Diagnosis: Loiasis
Based on the morphologic appearance of the organisms, a diagnosis of Loa loa infection was made (figures 1, 2, and 3; see also video 1, available in the electronic edition of Clinical Infectious Diseases) [1]. The patient subsequently had an endometrial biopsy performed that demonstrated foci of chronic inflammation suggestive of chronic endometritis. Skin snips of the bilateral scapular and iliac crest areas were performed and revealed a single, motile microfilaria consistent with Onchocerca volvulus. An ophthalmologic examination revealed only optic disk changes suspicious for glaucoma. The patient was referred to the Laboratory of Parasitic Diseases, the National Institutes of Health (Bethesda, MD), for further care. Additional diagnostic testing performed at the National Institutes of Health included a quantitative blood smear, which showed 3270 L. loa microfilariae/mL; a rapid diagnostic card test (Ov-16) that detects Onchocerca-specific antibodies, which had a positive result; and a Wuchereria bancrofti antigenemia test, which had a negative result.
Figure 1.
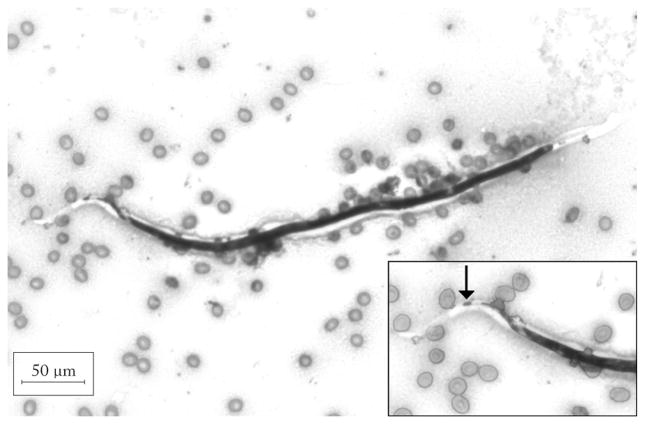
Photomicrograph of Wright-Giemsa stain of follicular fluid demonstrating a Loa loa microfilaria. L. loa are 185–300 μm in length and 5–8 μm in diameter and are best distinguished from other sheathed microfilariae (e.g., Wuchereria bancrofti and Brugia malayi) by examination of the tips of their tails (inset). L. loa uniquely possess nuclei that extend to the tip of a pointed tail and the terminal nucleus is elongate (arrow) (original magnification, ×200; inset original magnification, ×400).
Figure 2.
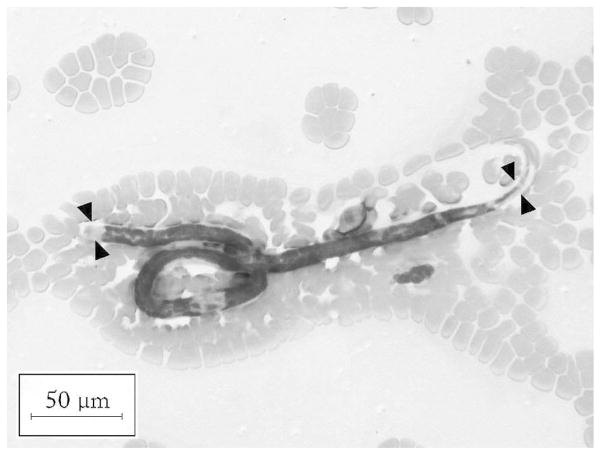
Photomicrograph of Wright-Giemsa stain of peripheral blood smear demonstrating a Loa loa microfilaria. L. loa possess a sheath that characteristically stains poorly with Wright-Giemsa; however, the sheath (arrowheads) can be appreciated by its deformation of other structures, such as RBCs (original magnification, ×200).
Figure 3.
