Abstract
The 22q13 deletion syndrome is characterised by intellectual disability (ID), delayed or absent speech, autistic-like behaviour and minor, nonspecific dysmorphic features. The deletion of the SHANK3 gene is thought to be responsible for these features. In this study, the clinical data of 7 patients with the 22q13 deletion syndrome are presented, obtained by clinical genetic examination, direct behavioural observation and by interview of family members and/or caregivers, complemented by behavioural questionnaires. The specific focus was on behaviour, psychopathology and the level of functioning during life course in order to determine common features that might contribute to the delineation of the syndrome. Major findings were a high incidence of psychiatric disorders, more in particular bipolar disorder (BPD) and attention deficit hyperactivity disorder (ADHD), and a sudden deterioration after acute events, in addition to a progressive loss of skills over years. Therefore, a deletion of SHANK3 may result in a dysfunctional nervous system, more susceptible to developmental problems and psychiatric disorders on the one hand, less able to recuperate after psychiatric and somatic events, and more vulnerable to degeneration at long term on the other hand. These results are exploratory and need to be confirmed in a larger sample.
Key Words: Behaviour, Bipolar disorder, Deletion 22q13, Life course, Phelan-McDermid, Psychiatric disorders, SHANK3
Recent advances in genetic testing enable the determination of the underlying genetic defect responsible for the phenotype in many patients. Several new microdeletion syndromes are being delineated, such as the 22q13 deletion syndrome, also known as the Phelan-McDermid syndrome. The clinical characteristics of this syndrome consist of intellectual disability, delayed or absent speech, autistic-like behaviour and minor, non-specific dysmorphic features [Phelan et al., 2001; Havens et al., 2004; Manning et al., 2004; Cuzmano-Ozog et al., 2007]. Although deletion sizes differ between patients, the absence of SHANK3 is considered to be responsible for the neurological symptoms of the syndrome [Bonaglia et al., 2001].
The SHANK3 gene is preferentially expressed in the cerebral cortex and cerebellum and encodes a multidomain scaffolding protein, ProSAP2 or SHANK3, in the postsynaptic density [Bonaglia et al., 2001]. Beside anchoring and clustering glutamate receptors exactly opposite to the presynaptic neurotransmitter release site, this protein also interacts with the cytoskeleton. Therefore, it is thought to play a significant role in the assembly of the postsynaptic density during synaptogenesis, in synaptic plasticity and regulation of dendritic spine morphology [Boeckers et al., 2002].
This study presents the clinical data of 7 patients with the 22q13 deletion syndrome, obtained by clinical examination by a clinical geneticist, direct behavioural observation, and by interview of family members and/or caregivers, complemented by behavioural questionnaires. The main focus was behaviour, psychopathology and the level of functioning during the life course, in order to determine common features that might contribute to the delineation of the syndrome.
Materials and Methods
Study Population
Over the last 6 years, almost 4,000 patients with intellectual disability (ID) underwent a diagnostic full genome array at the Centre for Human Genetics of the University Hospitals Leuven, Belgium. Informed consent was obtained from the parents or legal guardians of the affected patients and their healthy family members. Genomic DNA was extracted from peripheral leukocytes of EDTA-treated blood according to standard procedure guidelines.
The genomic DNA of the patients was either screened using the BAC/PAC-array [Menten et al., 2006] or the Oxford Gene Technology (OGT) CytoSure™ ISCA oligoarray set (Oxford Gene Technology, Oxford, UK) containing either 105k or 180k DNA oligonucleotides with a minimum resolution of 200 kb. All genome coordinates were according to NCBI human genome build 37 (hg19, February 2009).
In 7 patients, a 22q13 microdeletion was identified. In 1 patient (patient 7), an additional small copy number variation of 31 kb of unknown significance at 5q35.3 was also detected. The ages of the patients range from 5 to 51 years, and 3 patients were male, 4 female. All patients were examined at one or more occasions by an experienced clinical geneticist. Medical history was obtained from the parents, grand-parents, care-givers, and referring physicians. If parental DNA was available, array comparative genome hybridisation or FISH was performed to determine whether the deletion was inherited or not.
