Abstract
Background/Aim
The contribution of cerebrovascular dysfunction to the manifestation of dementia and cognitive decline in late life is gaining increased attention. We aimed to systematically review evidence for associations between dementia or aging and cerebrovascular function as measured using transcranial Doppler (TCD) examination.
Methods
A total of 1,172 articles were retrieved from PsychInfo and PubMed searches, and 34 relevant articles were identified using a variety of TCD methods.
Results
The pulsatility index (vessel resistance), spontaneous emboli and cerebrovascular reactivity to hyper-/hypocapnia appeared good discriminators of dementia. Aging was associated with a slowing in blood flow velocity.
Conclusion
TCD ultrasonography is inexpensive, portable and well tolerated by aged and demented subjects. The technique stands to make a valuable contribution to the knowledge regarding the underlying functional biology of age-related cognitive change and dementia.
Key Words: Aging, Dementia, Alzheimer's disease, Transcranial Doppler studies, Cerebrovascular function
Introduction
Aging and dementia are associated with changes in cerebrovascular structure and function which contribute to associated cognitive and functional declines [1, 2, 3]. Recent autopsy studies have stressed the important role of vascular pathologies in the manifestation of late-onset dementia [4, 5, 6]. Further, systematic reviews and meta-analyses have highlighted the importance of vascular risk factors (e.g. hypertension and stroke) on dementia onset and progression [7, 8, 9, 10]. Research investigating functional cerebrovascular contributions to cognitive performance in age-related decline and dementias such as Alzheimer's disease (AD) and vascular dementia (VaD) have generally reported reduced cerebral perfusion [11, 12]. These studies have employed techniques such as functional magnetic resonance imaging, positron emission tomography or single-photon emission computed tomography. However, these techniques are expensive, and there are feasibility issues which are particularly problematic for older populations, including the need for individuals to be sufficiently mobile to attend a research facility, lie still for a prolonged duration and have no metal implants.
Transcranial Doppler (TCD) ultrasonography is a non-invasive, inexpensive and portable technique with high temporal resolution, allowing continuous and bilateral recording of cerebral blood flow velocity through the major arteries (e.g. medial, anterior, posterior and basilar). Measurements can be taken at rest, during hypercapnia or hypocapnia (to assess cerebrovascular reactivity), or during cognitive tasks. TCD data collected during cognitive operations is commonly referred to as functional TCD (fTCD) and is the assessment of blood flow velocity change in response to a specific cognitive stimulus or mental operation. Resultant graphs displaying blood flow velocity versus time are known as evoked-flows and are generated in a similar manner to event-related potentials (ERPs) derived from electroencephalogram data where multiple trials are averaged relative to the presentation of cognitive stimuli [13]. Pioneered in the 1980s [14], there has been a recent resurgence in the use of the TCD technology, particularly in the aging and dementia fields.
Haemodynamic abnormalities may be critical markers of dementia and cognitive decline in elderly individuals. Chronic cerebral hypoperfusion could affect cellular health within the brain and the development of neurodegenerative pathologies [15, 16]. TCD methods have much to provide the assessment of functional cerebrovascular contributions to cognitive impairment in dementia and aging and may help in the differentiation of dementia from normal aging and between the subtypes such as AD and VaD. This paper aims to systematically review previous research assessing dementia [including mild cognitive impairment (MCI), an intermediate state between normal aging and dementia] and aging using TCD techniques, and, in doing so, summarise key protocols, metrics and consistent findings to point to areas of future research.
Search Strategy and Selection Criteria
The PubMed and PsychInfo databases were searched on March 19, 2012, using the search terms: (‘transcranial doppler’) AND (dementia OR age OR ageing OR aging OR Alzheim* OR ‘mild cognitive impairment’). A total of 1,172 articles were retrieved. Titles and abstracts were read by at least two of the authors. Articles were retained if they collected TCD data at rest, hyper-/hypocapnia or during a cognitive task from any cerebral artery accessible via TCD [the anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA) or basilar artery] and assessed dementia (differences between subtypes or with normal aging; or changes associated with clinical progression) or aging in those over 50 years of age. Articles were included if they assessed general late-onset dementia (i.e. without subtype classification) or AD and VaD (and its historical counterpart multi-infarct dementia) subtypes. Articles were excluded if they were not written in English or were case reports. Articles with TCD as an outcome in a clinical trial of a pharmaceutical compound were also excluded unless they presented baseline (i.e. prior to drug administration schedule) comparison data (e.g. with a healthy control or another subtype of dementia).
Data Extraction
Details from each included study are summarised in table 1 and include: study sample (including sex, age and diagnoses), TCD protocol, vessel(s) investigated, TCD metrics analysed and key findings.
Table 1.