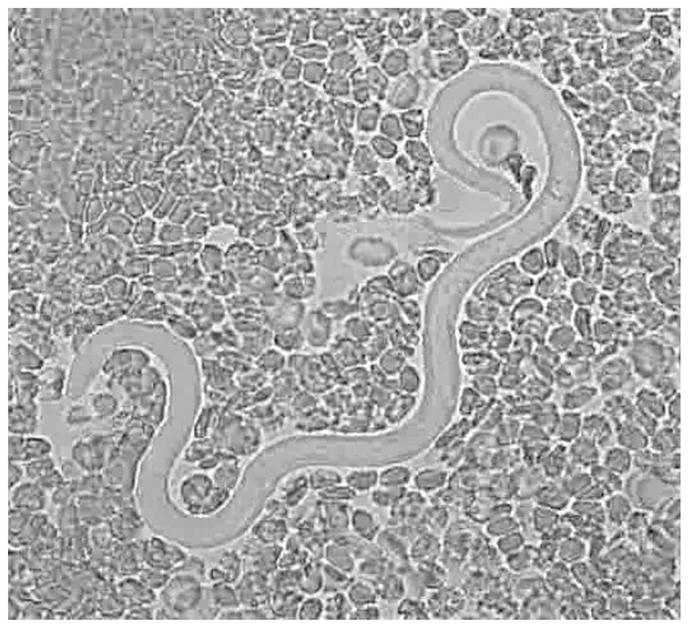
Loa loa microfilaria in a fresh peripheral blood sample. This is an image from video 1, available in the electronic edition of Clinical Infectious Diseases.
To reduce the L. loa microfilarial burden, the patient underwent apheresis procedures on 2 successive days that reduced the microfilarial levels to 92 microfilariae/mL. After apheresis, the patient was initially treated with a single dose of ivermectin (150 μg/kg). Subsequently, the patient received 3 weeks of therapy with diethylcarbamazine. An additional peripheral blood smear examination after treatment demonstrated no detectable microfilariae, and the patient resumed fertility treatments.
L. loa is a filarial nematode that is endemic in central and western Africa. Infection is transmitted through the bite of an infected Chrysops fly. Most infected persons are asymptomatic, but common and pathognomonic clinical findings include Calabar swellings—evanescent migratory angioedema related to migrating adult worms and the associated immune response—and subconjunctival migration of the adult worm (“eyeworm”). Calabar swellings and other allergic manifestations, including bronchospasm, pruritus, and urticaria, are more commonly seen among nonimmune visitors to areas of endemicity than among chronically exposed residents [2]. Cardiomyopathy, nephropathy, encephalitis, arthritis, lymphadenitis, and entrapment neuropathy may occasionally develop as a consequence of infection. Peripheral eosinophilia is a frequent laboratory finding [2, 3].
Diagnosis of loiasis is best made by examination of quantitative midday blood smears, because L. loa microfilariae are diurnally periodic. Most serological tests for L. loa have a high degree of sensitivity, but they typically cannot diagnose dual infections, distinguish among filarial infections due to organisms of differing genus or species, or distinguish between active and past infections in populations where the disease is endemic. A PCR test with excellent sensitivity and specificity exists, but it is only available in research settings [4]. The preferred treatment of loiasis is with diethylcarbamazine. However, because treatment of patients with high levels of circulating microfilariae has been associated with potentially fatal encephalitis, optimal management, where available, involves first decreasing the microfilarial load with apheresis or using small initial doses of diethylcarbamazine, with dose escalation over the first week of therapy.
O. volvulus is a filarial nematode that is endemic in Africa, Yemen, and Latin America. Infection is transmitted through the bite of an infected Simulium blackfly. Many infected persons are asymptomatic, whereas others develop skin and ocular disease, which can lead to progressive keratitis and blindness. Glaucoma, which this patient had signs of, has been proposed as another ocular complication of onchocerciasis [5]. Patients with onchocerciasis also often have peripheral eosinophilia.
Notably, O. volvulus is typically not found in the blood. Diagnostic tests for onchocerciasis include microscopic examination of skin snips, which is an insensitive test that is specific if there is no contamination with peripheral blood. Slit lamp examination can reveal clinically silent intraocular microfilariae. The microfilariae of O. volvulus are similar in size to those of L. loa and also possess an elongate terminal nucleus but, in contrast with L. loa, are not sheathed, and their tails are devoid of nuclei. Historically, O. volvulus immunodiagnosis has been of little use because of cross-reactivity between filarial species and the inability to distinguish between past and active infections. A newer rapid-format Ov-16 card test detects IgG4 to a recombinant antigen and is both sensitive and specific [6]. A highly specific PCR assay also exists, but is only available in research settings [7]. The recommended treatment for onchocerciasis is with ivermectin.