Observation and Questionnaires
All patients were directly observed in their everyday environment by 2 observers during at least 1 h. Parents and/or caregivers were also interviewed to obtain data regarding the developmental course, former and present level of functioning, medical and psychopathological history. In addition, adaptive functioning, autism spectrum symptoms and psychopathology in all 7 individuals were assessed by questionnaires. Family members and/or caregivers completed the Dutch versions of the Vineland Adaptive Behaviour Scale for individuals with ID (VABS, Vineland-Z) [de Bildt and Kraijer, 2003], the Scale of Pervasive Developmental Disorders in Mentally Retarded Persons (PDD-MRS) [Kraijer and de Bildt, 2005] and the Developmental Behaviour Checklist (DBC) [Einfeld and Tonge, 1995].
The Vineland-Z includes 225 items, divided in 3 domains: communication (67 items), daily living skills (92 items) and socialisation (66 items). In each domain, the items are ranked by level of difficulty in developmental order. Scores are determined by means of an open-ended interview with the personnel as respondents. Item-scores (2, 1 or 0) are calculated per domain in which they indicate the following: 2 = behaviour is usually performed, 1 = behaviour is sometimes or partly performed, 0 = behaviour is not performed. The Vineland-Z total score includes the 3 separate domain-scores.
The PDD-MRS is a simple classification and screening instrument for identification of autistic disorders (of the entire spectrum) in persons with ID ranging from mild to profound with an age-range of 2–55 years.
The DBC is a validated measure of psychopathology in young people with ID. Each item is rated as follows: not true, somewhat true or very true. Information from a Total Behaviour Problem Score and 5 sub-scales (self-absorbed, disruptive, communication disturbance, social relating and anxiety) was analysed.
Results
The overview of the clinical and molecular data is presented in table 1. There were no peculiarities during pregnancy, birth or neonatal period. Except for patient 3, each individual was able to walk independently before the age of 18 months. In all patients, language development was severely retarded (first words between 2 years and 6 months and 6 years). Currently, all function at a severe to profound level of ID. Five are in residential care, the 2 youngest attend special education and live in their home environment, combined with semi-residential care. All of them attended special education, and none are able to work in a sheltered environment.
Table 1.
Overview of clinical characteristics and developmental data
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| Age | 5 yrs 9 mo | 6 yrs 10 mo | 17 yrs 8 mo | 24 yrs 9 mo | 43 yrs 10 mo | 46 yrs 6 mo | 51 yrs 11 mo | |
| Sex | M | F | M | F | F | M | F | |
| Family history | negative | both parents moderate ID | negative | negative | negative | negative | limb defects in brother and brother of maternal grandfather | |
| Inheritance | de novo | maternally inherited | de novo | de novo | parents NT, sibs normal | parents NT, sibs normal | NT | |
| Deletion size | 49.284.146-49.501.571 217 kb | 49.425.750-49.624.810 199 kb | 49.492.103-49.567.789 76 kb | 49.470.363-49.567.789 97 kb | 47.740.349-49.453.810 1.7 Mb | 48.181.791-49.453.810 1.2 Mb | 46.163.519-49.567.789 3.4 Mb | |
| Gene content | SHANK3 + 11 other genes | SHANK3, ACR, RABL2B | SHANK3, ACR, RABL2B | SHANK3, ACR, RABL2B | SHANK3, MLC1, BRD1 + 30 other genes | SHANK3, MLC1, BRD1 + 30 other genes | SHANK3, MLC1, BRD1 + 34 other genes | |
| Array platform | OGT 180K | 1 Mb array/FISH | OGT 105K | OGT 180K | 1 Mb array/FISH | 1 Mb array/FISH | OGT 105K | |