Summary of the articles using resting, cerebrovascular reactivity and functional/cognitive TCD measures to investigate aging (over 50 years) or dementia
| Article | Participants | Vessel | TCD protocol (resting/vasoreactivity/ fTCD) | Metrics | Major findings |
|---|---|---|---|---|---|
| Purandare et al. [47], 2012 | Male and female; n = 144: 84 with AD and 60 with VaD (mean age 75 ± 7 years); some drop-outs over 24 months, with n = 99 in final testing wave | Bilateral MCA | Resting | Presence of spontaneous emboli | Spontaneous cerebral emboli detected in 43% of AD and 45% of VaD cases; presence of emboli was significantly associated with cognitive and functional decline over 2 years, as well as a larger increase in psychiatric symptoms |
| Anzola et al. [39], 2011 | Male and female; n = 15 with MCI (mean age 72 ± 9 years) and n = 28 controls (mean age 67 ± 10 years) | Right MCA | Resting | MV | No differences between groups |
| CVR: room air + 7% CO2 gas | MV | No differences between groups | |||
| Silvestrini et al. [48], 2011 | Male and female; n = 41 with AD + severe right carotid artery stenosis (median age 71 years, range 65–78) and n = 57 with AD + severe left carotid artery stenosis (median age 70 years, range 65–71); TCD not collected in control group (with AD and no stenosis) | Ipsilateral to stenosis (vessel not reported) | CVR: breath hold | BHI | Those with stenosis were more likely to develop severe dementia over 12 months, and this was related to BHI ipsilateral to stenosis |
| Roher et al. [29], 2011 | Male and female; n = 42 with AD (mean age 80 ± 7 years), n = 11 with MCI (mean age 80 ± 5 years) and n = 50 controls (mean age 79 ± 6 years) | Bilateral segments (8) of circle of Willis | Resting | PI and MV | Generally, PI higher and MV lower in AD as compared to control group; no significant differences involving MCI group unless restricting to only amnestic MCI |
| Kong et al. [30], 2011 | Male and female; n = 30 with AD (mean age 71 ± 2 years), n = 34 with VaD (mean age 72 ± 3 years) and n = 40 controls (mean age 71 ± 3 years) | Bilateral MCA | Resting | MV | Decreased MV in demented (AD and VaD) groups as compared to controls; no differences between dementia subtypes |
| Gucuyener et al. [35], 2010 | Male and female; n = 13 with ‘pseudodementia’/severe depression (mean age 65 ± 6 years), n = 11 with AD (mean age 66 ± 6 years) and n = 10 controls (mean age 64 ± 6 years) | PCA | Resting | MV | MV significantly lower in AD and pseudodementia group as compared to controls |
| fTCD: visual stimulation with shapes, with target image needing to be identified | Relative change in MV [(stimulation – rest)/rest] | Relative MV only lower in AD group as compared to controls | |||
| Gur et al. [21], 2010 | Male and female; n = 37 with first-ever acute ischemic stroke within 72 h of onset: n = 20 did not progress to dementia 3–6 months after stroke (mean age 70 ± 7 years) and n = 17 did progress to dementia 3–6 months after stroke (mean age 66 ± 5 years) | Bilateral MCA | Resting | MV | No significant differences in MV between demented and non-demented groups |
| CVR: following acetazolamide injection | CVR | No significant differences in CVR between demented and non-demented groups | |||
| van Beek et al. [22], 2010 | Male and female; n = 21 with AD (mean age 73 ± 6 years) and n = 20 controls (mean age 75 ± 3 years) | Bilateral MCA | Resting; pre-cholinesterase inhibitor use and 10 weeks after treatment | MV and resistance (mean BP/MV) | Cerebrovascular resistance increased in AD group as compared to controls, unchanged by cholinesterase inhibitor use |
| van Beek et al. [20], 2010 | Male and female; n = 21 with AD (mean age 72 ± 6 years) and n = 20 controls (mean age 75 ± 3 years); several participants were excluded for some measures | Bilateral MCA | Resting | MV and resistance (mean BP/MV) | No group differences in resting MV; increased cerebrovascular resistance in AD |
| Lee et al. [27], 2007 | Male and female; n = 17 with AD (mean age 67 ± 6 years) and n = 17 control (mean age 67 ± 6 years) | Bilateral MCA | Resting | MV and PI | No group differences |
| CVR: via rebreathing | MV | CVR significantly decreased in AD group as compared to controls | |||
| Likitjaroen et al. [37], 2009 | Male and female; n = 9 diagnosed with AD (median age 75 years, range 68–83) and n = 9 with VaD (median age 66 years, range 52–86) | Unilateral MCA | Resting | Baseline end DV, mid SV and peak SV before and after compound | No significant differences between AD and VaD groups |
| CVR: following acetazolamide injection | As above | No significant differences between AD and VaD groups | |||
| Menendez-Gonzalez et al. [49], 2009 | Male and female; n = 23 with AD and n = 25 controls with age around 74 years | Bilateral MCA and PCA | CVR: breath hold | BHI | AD group had significantly lower BHI than controls for all vessels |
| Stefani et al. [31], 2009 | Male and female; n = 40 diagnosed with AD (mean age 71 ± 6 years) and n = 40 controls (mean age 69 ± 8 years) | Bilateral MCA | Resting | MV and PI | MV significantly lower in AD group as compared to controls; PI displayed opposite effect (higher in AD group) |
| CVR: breath hold | BHI | BHI significantly lower in AD group as compared to controls; BHI correlated with cognition (MMSE); no differences between AD without white matter abnormalities, as compared to AD with white matter abnormalities | |||
| Claassen et al. [40], 2009 | Male and female; n = 9 with mild AD (mean age 68 ± 6 years) and n = 8 controls (mean age 65 ± 4 years) | Unilateral MCA | Resting | MV and RI | Resting velocity was lower in AD group but failed to reach conventional significance levels in small sample, not explained by brain atrophy; RI was higher in AD group |
| Purandare et al. [42], 2008, a review of relevant articles using the same cohort [43, 44, 46] | Male and female; n = 85 with AD (mean age 75 ± 8 years), n = 85 with VaD (mean age 78 ± 6 years) and n = 150 matched non-demented controls (some drop-outs over follow-up and recruitment of new patients) | Bilateral or unilateral MCA | Resting | Presence of spontaneous emboli | Spontaneous cerebral emboli significantly associated with dementia, adjusting for vascular risk factors; detected in 40% of AD and 37% of VaD cases, compared to 12% in controls; presence of emboli associated with depression in dementia; presence of emboli associated with worse cognitive decline in controls over 6 months but not over 2.5 years |
| Vicenzini et al. [32], 2007 | Male and female; n = 118 with dementia (60 with AD (mean age 69 ± 3 years) and 58 VaD (mean age 71 ± 2 years)) and n = 62 matched controls (mean age 69 ± 3 years) | Bilateral MCA | Resting | MV and PI | Those with dementia (AD or VaD) showed lower flow velocities and higher PIs |
| CVR: CO2 mixture inhalation (6%) for hypercapnia followed by hyperventilation for hypocapnia | MV | Those with dementia (AD or VaD) showed lower total vasomotor ranges (i.e. taking into account hypercapnia and hypocapnia) as compared to those without dementia | |||
