Abstract
Objective
To analyze the association between perioperative normothermia (temperature ≥36°C) and surgical site infections (SSIs) after gastrointestinal (GI) surgery.
Summary of Background Data
Although active warming during colorectal surgery reduces SSIs, there is limited evidence that perioperative normothermia is associated with lower rates of SSI. Nonetheless, hospitals participating in the Surgical Care Improvement Project must report normothermia rates during major surgery.
Methods
We conducted a nested, matched, case-control study; cases consisted of GI surgery patients enrolled in our National Surgical Quality Improvement Program database between March 2006 and March 2009 who developed SSIs. Patient/surgery risk factors for SSI were obtained from the National Surgical Quality Improvement Program database. Perioperative temperature/antibiotic/glucose data were obtained from medical records. Cases/controls were compared using univariate/random effects/logistic regression models. Independent risk factors for SSIs were identified using multivariate/random effects/logistic regression models.
Results
A total of 146 cases and 323 matched controls were identified; 82% of patients underwent noncolorectal surgery. Cases were more likely to have final intraoperative normothermia compared with controls (87.6% vs. 77.8%, P = 0.015); rates of immediate postoperative normothermia were similar (70.6% vs. 65.3%, respectively, P = 0.19). Emergent surgery/higher wound class were associated with higher rates of intraoperative normothermia. Independent risk factors for SSI were diabetes, surgical complexity, small bowel surgery, and nonlaparoscopic surgery. There was no independent association between perioperative normothermia and SSI (adjusted odds ratio, 1.05; 95% confidence interval, 0.48–2.33; P = 0.90).
Conclusions
Pay-for-reporting measures focusing on perioperative normothermia may be of limited value in preventing SSI after GI surgery. Studies to define the benefit of active warming after noncolorectal GI surgery are warranted.
Surgical site infections (SSIs) are the third leading cause of nosocomial infections, accounting for 14% to 16% of all hospital-acquired infections, and are the leading cause of nosocomial infection among surgical patients.1 SSIs are a major cause of postoperative morbidity and are associated with a 2- to 12-fold increased risk of postoperative mortality.2,3 Furthermore, SSIs represent a significant burden to the health care system. SSIs increase postoperative length of stay by an average of 4 days and result in attributable direct costs of up to $8,000 per case.4 It is estimated that SSIs accounted for up to $10 billion in direct costs in 2007 alone.5
Several measures have been shown to prevent the development of SSIs, including administration of prophylactic antibiotics within 1 hour prior to incision, use of hair clippers (rather than razors), preparation of the surgical field with appropriate antiseptics, and avoidance of postoperative hyperglycemia.6 Mild perioperative hypothermia, which is common during major surgery, has been hypothesized to predispose patients to SSIs by triggering thermo-regulatory vasoconstriction, which may decrease partial pressure of oxygen in tissues, impair oxidative killing by neutrophils, and interfere with collage deposition, resulting in impaired wound healing.7–13 The effect of active warming to prevent perioperative hypothermia was tested in a 1996 clinical trial by Kurz et al in which 200 patients undergoing colorectal surgery were randomized to active warming using forced-air warmer and fluid warmers to maintain patients’ core temperatures near 36.5°C (the “normothermia group”) or routine intraoperative thermal care (the “hypothermia group”).14 Of patients in the normothermia group, 6% developed SSIs, compared with 19% of patients in the hypothermia group (P = 0.009). In addition, mean final intraoperative core temperature in the normothermia group was 36.6°C, compared with 34.7°C in the hypothermia group (P = 0.002).