| Dysmorphism | - | strabismus | coarse face, full lips, deep-set eyes straight eyebrows, low-set ears, hypotonic posture, joint hyperlaxity, clubfeet | - | hypotonic face, kyphoscoliosis, cutaneous syndactyly fingers 3 and 4, finger swanning, wrist dorsiflexion, thumb adduction | - | - | |
| Growth parameters | ||||||||
| Age | 5 yrs 1 mo | 6 yrs | 15 yrs | 24 yrs | 37 yrs | 41 yrs | 55 yrs | |
| Weight (kg) | 17.7 (P25) | 19.3 (P10) | 55 (P25) | 53 (P10–25) | 52 (P10–25) | 63.5 (P10–25) | 64.3 (P50–75) | |
| Length (cm) | 105 (P10) | 114 (P3–10) | 170 (P25) | 161 (P10–25) | 158 (P10) | 176 (P25) | 168 (P50–75) | |
| OFC (cm) | 52 (P75) | 49.9 (P25) | 54.5 (P25) | 54.7 (P50) | 54 (P25–50) | 57 (P50–75) | 55 (P50) | |
| Somatic diagnoses/age | - | - | eczema, drop attacks of unknown etiology | - | Crohn's disease/28 yrs, metrorraghia | mumps encephalitis/18 mo, NMS/27 yrs, DVT/34 yrs, septic shock/40 yrs | epilepsy, epileptic state/45 yrs, gastric reflux | |
| Neuroimaging/age | - | MRI/2 yrs 8 mo: normal | MRI/22 mo: normal | MRI/9 yrs: normal CT/19 yrs: normal | CT/19, 25, and 41 yrs: corticosubcortical atrophy | CT/19, 30 yrs: normal CT/40 yrs: infarctions basal ggl. | CT/43 yrs: mild corticosubcortical atrophy | |
| Developmental testing/age | BSID/25 mo: 11.5 mo BSID/3 yrs 10 mo: 15mo | BSID/23 mo: 15 mo BSID/2 yrs 4 mo: 16 mo BSID/3 yrs 3 mo: 16 mo, OI <55 | BSID/17 yrs: <3mo | SON/9 yrs 5 mo: 3–3 yrs 6 mo Terman IQ 39 | IQ/7 yrs: 850 IQ 30 | SON-IQ/18 yrs: 18 | Terman/10 yrs <2 yrs BSID/34 yrs: 12 mo BSID/36 yrs: 11 mo | |
| Language testing/age | NNST/26 mo: R+E <12 mo NNST/38 mo: R: <12 mo, E: 15 mo | Reynell/3 yrs 4 mo: 1 yr 5 mo NNST/4 yrs 8 mo: R: 12 mo, E <Pc1 | Reynell/9 yrs 5 mo: R: 2 yrs 9 mo E: 3 yrs 6 mo | |||||
| Motor testing/age | PDMS/26 mo: 18 mo PDMS/38 mo: G: 18 mo, F: 36 mo, VMI: 16 mo | |||||||
| Progressive loss of skills | + | + | + | + | + | + | + | |
| Current level of functioning | ||||||||
| ID | severe | severe | profound | severe | severe | profound | profound | |
| Language | short sentences | single words | no speech | single words | single words | no speech | no speech | |
| Motor function | walking | walking | walking | walking | walking | wheelchair bound | bedridden | |
| Current behaviour | ||||||||
| PDD-symptoms | + | + | + | + | + | + | + | |
| ADHD symptoms | + | + | - | - | - | - | - | |
| Psychotic symptoms | - | - | - | + | - | - | - | |
| Other | defiant, low frustration tolerance, mouthing | mouthing | shouting, bursts of anger, mouthing | extreme swings of mood and activity level, chaotic and disorganized, mouthing | - | varying mood and sleep, irritability | - | |
| Psychiatric diagnosis/age | - | ADHD/5 yrs | - | rapid cycling BPD/19 yrs, catatonic state/24 yrs | BPD/30 yrs | BPD/16 yrs | BPD/22 yrs | |
| Familial psychiatric diagnoses | - | - | - | - | brother of father psychiatric care, no known diagnosis | - | not known | |
| Current psychiatric medication | - | methylphenidate | mianserin | lithium, valproate, carbamazepine | - | valproate | - | |
yr/yrs = Year(s); mo = months; NT = not tested; - = absent; + = present; NMS = neuroleptic malignant syndrome; DVT = deep venous thrombosis; ggl. = ganglia; BSID = Bayley Scales of Infant Development, Dutch version; SON = Snijders-Oomen Nonverbal Intelligence Test; NNST = Dutch Nonspeech Test; Reynell R = receptive language, E = expressive language; PDMS = Peabody Developmental Motor Scale; G = gross motor skills; F = fine motor skills; VMI = visuomotor integration; PDD-symptoms = low social reciprocity, poor nonverbal skills, stereotypic behaviour, rigidity; ADHD-symptoms = short attention span, hyperactivity; Psychotic symptoms = hallucinations, delusions; BPD = bipolar disorder.