| Roher et al. [23], 2006 | Male and female; n = 21 with AD (mean age 79 ± 6 years) and n = 26 controls (mean age 81 ± 6 years) | Bilateral segments (8) of circle of Willis; resting | Resting | MV and PI | AD group had higher PIs (6/16 segments), most notably for anterior segments |
| Rosengarten et al. [53], 2006 | Male and female; n = 8 with AD (mean age 74 ± 4 years) and n = 16 controls (mean age 69 ± 7 years) | Bilateral PCA and MCA | fTCD: visual perception | SV at rest, gain, attenuation (stiffness of vascular system), natural frequency and rate time | AD group displayed a significantly reduced response to visual task as compared to controls |
| Silvestrini et al. [51], 2006 | Male and female; n = 53 with AD (mean age 70 ± 6 years) | Bilateral MC | CVR: breath hold | BHI | BHI significantly predicted cognitive decline over a 12-month period – the lower the BHI the worse the prognosis |
| Asil and Uzuner [26], 2005 | Male and female; n = 15 with AD (mean age 70 years), n = 12 with VaD (mean age 58 years) and n = 9 healthy controls (mean age 58 years) | Bilateral PCA | Resting | MV | No significant differences in MV between groups |
| fTCD: visual perception | Relative increase from baseline to activation in MV for each task | Response similar between healthy controls and those with AD, but attenuated in those with VaD | |||
| Ruitenberg et al. [15], 2005 | Male and female; n = 1,730 (mean age 71 ± 6 years), of whom n = 14 had dementia (13 with AD, 1 with VaD); population-based sample (Rotterdam Study) | Bilateral MCA | Resting | MV | Those with dementia had lower MV |
| CVR: CO2 mixture (5%) | CVR | CVR did not differ between demented and non-demented persons; in non-demented group, lower cerebral blood flow velocity was associated with (1) preceding cognitive impairment and (2) smaller hippocampal and amygdala volumes; lower CVR was associated with preceding cognitive decline | |||
| Purandare et al. [45], 2005 | Male and female; n = 24 with AD and n = 17 with VaD (overall mean age 72 ± 7 years), n = 16 controls (mean age 72 ± 6) | Bilateral MCA | Resting | Presence of spontaneous emboli | Emboli detected in 17% of AD cases, 41% of VaD cases and in 7% of controls (1/16); presence of emboli was significantly associated with VaD |
| Bakker et al. [17], 2004 | Male and female; n = 1,720 (mean age 71 ± 6 years); population-based sample (Rotterdam Study) | Bilateral MCA | Resting | DV, SV and PI | MV, DV and SV declined significantly with age, while PI increased significantly with age |
| CVR: CO2 mixture (5%) | MV | CVR significantly decreased with age | |||
| Matteis et al. [52], 1998 | Male and female; n = 10 with AD (mean age 62 ± 9 years), n = 10 with multi-infarct dementia (mean age 68 ± 8 years) and n = 20 controls (mean age 63 ± 12 years) | MCA | CVR: breath hold | BHI | Significantly lower in multi-infarct group compared to AD and controls |
| fTCD: verbal and visual discrimination | Relative increase from baseline to activation in MV for each task | No group differences in average velocity responses; however, only the control group showed lateralised responses (left for verbal, right for visual) | |||
| Sattel et al. [18], 1996 | Male and female; n = 46 with AD (median age 80 years, range 67–92) and n = 44 with multi-infarct dementia (median age 79 years, range 69–90) | Bilateral MCA, PCA and ACA, and basilar | Resting | MV and PI | PI of all arteries was higher in the multi-infarct group as compared to AD; age positively correlated with PI (of basilar) |
| Franceschi et al. [33], 1995 | Male and female; n = 17 with AD (mean age 66 ± 7 years) and n = 20 healthy controls (mean age 63 ± 8 years) | Bilateral MCA | Resting | MV and flow asymmetry index | Velocities slower and displayed more asymmetry in AD group as compared to controls |
| Biedert et al. [24], 1995 | Male and female; n = 23 with AD, n = 19 with multi-infarct dementia and n = 36 controls, all between 60 and 69 years | Bilateral MCA and basilar | Resting | MV and PI | Multi-infarct dementia group displayed higher PIs than AD group (no significant differences with controls) |
| Heun et al. [19], 1994 | Male and female; n = 24 with AD and no evidence of cardio-/cerebrovascular disease (mean age 75 ± 10 years) | Bilateral ACA, MCA and PCA | Resting | Resting MV, SV, DV and RI | Left MCA SV and DV correlated negatively with age; left MCA SV, DV and MV correlated positively with MMSE score (along with some other cognitive measures, but associations not as consistent) |
| Biedert et al. [28], 1993 | Male and female; n = 32 with dementia (23 with AD and 19 with multi-infarct dementia) and n = 36 controls, all between 60 and 69 years | Bilateral MCA and basilar | Resting | MV, SV and PI | MCA and basilar PI increased in multi-infarct dementia group as compared to AD group (no significant differences from controls); no significant group differences for MV and SV |
| Ries et al. [38], 1993 | Male and female; n = 17 with multi-infarct dementia (mean age 69 ± 9 years), n = 24 with AD (mean age 66 ± 9 years) and n = 64 controls (mean age 61 ± 11 years) | Bilateral MCA (in 6 individuals, this was unilateral) | Resting | SV, DV and MV | MV and DV lower in multi-infarct dementia group compared to other groups |
| Caamano et al. [34], 1993 | Male and female; n = 12 with AD (mean age 64 ± 7 years), n = 12 with multi-infarct dementia (mean age 57 ± 8 years) and n = 12 controls (mean age 57 ± 8 years) | Bilateral MCA and basilar | Resting | MV, SV, DV and PI | Demented groups displayed reduced bilateral MCA MV, SV and DV; the multi-infarct group also displayed increased PIs in MCAs; results from the basilar were weak; no significant differences between dementia groups |
| Bressi et al. [41], 1992 | Male and female; n = 23 with AD (mean age 64 ± 9 years) and n = 10 controls (mean age 62 ± 7 years) | Bilateral ACA, MCA and PCA | Resting | SV, MV, DV, PI and laterality index | Velocities (MV, SV and DV) were slower in the MCA in AD group as compared to controls; no group differences for ACA or PCA, or for PI; MCA velocities correlated with some neuropsychological test scores (one significant for ACA) |
| Provinciali et al. [36], 1990 | Male and female; n = 20 with AD (mean age 68 ± 5 years), n = 20 with multi-infarct dementia (mean age 65 ± 7 years) and n = 25 controls | Bilateral MCA | Resting | PI and MV | PIs higher in both dementia groups; MV slower in multi-infarct group as compared to controls |
| CVR: hyperventilation/hypocapnia, breath hold/hypercapnia and close-circuit rebreathing | MV (highest % increase in MV between rest and hypercapnia/hypocapnia) | Hypocapnia-induced velocity decrease lower in demented groups as compared to controls; hypercapnia-included changes in velocity only lower in multi-infarct group as compared to controls | |||
| Foerstl et al. [25], 1989 | Male and female; n = 18 with dementia, of whom 9 with AD and 9 with multi-infarct dementia; n = 14 controls | MCA and basilar (unclear if uni- or bilateral) | Resting | PI and MV | PI increased in multi-infarct dementia group as compared to controls |
Clinic or convenience samples unless otherwise stated.