Excluding coinfection due to multiple filarial species is an important first step in the clinical assessment of patients with filarial infections. In particular, coinfection with L. loa and O. volvulus is not uncommon, and severe adverse events have been associated with treatment of onchocerciasis in patients who are not recognized to be coinfected with L. loa (and vice versa). Specifically, treatment of onchocercisis with ivermectin in coinfected patients has led to fatal encephalopathy and is not recommended unless the L. loa burden is low. Relatedly, diethylcarbamazine is potently filariacidal for O. volvulus, and its use to treat loiasis can lead to hypotension and death in coinfected patients. The patient reported here was initially treated with ivermectin for presumptive onchocerciasis at a time when her L. loa microfilarial counts were at their nadir. She tolerated treatment with ivermectin, as well as subsequent diethylcarbamazine therapy, without any adverse events.
Finally, this case is the second report, to our knowledge, involving L. loa isolated from ovarian follicular fluid. The first report of L. loa aspirated during oocyte retrieval was in an African woman undergoing in vitro fertilization in Belgium; this patient was not treated and underwent a spontaneous abortion after embryo implantation [8]. Other filarial infections discovered during in vitro fertilization include Mansonella perstans infection in a white woman with a history of travel to central and western Africa and W. bancrofti infection in a white woman with a history of travel to Guinea [9, 10]. This last patient initially experienced implantation failure during in vitro fertilization cycles but successfully conceived after antifilarial treatment. The patient described here had idiopathic endometrial inflammation; an additional endometrial biopsy performed after antifilarial treatment was nondiagnostic. The possible association between filarial infection and infertility is an intriguing observation that may deserve further investigation [11].
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.McPherson T, Nutman TB. Filarial nematodes. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, editors. Manual of clinical microbiology. 9. Vol. 2. Washington, DC: ASM Press; 2007. pp. 2156–65. [Google Scholar]
- 2.Klion AD, Massougbodji A, Sadeler BC, Ottesen EA, Nutman TB. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis. 1991;163:1318–25. doi: 10.1093/infdis/163.6.1318. [DOI] [PubMed] [Google Scholar]
- 3.Nutman TB, Miller KD, Mulligan M, Ottesen EA. Loa loa infection in temporary residents of endemic regions: recognition of a hyperresponsive syndrome with characteristic clinical manifestations. J Infect Dis. 1986;154:10–8. doi: 10.1093/infdis/154.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Nutman TB, Zimmerman PA, Kubofcik J, Kostyu DD. A universally applicable diagnostic approach to filarial and other infections. Parasitol Today. 1994;10:239–43. doi: 10.1016/0169-4758(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 5.Egbert PR, Jacobson DW, Fiadoyor S, Dadzie P, Ellingson KD. Onchocerciasis: a potential risk factor for glaucoma. Br J Ophthalmol. 2005;89:796–8. doi: 10.1136/bjo.2004.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipner EM, Dembele N, Souleymane S, et al. Field applicability of a rapid-format anti-Ov-16 antibody test for the assessment of onchocerciasis control measures in regions of endemicity. J Infect Dis. 2006;194:216–21. doi: 10.1086/505081. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman PA, Guderian RH, Aruajo E, et al. Polymerase chain reaction–based diagnosis of Onchocerca volvulus infection: improved detection of patients with onchocerciasis. J Infect Dis. 1994;169:686–9. doi: 10.1093/infdis/169.3.686. [DOI] [PubMed] [Google Scholar]
- 8.Wisanto A, Laureys M, Camus M, Devroey P, Verheyen G, Van Steir-teghem AC. Loa loa microfilariae aspirated during oocyte retrieval. Hum Reprod. 1993;8:2096–7. doi: 10.1093/oxfordjournals.humrep.a137988. [DOI] [PubMed] [Google Scholar]
- 9.Bazi T, Finan R, Zourob D, Sabbagh AS, Nasnas R, Zreik TG. Filariasis infection is a probable cause of implantation failure in in vitro fertilization cycles. Fertil Steril. 2006;85:1822, e13–5. doi: 10.1016/j.fertnstert.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 10.Goverde AJ, Schats R, van Berlo PJ, Claessen FA. An unexpected guest in follicular fluid. Hum Reprod. 1996;11:531–2. doi: 10.1093/humrep/11.3.531. [DOI] [PubMed] [Google Scholar]
- 11.Bernhard P, Makunde RW, Magnussen P, Lemnge MM. Genital manifestations and reproductive health in female residents of a Wuchereria bancrofti–endemic area in Tanzania. Trans R Soc Trop Med Hyg. 2000;94:409–12. doi: 10.1016/s0035-9203(00)90123-8. [DOI] [PubMed] [Google Scholar]