Patient 2 inherited the deletion from her mother; this 36-year-old mother with a moderate ID had normal early motor milestones, and her early language milestones were not markedly delayed either. At present, she is able to construct elementary sentences, works in a sheltered environment and needs major assistance from her parents to take care of her daughter. There is no history of somatic or psychiatric problems.
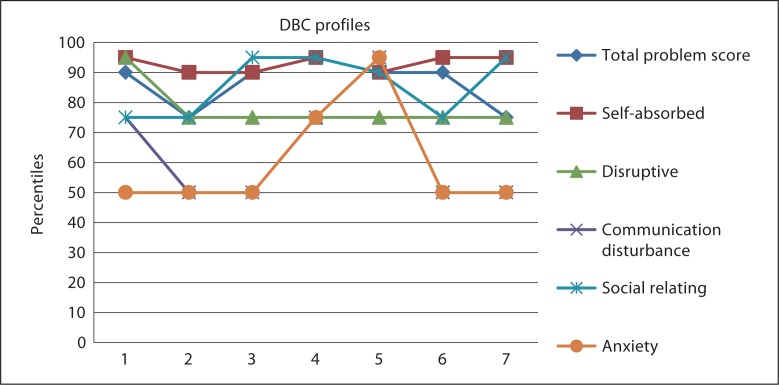
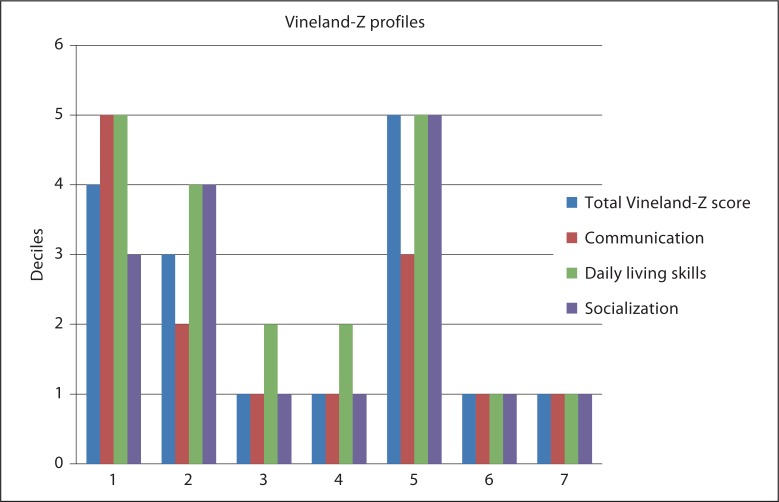
The results of the questionnaires regarding the 7 observed patients are summarized in figures 1 and 2, and table 2.
Fig. 1.
DBC-profiles in del22q13.3 (n = 7).
Fig. 2.
Vineland-Z profiles in del22q13.3 (n = 7).
Table 2.
Scores of the 7 individuals with del22q13.3 on the PDD-MRS
| Subject | Gender | Age (years/months) | PDD-MRS | Classification |
|---|---|---|---|---|
| 1 | male | 5/9 | 11 | PDD |
| 2 | female | 6/10 | 9 | PDD |
| 3 | male | 17/8 | 15 | PDD |
| 4 | female | 24/9 | 14 | PDD |
| 5 | female | 43/10 | 5 | N |
| 6 | male | 46/6 | 6 | N |
| 7 | female | 51/11 | 6 | N |
N = normal range.
In figure 1, the DBC-profiles of all 7 patients with a del22q13.3 are shown. All individuals show severe challenging behaviour; all have a total problem score between Pc. 75–90. Most prominent problem behaviours are self-absorbing behaviour, problems with social relating and disruptive behaviour. In 1 individual (patient 5), anxiety is the most problematic.