BP = Blood pressure; CVR = cerebrovascular reactivity; DV = diastolic velocity; MMSE = Mini-Mental State Examination; PI = pulsatility index; RI = resistance index; SV = systolic velocity; MV = mean flow velocity.
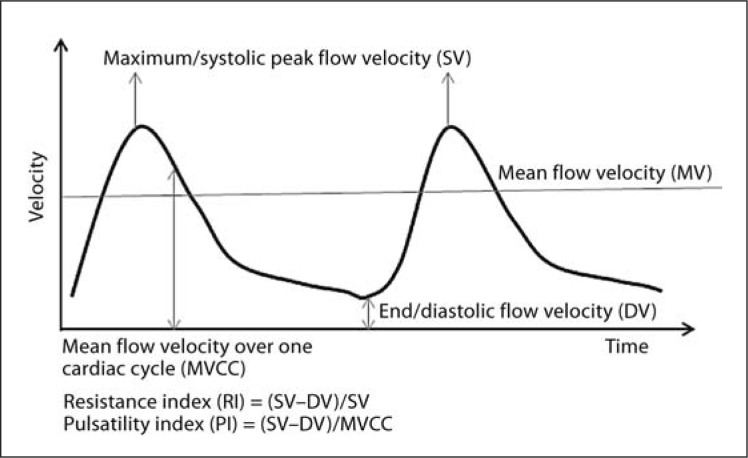
The calculation of common resting TCD metrics employed are summarised in figure 1, including systolic peak flow velocity, end diastolic velocity, mean flow velocity (MV) and the pulsatility index and resistance index. Cerebrovascular reactivity to hypocapnia (reduced CO2 in blood) or hypercapnia (increased CO2 in blood) was calculated as the difference between mean flow velocity during hypo-/hypercapnia and resting mean flow velocity, divided by resting mean flow velocity [i.e. (MV during capnia − resting MV)/resting MV]. The periods of time where resting or hypo-/hypercapnia mean flow velocities were measured varied between studies. The breath-holding index (BHI) was also commonly used to assess velocity changes in response to hypercapnia which was calculated as the difference between mean flow velocity at the end of a breath hold (usually at least 30 s) and resting mean flow velocity, divided by resting mean flow velocity [i.e. (MV at end of breath hold − resting MV)/resting MV].
Fig. 1.
Illustration of two cardiac cycles as measured via TCD. Key resting TCD metrics and the calculation of derivatives are shown. MVCC = Mean flow velocity over one cardiac cycle; RI = resistance index; SV = systolic velocity; DV = diastolic velocity; PI = pulsatility index.
Results
Thirty-four articles were selected for review: 29 assessed TCD measures during rest, 13 during hyper-/hypocapnia and 4 employed fTCD (cognitive) techniques (some articles presented multiple assessments). The vast majority of articles assessed differences between demented and non-demented groups rather than age-related changes (over 50 years). There was only one population-based sample used, the Rotterdam Study [15, 17], which comprised over 1,700 individuals. The remaining studies employed clinic or convenience recruited samples. All studies are summarised in table 1.
Resting TCD Measures
A number of TCD metrics were employed to assess cerebral blood flow at rest including mean flow velocity, systolic velocity, diastolic velocity, the pulsatility index and other resistance measures, and the flow asymmetry index (i.e. the difference between right and left arteries at rest). The majority of papers assessed the MCA or PCA. Other vessels investigated included the ACA and the basilar artery. Most papers assessed differences between those with and without dementia or dementia progression. However, some age associations in late life were reported: the pulsatility index increased with age [17, 18], while mean, diastolic and systolic flow velocities decreased with age in AD [19] and in the general population [17].
Most studies reported no significant differences in resting mean flow velocities between demented and control groups [20, 21, 22, 23, 24, 25, 26, 27, 28], or, lower mean flow velocities in demented groups including AD [29, 30, 31, 32, 33, 34, 35], VaD or multi-infarct dementia [30, 32, 34, 36], regardless of subtype [15]. Mean flow velocity measures in AD and VaD groups appeared similar [18, 24, 30, 32, 34, 36, 37], although this was not always the case. Ries et al. [38] reported that the multi-infarct dementia group displayed slower mean and diastolic flow velocities compared to both control and AD groups. Few studies reported flow velocities other than the mean; however, Caamaño et al. [34] reported that individuals with AD and multi-infarct dementia displayed reduced systolic and diastolic (as well as mean) flow velocities compared to healthy controls. The two articles which examined a MCI group assessed mean flow velocity and reported no differences between the MCI group and the control group [29, 39], unless restricting to amnestic MCI [29]. One study reported that mean flow velocities were more asymmetric in an AD group as compared to healthy controls [33]. In non-demented controls, lower mean flow velocities were associated with preceding cognitive impairment and smaller hippocampal and amygdala volumes [15].
The pulsatility index was the most commonly employed measure of vessel resistance and was found to be increased in AD [23, 29, 31, 32, 36, 40] and VaD or multi-infarct dementia patients [25, 32, 36] as compared to healthy controls, although this was not the case in three papers [24, 28, 41]. There were many reports that those with VaD or multi-infarct dementia displayed higher pulsatility indexes than those with AD [18, 24, 28, 34], although there were three reports of non-significant differences between subtypes [25, 32, 36]. There appeared to be no significant difference in the pulsatility index between a MCI group and controls [29]. Employing a different calculation of resistance, van Beek et al. [20] reported increased cerebrovascular resistance in an AD group.
