, Rosing DR, Blackwelder WC, Castro O, Kato GJ, Gladwin MT. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2006 Nov 3; doi: 10.1016/j.blre.2006.07.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, 6th, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006b Mar 15;107(6):2279–85. doi: 10.1182/blood-2005-06-2373. Epub 2005 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato GJ, Hsieh M, Machado R, Taylor J, 6th, Little J, Butman JA, Lehky T, Tisdale J, Gladwin MT. Cerebrovascular disease associated with sickle cell pulmonary hypertension. Am J Hematol. 2006c Jul;81(7):503–10. doi: 10.1002/ajh.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005 Jul 6;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 24.Joint National Committee. National High Blood Pressure Education Program, NIH Publication No 03–5233. Dec, 2003. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. [Google Scholar]
- 25.Castro OL, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–61. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 26.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 27.Anthi A, Machado RF, Jison ML, Taveira-DaSilva AM, Rubin LJ, Hunter L, Hunter CJ, Coles W, Nichols J, Avila NA, Sachdev V, Chen CC, Gladwin MT. Hemodynamic and functional assessment of sickle cell disease patients with pulmonary hypertension. AJRCCM. 2007 doi: 10.1164/rccm.200610-1498OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West MS, Wethers D, Smith J, Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992 Aug;45(8):893–909. doi: 10.1016/0895-4356(92)90073-v. [DOI] [PubMed] [Google Scholar]