Figure 2 shows the Vineland-Z profiles of all 7 patients with a del22q13.3. In 6 out of 7 patients, communication is very poor, and 5 out of 7 have prominent problems within the domain of socialisation. Daily living skills seem to be well developed in 5 out of 7 patients.
In table 2, the total PDD-MRS score of all 7 patients is reported. Four individuals have a score within the PDD-spectrum: most typical for these patients are the unpredictable outbursts, stereotypic behaviours (clapping, biting) and obsessive traits.
Although all individuals have always shown autistic-like behaviour, like low or absent social reciprocity, poor nonverbal skills, stereotypic behaviour and rigidity, none have been specifically examined and assessed for autism. This is probably due to the severe to profound level of ID. Our observations and gathered data indicate that the criteria for a diagnosis of PDD may be fulfilled in several individuals.
Four patients have been evaluated by a psychiatrist. Due to extreme overactive and impulsive behaviour with a very short attention span, patient 2 was diagnosed with ADHD, responding well to treatment with methylphenidate. Four others (patients 4, 5, 6, and 7) received a formal diagnosis of bipolar disorder (BPD) because of the presence of at least one manic episode with irritable mood, psychomotor agitation, decreased need for sleep, and increased speech (increased babbling and talking). The latter was unexpected because these patients otherwise did not talk.
Remarkably, many patients show a progressive loss of skills during their life. This is the most subtle in the 2 youngest children, with regard to language skills, e.g. losing acquired words when they are no longer practiced. The other individuals also showed deterioration in language over years, like a decline in receptive and expressive language or a regression in pronunciation. Several other developmental domains are also affected. Concerning gross and fine motor skills, parents, caregivers and therapists observed, for instance, deterioration in balance and coordination, progressive rigidity of the posture with shuffling gait, loss of the ability to do handwork, and to eat with a knife and fork. With regard to social skills, a reduced eye-contact and diminished social interest were perceived.
In addition, several patients showed severe and sudden loss of skills after acute events which could not always be regained.
Patient 4 was diagnosed with a BPD, rapid cycling type with psychotic symptoms. The response of neuroleptics and benzodiazepines to the excessive mood and activity swings was poor, thus requiring higher doses. Due to a rise in temperature and the fear of a neuroleptic malignant syndrome, the neuroleptics were stopped. Few days later, she was hospitalized for a day because of a sudden blood pressure fall with decreased consciousness, presumably because of an overdose of benzodiazepines. This was immediately followed by an apathetic and catatonic period, in which she stopped moving and talking. Afterwards, her level of functioning had become very different than before. She was not able to use the language she knew, stopped interacting with others and did no longer recognize her mother. The mood swings became even more explicit than they had been before. She stopped eating independently and also lost continence.
Patient 6 was hospitalized in intensive care for a malignant neuroleptic syndrome during a substantial manic episode at the age of 27, treated with high doses of haloperidol. Afterwards, he lost the ability to walk or eat independently, needing a long period of rehabilitation to recover. At 40 years, he was hospitalized again for septic shock due to aspiration pneumonia. Thereafter, he lost even more skills (loss of active and passive language, loss of walking, independently eating and dressing, and loss of continence) that could not be regained at all. Currently, he is spastic and wheelchair bound.
Patient 7 became totally dependent and bedridden after a prolonged epileptic state.
Discussion
At present, the psychiatric disorder the most frequently associated with the 22q13 deletion syndrome is autism. In our patient sample, all individuals show autistic-like behaviour and 4 patients have a PDD-MRS-score that falls within the PDD-spectrum. However, the most remarkable findings in our study are the high incidence of another psychiatric disorder, namely BPD, together with progressive loss of skills.
Bipolar Disorder
Surprisingly, 4 out of a group of 7 individuals (including 2 young children) were diagnosed with BPD. The diagnosis of paediatric BPD still remains controversial because the clinical presentation of mania in children and adults can be very distinct. Furthermore, the high incidence of comorbid disorders complicates the diagnostic process because of the significant symptom overlap in children. Due to the fact that clinicians are often more familiar with the clinical presentation of the comorbid disorder, like for instance ADHD, some presume that a considerable part of the children diagnosed with ADHD might actually have BPD or an unrecognised comorbid BPD [Youngstrom et al., 2005].