A series of papers by Purandare and colleagues [42, 43, 44, 45, 46, 47] assessed the significance of spontaneous emboli as detected during resting TCD to dementia and its progression and symptoms. In an article published in 2005, they reported the presence of emboli to be associated with VaD [45]. In a summary of a sequence of studies using the same large cohort [reviewed in [42]], the group went on to report emboli to be associated with both VaD (37% of cases) and AD (40% of cases), as compared to controls (12% of cases). Further, they showed that the presence of emboli was associated with depression symptomatology in dementia and worse cognitive decline over 6 months in controls (i.e. non-demented cases at baseline). Recently, the group has reported emboli to be associated with more rapid cognitive and functional decline in AD and VaD, as well as with an increased number of psychiatric symptoms over a 24-month period [47].
Cerebrovascular Reactivity TCD Measures (i.e. during Hypo- or Hypercapnia)
The BHI was commonly employed to assess the effects of hypercapnia [31, 48, 49, 50], along with the administration of gasses with varying %CO2 [15, 32, 39] or a pharmaceutical compound [21, 37], and closed-circuit rebreathing [27]. In a large population-based sample, cerebrovascular response to hypercapnia was found to significantly decrease with age [17]. Two papers reported that the BHI was lower in AD patients as compared to healthy controls [31, 49], similar to Lee et al. [27] who employed closed-circuit rebreathing, while another two papers reported the BHI was only lower in a multi-infarct dementia group (not an AD group) as compared to healthy controls [36, 51]. Silvestrini et al. [48] used the BHI to investigate differences between patients with mild/moderate AD with/without carotid stenosis. They reported that those with stenosis were more likely to develop severe dementia over 1 year, and this was related to the BHI ipsilateral to the stenosis. In another paper investigating progression, Silverstrini et al. [50] reported that, in a group diagnosed with AD, lower BHIs were associated with a more rapid cognitive decline.
Vicenzini et al. [32] used a gas mixture (with increased CO2) to induce hypercapnia, followed by hyperventilation to induce hypocapnia. They reported that the vasomotor range (i.e. taking into account flow velocities during hyper- and hypocapnia) was reduced in AD and VaD patients. Ruitenberg et al. [15] reported no differences in cerebrovascular reactivity using the same protocol between demented (regardless of subtype) and non-demented individuals in a large population-based sample. However, in this case there were only 14 individuals diagnosed with dementia (as compared to 1,730 non-demented subjects). Interestingly, lower cerebrovascular reactivity in the non-demented group was found to be associated with preceding cognitive decline [15]. Anzola et al. [39] reported no differences in cardiovascular response to hypercapnia between MCI and control groups.
Gur et al. [21] and Likitjaroen et al. [37] investigated cerebrovascular reactivity via the administration of acetazolamide, which indirectly induces hypercapnia. Likitjaroen et al. [37] reported no significant differences in cerebrovascular reactivity between AD and VaD groups using this compound (there was no healthy control group). Gur et al. [21] reported no significant differences in cerebrovascular reactivity in individuals who did and did not convert to dementia 3–6 months after a first-ever ischaemic stroke.
Cognitive TCD/fTCD Measures
No article was identified using fTCD metrics to investigate changes over the age of 50 years. Four fTCD studies compared dementia subtypes and controls: two found that individuals with AD had an attenuated response to cognitive demand [35, 52], and two found no differences between AD patients and controls [26, 51]. Matteis et al. [51], however, reported that their AD group displayed a reduction in lateralised function, and Asil and Uzuner [26] reported an attenuated response only in their VaD group.
Discussion
This systematic review revealed 34 articles using TCD methods to investigate aging or dementia. This technique appears to be a feasible method of investigating cerebrovascular function during rest, hyper-/hypocapnia and cognition in old and demented individuals. Measures of vessel resistance and the presence of emboli as detected via TCD appeared the best discriminators of dementia from normal aging. There was also evidence for cerebrovascular reactivity to hypo- and hypercapnia.
Measures of vessel resistance during resting TCD, particularly the pulsatility index, were consistently associated with the presence of dementia, both in AD [20, 23, 29, 31, 32, 36, 40] and VaD or multi-infarct dementia [25, 32, 36]. Further, the pulsatility index appeared to discriminate between these dementia subtypes [18, 24, 28, 34]. Subjects with VaD appeared to have the highest pulsatility indexes (associated with high vessel resistance), controls the lowest and AD patients sitting in between. Another consistent discriminator between those with and without dementia was the presence of spontaneous cerebral emboli as detected via TCD, although all findings come from one participant group [42, 43, 44, 45, 46, 47].
Cerebrovascular reactivity to hypo- or hypercapnia was also a good discriminator of dementia, using the BHI [31, 49], a gas [32] or closed-circuit rebreathing [27]. Effects may be stronger in VaD [36], similar to pulsatility index findings. Ruitenberg et al. [15] reported cerebrovascular reactivity was a good marker of future cognitive decline in non-demented individuals. However, they reported no differences in cerebrovascular reactivity between demented and non-demented groups using gas stimuli. The sample in this study was large and population based, however lacked the necessary power to detect effects as only 14 individuals were reported to have dementia. In contrast to measures of reactivity to hypo- or hypercapnia, the administration of pharmaceutical compounds to directly induce cerebrovascular reactivity was not as successful in the discrimination of dementia from normal aging and in predicting future decline [21, 37], possibly due to their indirect effect and inter-subject metabolic differences.