In 2006, the Center for Medicare and Medicaid Services launched the Surgical Infection Prevention Project, which later became the Surgical Care Improvement Project (SCIP), a pay-for-reporting program designed to reducing preventable perioperative morbidity and mortality by 25% by 2010. Hospitals participating in SCIP currently report their compliance with various evidence-based, process measures aimed at reducing perioperative SSIs, catheter-related urinary tract infections, cardiovascular complications, and venous thromboembolism. One such process measure, SCIP INF 7, initially required hospitals to report the percentage of patients undergoing colorectal surgery who had a first postoperative temperature greater than or equal to 36°C (ie, immediate postoperative normothermia).15 As of October 1, 2009, a new “normothermia” process measure was instituted: SCIP INF 10, Surgery patients with Perioperative Temperature Management.16 This measure requires hospitals to report the percentage of patients in whom active warming was used or who had at least one body temperature greater than or equal to 36°C within 30 minutes before or 15 minutes after anesthesia end time. Unlike SCIP INF 7, which focused solely on colorectal surgery patients, SCIP INF 10 includes all patients undergoing surgery under general or neuraxial anesthesia of greater than or equal to 60 minutes duration in its denominator. In their rationale, the authors of the measure cited the results of the active warming trial by Kurz, as well as research correlating unplanned hypothermia with adverse cardiac events, altered drug metabolism, and coagulopathy.17
Perioperative normothermia can be difficult to attain and often requires significant personnel, equipment, and time costs.14,18–20 Moreover, there is no conclusive evidence that perioperative normothermia is independently associated with a decreased risk of incisional SSIs.21 In the active warming trial by Kurz et al (the basis of evidence for the SCIP INF 7 and SCIP INF 10 process measures), the efficacy analysis was based on experimental group assignment (ie, active warming vs. routine intraoperative thermal care), not perioperative temperatures.14 The SCIP INF 7 and SCIP INF 10 process measures, on the other hand, focus on final intra- or first postoperative temperatures under the assumption that they are proxies for effective, active perioperative warming and that perioperative normothermia in and of itself carries a similar protective effect.15,16
The goal of this study was to explore the association between perioperative normothermia and incisional SSIs after gastrointestinal (GI) surgery. More specifically, we sought to determine the independent association between intra- and postoperative normothermia and SSI after colorectal and noncolorectal GI surgery, controlling for the effects of potential confounders, including patient- and surgery-related risk factors for SSI, antibiotic prophylaxis, and perioperative glucose control.
METHODS
We used a nested, matched, case-control study design to analyze the association between perioperative normothermia and incisional SSIs after GI surgery. After obtaining approval from the Medical University of South Carolina’s Institutional Review Board, we identified all patients who underwent GI surgery (based on Current Procedural Terminology [CPT] codes) at our institution between March 2006 and March 2009 and were entered into our American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. The NSQIP general approach to data collection and performance evaluation has been previously described in this journal.22 As part of our participation in ACS NSQIP (which began in March 2006), preoperative risk and laboratory data, intraoperative data, and 30-day postoperative morbidity and mortality data were collected on sample of patients undergoing major general or vascular operations under general, spinal, or epidural anesthesia during consecutive 8-day cycles. The first 40 consecutive, eligible operations in each 8-day cycle were included, with each cycle beginning on a different day of the week to improve sampling of cases. A surgical nurse reviewer trained in standardized definitions of each variable and data collection procedures collected and transmitted data to the Program, and conference calls and periodic site visits were conducted to ensure continual inter-rater reliability, data quality, and reliability.
For all patients entered into our ACS NSQIP database, data were collected on 135 variables, including 6 patient demographic variables, 11 surgical profile variables, 57 preoperative variables (including 44 risk factors and 13 laboratory variables), 19 intraoperative variables, and 20 types of postoperative occurrences (including superficial and deep SSI). Preoperative data were collected directly by nurse reviewers from the medical record, whereas intraoperative variables (including emergency status, American Society of Anesthesiologists [ASA] class, CPT code of the operation, and wound class) were obtained from the operative note and/or anesthesia records. Patients were followed by the nurse reviewer throughout their hospitalization and for up to 30 days after discharge to assess postoperative outcomes. Nurse reviewers also obtained information on outcomes by conducting periodic reviews of the medical record, attending morbidity and mortality conferences, contacting surgical attending physicians and primary care providers, and by communicating with patients after discharge from the hospital by follow-up letters and/or telephone calls.
For the purposes of this study, cases were defined as patients in the ACS NSQIP database who developed a superficial or deep incisional SSI after GI surgery (Table 1).23 Controls consisted of patients in the ACS NSQIP who also underwent GI surgery (matched by month/year of surgery) but did not develop a superficial or deep incisional SSI during the 30-day postoperative period. We allowed for a varying matching case-control ratio based on availability of controls. Patients with preoperative sepsis or septic shock and patients with pre-existing wound infections were excluded from the study.
TABLE 1.