If in our group only the adults are taken into consideration, the incidence of BPD is very high (4 out of 4 patients). One of the children (patient 2) is diagnosed with ADHD. She doesn't show manic episodes, but her further development and behaviour should be carefully monitored.
Presently, only little data regarding mood disorders in patients with 22q13 deletion syndrome are available in the literature. However, BPD has been associated with several chromosome regions, amongst others 22q13 [Kato, 2007]. In this region, 2 potential BPD candidate genes, MLC1 and BRD1, were put forward. The MLC1 gene is expressed in brain and encodes a putative nonselective cation channel. It is presumed to modulate neuronal functions [Verma et al., 2005]. BRD1, also expressed in brain, is considered a potential regulator of transcription with a presumed role in neurodevelopment [Severinsen et al., 2006]. In our group of bipolar patients, the deletion in patient 5, 6 and 7 involves beside SHANK3 also MLC1 and BRD1. However, in patient 4, the latter genes are not deleted, suggesting a possible role for SHANK3 in this disorder. This is in agreement with a previous finding where a patient with a ring chromosome 22 was described with a rapid cycling BPD [Sovner et al., 1996]. Although no further molecular studies were done in this patient, it is likely that SHANK3, due to its subtelomeric position, is deleted.
Loss of Skills
Another remarkable observation is the loss of skills in many of our patients. This regression is the most apparent in the oldest patients and occurs most dramatically after acute events, such as a septic shock, epileptic state, catatonic phase, or malignant neuroleptic syndrome. A similar severe neurological deterioration was observed in 2 individuals with epilepsy over 40 years in a group of 44 patients with the 22q13 deletion syndrome, and SHANK3 haploinsufficiency was considered as responsible for this [Bonaglia et al., 2011].
This raises the interesting question whether the deterioration in our patients is a consequence of acute incidents or less acute underlying pathology like BPD, or whether it might also be inherent to the 22q13 deletion syndrome. The latter may be the case since some of these patients already showed a progressive loss of functioning decennia before the acute deterioration. Furthermore, the loss of functioning is also reported in the patients with an uneventful clinical course. It is even noticeable, though in a more subtle manner, in the youngest patients, suggesting an onset during childhood. This observation might suggest a direct relation between the neurological deterioration and the SHANK3 deletion in the Phelan-McDermid syndrome. It might be possible that haploinsufficiency of SHANK3 renders the nervous system more vulnerable to degeneration on long term and less capable to recuperate after psychiatric and somatic events.
Strengths and Limitations of This Research
This research has several strengths. First, we were able to gather retrospective data in addition to data regarding the current level of functioning of our patients, and this enabled us to recognise the degenerative processes. Furthermore, we had direct contact with parents and caregivers besides the use of questionnaires. Moreover, the direct observation of the patients by 2 behavioural scientists led to a better understanding and interpretation of the data obtained by the interview and questionnaires.
The most important limitation of this research is the size of our sample, too small to draw firm conclusions. Second, the large age differences render the interpretation and comparison of data more difficult. Third, diagnostics are made by different psychiatrists at different moments. However, the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) have been used [APA, 2001].
Directives for the Future
First, in clinical practice, the possibility of a 22q13 deletion needs to be considered in severe to profound intellectually disabled individuals not only with autistic-like behaviour, but also in individuals with ADHD or ADHD- like behaviour, bipolar disorder, or loss of skills. Second, in patients with a known 22q13 deletion syndrome, caregivers need to be vigilant for the emergence of psychiatric symptomatology in general and for the development of BPD in particular. Third, continuously practising acquired skills might maintain them for a longer period of time, and rigorous medical follow-up seems important to prevent severe pathology that could lead to an acute loss of functioning.