Findings in relation to differentiating demented and non-demented groups using resting flow velocities were not consistent. Resting flow velocities including mean, systolic and diastolic velocities appeared similar [20, 21, 22, 23, 24, 25] or lower in demented groups including AD patients [29, 30, 31, 32, 33, 34, 35], VaD/multi-infarct dementia patients [30, 32, 34, 36] or general dementia patients [15], as compared to healthy controls. There appeared to be no significant differences in blood flow velocities between dementia subtypes [18, 24, 30, 32, 34, 36, 37]. Resistance, emboli and cerebrovascular reactivity TCD measures appeared better discriminators of dementia. However, this may be due to the selection of non-optimal resting flow measures. For example, Rosengarten et al. [53] along with Rosengarten and Kaps [54] reported that systolic velocity is less prone to artefacts and more sensitive to the regulation of blood flow than mean flow velocity.
fTCD metrics showed that individuals with dementia had an attenuated response to cognitive load [26, 35, 52] or a reduction in lateralised function [51] which may reflect compensatory cognitive ability [55, 56]. Interestingly, a study of resting TCD reported that mean flow velocities were more asymmetric in an AD group as compared to controls [33] – opposite to that reported in the fTCD paper [51]. Future studies are needed to confirm these findings. There were no identified articles that assessed fTCD changes after 50 years of age.
Abnormalities in TCD measures in demented groups could reflect a number of pathological processes such as cerebral amyloid angiopathy [6, 49], arteriolosclerosis or endothelial dysfunction, particularly within the microvascular system [1]. Attenuations in the responsiveness of the cerebrovascular system during cognitive tasks in old and demented subjects may also be a function of surrounding neurons and astrocytes (not signalling for sufficient supply). This abnormal cerebral blood flow may be a cause or consequence of age- and dementia-related neuropathology such as cerebral atrophy. For example, it could simply be that reduced blood flow velocities represent the reduced metabolism of an atrophied brain. Alternatively, reduced blood flow velocities and thus flow may directly lead to cellular dysfunction and death in vulnerable areas such as the hippocampus [15, 57]. Ruitenberg et al. [15] found that there was a negative association between resting mean velocity and hippocampal/amygdala volume, and that cerebrovascular disease did not mediate this relationship. This suggests that cerebral blood flow velocity may be directly associated with the volume of brain structures.
Future studies need to investigate neuronal and vascular systems in parallel to assess neurovascular coupling. It is possible to simultaneously record electrical brain activity (via ERPs) and blood flow velocity (fTCD) to assess coupling [52, 53, 58, 59]. The combined ERP-TCD protocol provides an inexpensive, non-invasive method for measuring neurovascular coupling which has the potential to be significantly developed.
One limitation of the TCD method is the assumption that the artery diameter remains constant and therefore any change in velocity represses a change in flow. It has been reported, however, that the diameter of the MCA does not significantly change during moderate alterations in blood pressure (e.g. around 30 mm Hg) [60, 61] and therefore any change in velocity reflects a change in blood volume through the artery. Another potential limitation relates to the structure of the temporal window. With age, the temporal window where the TCD ultrasound probe is placed thickens, making recording more difficult. A population-based study reported 25% of participants were lost due to failure to obtain an adequate TCD signal, especially marked in older women [15, 17]. These failure rates should be taken into account when planning TCD-based studies. The technique is, however, well tolerated, portable, does not require participants to remain still and allows metal implants to remain in place, unlike expensive and high-spatial resolution cerebral blood flow imaging techniques such as positron emission tomography and single-photon emission computed tomography. Furthermore, TCD equipment is widespread in clinical and research facilities around the world.
Dementia appears to be associated with increased vessel resistance, the presence of spontaneous emboli and a reduced cerebrovascular response to increased/decreased environmental CO2 using TCD methods. Blood flow velocities appear to decrease with age in late life. These patterns of TCD findings correspond to known structural vascular changes [16, 62]. TCD techniques stand to make a valuable contribution to the understanding of underlying cerebrovascular contributions to age-related cognitive impairment and dementia. Further, TCD measures may assist in the development of novel therapeutic strategies addressing cerebral vasoreactivity or may be reliable methods to differentiate between dementia subtypes or predict clinical progression of cognitive decline [15, 48, 50].
Acknowledgements
H.A.D.K. is supported by an Australian National Health and Medical Research Council Training Fellowship (568890). This research was funded by the Brain Foundation, Australia (2012 Research Grant), and the Australian Association of Gerontology (2012 RM Gibson Scientific Research Award).
References
- 1.Sonntag W, Eckman D, Ingraham J, Riddle D. Regulation of cerebrovascular aging. In: Riddle D, editor. Brain Aging: Models, Methods and Mechanisms. Boca Raton: CRC Press; (2007). [PubMed] [Google Scholar]
- 2.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer's disease patients and experimental models. J Cereb Blood Flow Metab. 2011;31:1354–1370. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz SK, O'Leary DS, Ponto LLB, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to midlife adults. Neuroreport. 1999;10:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- 4.Neuropathology Group Medical Research Council Cognitive Function and Aging Study: Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 6.Keage H, Carare R, Friedland R, Ince P, Love S, Nicoll J, Wharton S, Weller R, Brayne C. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 2009;9:3. doi: 10.1186/1471-2377-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharp SI, Aarsland D, Day S, Sønnesyn H. Alzheimer's Society Vascular Dementia Systematic Review G, Ballard C: Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26:661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 9.Keage HAD, Gupta S, Brayne C. Alzheimer's Society Systematic Review Group: Risk for dementia and age at measurement. Int J Geriatr Psychiatry. 2011;26:329–330. doi: 10.1002/gps.2492. [DOI] [PubMed] [Google Scholar]
- 10.Savva GM, Stephan BC, Alzheimer's Society Vascular Dementia Systematic Review Group Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 11.Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luckhaus C, Flüb MO, Wittsack HJ, Grass-Kapanke B, Jänner M. Khalili-Amiri R. Friedrich W. Supprian T. Gaebel W. Mödder U, Cohnen M. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer's dementia by perfusion-weighted magnetic resonance imaging. Neuroimage. 2008;40:495–503. doi: 10.1016/j.neuroimage.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 13.Rosengarten B, Paulsen S, Burr O, Kaps M. Effect of ApoE epsilon4 allele on visual evoked potentials and resultant flow coupling in patients with Alzheimer. J Geriatr Psychiatry Neurol. 2010;23:165–170. doi: 10.1177/0891988710363711. [DOI] [PubMed] [Google Scholar]
- 14.Aaslid R, Markwalder R, Nornes H. Noninvasive transcranial ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 15.Ruitenberg A, den Heijer T. Bakker SL. van Swieten JC. Koudstaal PJ. Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 16.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68:S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker SL, de Leeuw FE. den Heijer T. Koudstaal PJ, Hofman A. Cerebral haemodynamics in the elderly: the Rotterdam Study. Neuroepidemiology. 2004;23:178–184. doi: 10.1159/000078503. [DOI] [PubMed] [Google Scholar]
- 18.Sattel H, Forstl H, Biedert S. Senile dementia of Alzheimer type and multi-infarct dementia investigated by transcranial Doppler sonography. Dementia. 1996;7:41–46. doi: 10.1159/000106851. [DOI] [PubMed] [Google Scholar]
- 19.Heun R, Knappertz V, Kraemer G. Transcranial Doppler sonography in dementia of Alzheimer type. Dementia. 1994;5:327–333. doi: 10.1159/000106742. [DOI] [PubMed] [Google Scholar]
- 20.van Beek AH, Lagro J, Olde-Rikkert MG, Zhang R, Claassen JA. Oscillations in cerebral blood flow and cortical oxygenation in Alzheimer's disease. Neurobiol Aging. 2012;33:428.e421–428.e431. doi: 10.1016/j.neurobiolaging.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Gur AY, Gücüyener D, Korczyn AD, Üzüner N, Gilutz Y, Özdemir G, Bornstein NM. Cerebral vasomotor reactivity and dementia after ischemic stroke. Acta Neurol Scand. 2010;122:383–388. doi: 10.1111/j.1600-0404.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 22.van Beek AH, Sijbesma JC, Jansen RW. Rikkert MG, Claassen JA. Cortical oxygen supply during postural hypotension is further decreased in Alzheimer's disease, but unrelated to cholinesterase-inhibitor use. J Alzheimers Dis. 2010;21:519–526. doi: 10.3233/JAD-2010-100288. [DOI] [PubMed] [Google Scholar]
- 23.Roher A, Garami Z, Alexandrov A, Kokjohn T, Esh C, Kalback W, Vedders L, Wilson J, Sabbagh M, Beach T. Interaction of cardiovascular disease and neurodegeneration: transcranial Doppler ultrasonography and Alzheimer's disease. Neurol Res. 2006;28:672–678. doi: 10.1179/016164106X130470. [DOI] [PubMed] [Google Scholar]
- 24.Biedert S, Förstl H, Hewer W. Multiinfarct dementia versus Alzheimer's disease: sonographic criteria. Angiology. 1995;46:129–135. doi: 10.1177/000331979504600206. [DOI] [PubMed] [Google Scholar]
- 25.Foerstl H, Biedert S, Hewer W. Multiinfarct and Alzheimer-type dementia investigated by transcranial Doppler sonography. Biol Psychiatry. 1989;26:590–594. doi: 10.1016/0006-3223(89)90084-x. [DOI] [PubMed] [Google Scholar]
- 26.Asil T, Uzuner N. Differentiation of vascular dementia and Alzheimer disease: a functional transcranial Doppler ultrasonographic study. J Ultrasound Med. 2005;24:1065–1070. doi: 10.7863/jum.2005.24.8.1065. [DOI] [PubMed] [Google Scholar]
- 27.Lee ST, Jung KH, Lee YS. Decreased vasomotor reactivity in Alzheimer's disease. J Clin Neurology. 2007;3:18–23. doi: 10.3988/jcn.2007.3.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biedert S, Förstl H, Hewer W. The value of transcranial Doppler sonography in the differential diagnosis of Alzheimer disease versus multi-infarct dementia. Mol Chem Neuropathol. 1993;19:15–23. doi: 10.1007/BF03160165. [DOI] [PubMed] [Google Scholar]
- 29.Roher AE, Garami Z, Tyas SL, Maarouf CL, Kokjohn TA, Belohlavek M, Vedders LJ, Connor D, Sabbagh MN, Beach TG, Emmerling MR. Transcranial doppler ultrasound blood flow velocity and pulsatility index as systemic indicators for Alzheimer's disease. Alzheimers Dement. 2011;7:445–455. doi: 10.1016/j.jalz.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong XD, Zhang Y, Liu L, Sun N, Zhang MY, Zhag JN. Endothelial progenitor cells with Alzheimer's disease. Chin Med J. 2011;124:901–906. [PubMed] [Google Scholar]
- 31.Stefani A, Sancesario G, Pierantozzi M, Leone G, Galati S, Hainsworth AH, Diomedi M. CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer's and mixed dementia. J Neurol Sci. 2009;283:109–115. doi: 10.1016/j.jns.2009.02.343. [DOI] [PubMed] [Google Scholar]
- 32.Vicenzini E, Ricciardi M, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi G. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur Neurol. 2007;58:84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- 33.Franceschi M, Alberoni M, Bressi S, Canal N, Comi G, Fazio F, Grassi F, Perani D, Volonté MA. Correlations between cognitive impairment, middle cerebral artery flow velocity and cortical glucose metabolism in the early phase of Alzheimer's disease. Dementia. 1995;6:32–38. doi: 10.1159/000106919. [DOI] [PubMed] [Google Scholar]