Because of the lack of specific dysmorphic features in the 22q13 deletion syndrome, future research to delineate the phenotype should also focus on behavioural and psychiatric aspects. Therefore, larger groups of 22q13 deleted patients need a systematic and longitudinal psychiatric assessment. Moreover, closer investigation should demonstrate whether SHANK3 might be a possible candidate gene for BPD and whether the degeneration is part of the phenotype.
Acknowledgements
We would like to thank the patients and their families, the medical staff and caregivers of the institutions for their kind and helpful cooperation in this study. H.V.E. and K.D. are clinical investigators of FWO Vlaanderen.
References
- 1.American Psychiatric Association . (Washington: American Psychiatric Association); 2001. Diagnostic and statistical manual of mental disorders, ed 4. [Google Scholar]
- 2.Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins – a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. (2002);81:903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, et al. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. (2001);69:261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaglia MC, Giorda R, Beri S, De Agostini C, Novara F, et al. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet. (2011);7:e1002173. doi: 10.1371/journal.pgen.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusmano-Ozog K, Manning MA, Hoyme HE. 22q13.3 deletion syndrome: a recognizable malformation syndrome associated with marked speech and language delay. Am J Med Genet Part C Semin Med Genet. (2007);145C:393–398. doi: 10.1002/ajmg.c.30155. [DOI] [PubMed] [Google Scholar]
- 6.de Bildt A, Kraijer D. (PITS: Leiden); 2003. Vineland Adaptive Behavior Scales (Vineland-Z) [DOI] [PubMed] [Google Scholar]
- 7.Einfeld SL, Tonge BJ. The Developmental Behavior Checklist: the development and validation of an instrument to assess behavioral and emotional disturbance in children and adolescents with mental retardation. J Autism Dev Disord. (1995);25:81–104. doi: 10.1007/BF02178498. [DOI] [PubMed] [Google Scholar]
- 8.Havens JM, Visootsak J, Phelan MC, Graham JM. 22q13 deletion syndrome: an update and review for the primary pediatrician. Clin Pediatr. (2004);49:43–53. doi: 10.1177/000992280404300106. [DOI] [PubMed] [Google Scholar]
- 9.Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. (2007);61:3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 10.Kraijer D, de Bildt A. The PDD-MRS: an instrument for identification of autism spectrum disorders in persons with mental retardation. J Autism Dev Disord. (2005);35:499–513. doi: 10.1007/s10803-005-5040-0. [DOI] [PubMed] [Google Scholar]
- 11.Manning MA, Cassidy SB, Clericuzio C, Cherry AM, Schwartz S, et al. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. (2004);114:451–457. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- 12.Menten B, Maas N, Thienpont B, Buysse K, Vandesompele J, et al. Emerging patterns of cryptic chromosomal imbalance in patients with idiopathic mental retardation and multiple congenital anomalies: a new series of 140 patients and review of published reports. J Med Genet. (2006);43:625–633. doi: 10.1136/jmg.2005.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, et al. 22q13 deletion syndrome. Am J Med Genet. (2001);101:91–99. doi: 10.1002/1096-8628(20010615)101:2<91::aid-ajmg1340>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Severinsen JE, Bjarkam CR, Kiaer-Larsen S, Olsen IM, Nielsen MM, et al. Evidence implicating BRD1 with brain development and susceptibility to both schizophrenia and bipolar affective disorder. Mol Psychiatry. (2006);11:1126–1138. doi: 10.1038/sj.mp.4001885. [DOI] [PubMed] [Google Scholar]
- 15.Sovner R, Stone A, Fox C. Ring chromosome 22 and mood disorders. J Intellect Disabil Res. (1996);40:82–86. doi: 10.1111/j.1365-2788.1996.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 16.Verma R, Mukerji M, Grover D, B-Rao C, Das SK, et al. MLC1 gene is associated with schizophrenia and bipolar disorder in southern India. Biol Psychiatry. (2005);58:16–22. doi: 10.1016/j.biopsych.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Youngstrom EA, Findling RL, Youngstrom JK, Calabrese JR. Toward an evidence-based assessment of pediatric bipolar disorder. J Clin Child Adolesc Psychol. (2005);34:433–448. doi: 10.1207/s15374424jccp3403_4. [DOI] [PubMed] [Google Scholar]