- 34.Caamaño J, Gómez M, Cacabelos R. Transcranial Doppler ultrasonography in senile dementia: neuropsychological correlations. Methods Find Exp Clin Pharmacol. 1993;16:193–199. [PubMed] [Google Scholar]
- 35.Gucuyener DO, Yenilmez C, Ayranci U, Ozdemir F, Uzuner N, Ozkan S, Kaptanoglu C, Ozdemir G. An analysis of changes in cerebral blood flood velocities in depressive pseudo-dementia and Alzheimer disease patients. Neurologist. 2010;16:358–363. doi: 10.1097/NRL.0b013e3181a2eace. [DOI] [PubMed] [Google Scholar]
- 36.Provinciali L, Minicotti P, Ceravolo G, Angeleri F, Sanguinetti CN. Transcranial Doppler sonography as a diagnostic tool in vascular dementia. Eur Neurol. 1990;30:98–103. doi: 10.1159/000117320. [DOI] [PubMed] [Google Scholar]
- 37.Likitjaroen Y, Suwanwela NC, Phanthumchinda K. Vasoreactivity induced by acetazolamide in patients with vascular dementia versus Alzheimer's disease. J Neurol Sci. 2009;283:32–35. doi: 10.1016/j.jns.2009.02.363. [DOI] [PubMed] [Google Scholar]
- 38.Ries F, Horn R, Hillekamp J, Honisch C, Konig M, Solymosi L. Differentiation of multi-infarct and Alzheimer dementia by intracranial hemodynamic parameters. Stroke. 1993;24:228–235. doi: 10.1161/01.str.24.2.228. [DOI] [PubMed] [Google Scholar]
- 39.Anzola GP, Galluzzi S, Mazzucco S, Frisoni GB. Autonomic dysfunction in mild cognitive impairment: a transcranial Doppler study. Acta Neurol Scand. 2011;124:403–409. doi: 10.1111/j.1600-0404.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 40.Claassen JA, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R. Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using transcranial Doppler. J Alzheimers Dis. 2009;17:621–629. doi: 10.3233/JAD-2009-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bressi S, Volonte MA, Alberoni M, Canal N, Franceschi M. Transcranial Doppler sonography in the early phase of Alzheimer's disease. Dementia. 1992;3:25–31. doi: 10.1159/000106919. [DOI] [PubMed] [Google Scholar]
- 42.Purandare N, Burns A. Cerebral emboli in the genesis of dementia. J Neurol Sci. 2009;283:17–20. doi: 10.1016/j.jns.2009.02.306. [DOI] [PubMed] [Google Scholar]
- 43.Purandare N, Burns A, Daly KJ, Hardicre J, Morris J, Macfarlane G, McCollum C. Cerebral emboli as a potential cause of Alzheimer's disease and vascular dementia: case-control study. BMJ. 2006;332:1119–1124. doi: 10.1136/bmj.38814.696493.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purandare N, Voshaar RC, Morris J, Byrne JE, Wren J, Heller RF, McCollum CN, Burns A. Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry. 2007;62:339–344. doi: 10.1016/j.biopsych.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Purandare N, Welsh S, Hutchinson S, Riding G, Burns A, McCollum C. Cerebral emboli and paradoxical embolisation in dementia: a pilot study. Int J Geriatr Psychiatry. 2005;20:12–16. doi: 10.1002/gps.1202. [DOI] [PubMed] [Google Scholar]
- 46.Voshaar RC, Purandare N, Hardicre J, McCollum C, Burns A. Asymptomatic spontaneous cerebral emboli and cognitive decline in a cohort of older people: a prospective study. Int J Geriatr Psychiatry. 2007;22:794–800. doi: 10.1002/gps.1744. [DOI] [PubMed] [Google Scholar]
- 47.Purandare N, Burns A, Morris J, Perry E, Wren J, McCollum C. Association of cerebral emboli with accelerated cognitive deterioration in Alzheimer's disease and vascular dementia. Am J Psychiatry. 2012;169:300–308. doi: 10.1176/appi.ajp.2011.11010009. [DOI] [PubMed] [Google Scholar]
- 48.Silvestrini M, Viticchi G, Falsetti L, Balucani C, Vernieri F, Cerqua R, Luzzi S, Bartolini M, Provinciali L. The role of carotid atherosclerosis in Alzheimer's disease progression. J Alzheimers Dis. 2011;25:719–726. doi: 10.3233/JAD-2011-101968. [DOI] [PubMed] [Google Scholar]
- 49.Menendez-Gonzalez M, Garcia-Garcia J, Calleja S, Rojo J, Ribacoba R. Vasomotor reactivity is similarly impaired in patients with Alzheimer's disease and patients with amyloid hemorrhage. J Neuroimaging. 2011;21:e83–e85. doi: 10.1111/j.1552-6569.2009.00438.x. [DOI] [PubMed] [Google Scholar]
- 50.Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37:1010–1015. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 51.Matteis M, Silvestrini M, Troisi E, Bragoni M, Vernieri F, Caltagirone C. Cerebral hemodynamic patterns during stimuli tasks in multi-infarct and Alzheimer types of dementia. Acta Neurol Scand. 1998;97:374–380. doi: 10.1111/j.1600-0404.1998.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 52.Rosengarten B, Molnar S, Trautmann J, Kaps M. Simultaneous VEP and transcranial Doppler ultrasound recordings to investigate activation-flow coupling in humans. Ultrasound Med Biol. 2006;32:1171–1180. doi: 10.1016/j.ultrasmedbio.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Rosengarten B, Aldinger C, Kaufmann A, Kaps M. Comparison of visually evoked peak systolic and end diastolic blood flow velocity using a control system approach. Ultrasound Med Biol. 2001;27:1499–1503. doi: 10.1016/s0301-5629(01)00464-1. [DOI] [PubMed] [Google Scholar]
- 54.Rosengarten B, Kaps M. Peak systolic velocity Doppler index reflects most appropriately the dynamic time course of intact cerebral autoregulation. Cerebrovasc Dis. 2002;13:230–234. doi: 10.1159/000057848. [DOI] [PubMed] [Google Scholar]
- 55.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 56.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de la Torre JC. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer's pathogenesis. Neurobiol Aging. 2000;21:331–342. doi: 10.1016/s0197-4580(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 58.Rosengarten B, Aldinger C, Spiller A, Kaps M. Neurovascular coupling remains unaffected during normal aging. J Neuroimaging. 2003;13:43–47. [PubMed] [Google Scholar]
- 59.Rosengarten B, Kaps M. A simultaneous EEG and transcranial Doppler technique to investigate the neurovascular coupling in the human visual cortex. Cerebrovasc Dis. 2010;29:211–216. doi: 10.1159/000267840. [DOI] [PubMed] [Google Scholar]
- 60.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- 61.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 62.Kalaria R. Similarities between Alzheimer's disease and vascular dementia. J Neurol Sci. 2002;203–204:29–34. doi: 10.1016/s0022-510x(02)00256-3. [DOI] [PubMed] [Google Scholar]