Definition of Superficial and Deep Surgical Site Infections
| Superficial incisional SSI is an infection that occurs within 30 days after operation, and infection involves only skin or subcutaneous tissue of the incision and at least one of the following: |
| Purulent drainage, with or without laboratory confirmation, from the superficial incision |
| Organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision |
| At least one of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness or heat and superficial incision is deliberately opened by the surgeon, unless incision is culture-negative |
| Diagnosis of superficial incisional SSI by the surgeon or attending physician |
| Deep incisional SSI is an infection that occurs within 30 days after operation, appears to be related to the operation, and involves deep soft tissues (eg, fascia and muscle layers) of the incision and at least one of the following: |
| Purulent drainage from the deep incision but not from the organ/space component of the surgical site |
| A deep incision spontaneously dehisces or is deliberately opened by a surgeon when the patient has at least one of the following signs or symptoms: fever (>38°C), localized pain, or tenderness, unless site is culture-negative |
| An abscess or other evidence of infection involving the deep incision is found on direct examination, during reoperation, or by histopathologic or radiologic examination |
| Diagnosis of a deep incisional SSI by a surgeon or attending physician |
SSI indicates surgical site infections.
Perioperative temperature data (including lowest intraoperative temperature, final intraoperative temperature, and first postoperative temperature) was obtained from the anesthesia and/or nursing records. Intraoperative core temperatures were measured in the operating room using esophageal temperature probes, whereas first postoperative temperatures were measured in the postanesthesia care unit or intensive care units at the tympanic membrane. Information regarding patient- and surgery-related characteristics (including potential confounding risk factors for SSI) was obtained from the ACS NSQIP database and the medical record.23 Timing and type of perioperative antibiotic prophylaxis was obtained from the intraoperative nursing and/or anesthesia record. Perioperative serum glucose values (including highest intraoperative glucose, first postoperative glucose, highest glucose during the first postoperative 48 hours, and highest postoperative glucose during the patient’s length of stay) were obtained from the electronic laboratory system. All manually collected data were entered into a Research Electronic Data Capture (REDCap) database housed at Medical University of South Carolina.24 REDCap provides a secure internet location for gathering data and a means of importing additional data and exporting data to statistical software programs. Patient records in the ACS NSQIP database and the resulting REDCap database were electronically linked prior to analysis.
Statistical Analysis
Patients with a lowest intraoperative, final intraoperative, or first postoperative temperatures ≥36°C were defined as normothermic at each time point. “Perioperative normothermia” was defined as either a final intraoperative or first postoperative temperature ≥36°C (to approximate the functional definition of normothermia used in SCIP INF 10). As per ACS NSQIP protocol, patients who smoked within 1 year before surgery were defined as “current smokers.” For the purposes of this study, patients in the ACS NSQIP database who were recorded as having more than 2 alcoholic drinks per day within 2 weeks before surgery were defined as having “significant alcohol use.” Patients were considered to have received antibiotic prophylaxis only if administration of a specific agent was documented in the nursing and/or anesthesia record. Patients were considered to have received “correct” antibiotic prophylaxis based on the agent administered relative to the type of procedure performed.25 Surgical complexity, measured by work relative value units (work RVUs), was calculated based on the CPT code of the primary procedure performed. Length of surgery and estimated blood loss were recorded based on the highest value reported in the ACS NSQIP database or intraoperative records.
Univariate, random effects, logistic regression models with random intercepts for each matched set were used to identify the variables associated with case/control status. Multivariate, random effects, logistic regression models with random intercepts for each matched set were constructed to assess the independent association between perioperative normothermia and SSI, adjusting for all the variables associated with SSI on univariate analyses as well as other clinically significant variables. Assuming that the “protective effect” of perioperative normothermia on SSI approximated that of intraoperative active warming observed by Kurz et al, our study had 99% power to detect a difference in perioperative normothermia rates between cases and controls (if it existed). Statistical significance was defined as a 2-sided P < 0.05. Analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Our final study cohort consisted of 469 patients: 146 cases and 323 controls. Overall, the majority (82%) of our cohort underwent noncolorectal surgery. The patient characteristics of cases and controls are shown in Table 2. Cases were slightly older than controls, and the percentage of patients who were male was not statistically significant. Although the median body mass index in our cohort was relatively high, it did differ significantly in cases versus controls. Cases were more likely to have a history of diabetes and presented with advanced ASA class. Cases were no more likely than controls to have disseminated cancer, to have received chemotherapy, radiation therapy, or steroid therapy prior to surgery, or to be current smokers. In contrast, cases were more likely to have a significant history of alcohol use compared with controls. Highest preoperative bilirubin and lowest preoperative albumin levels (independent predictors of SSI in a previous study) were similar between cases and controls in our cohort.23
TABLE 2.
Patient Characteristics of Cases and Controls
| Characteristic (nm = Cases/Controls) | Cases n = 146 | Controls n = 323 | OR (95% CI) | P |
|---|---|---|---|---|
| Age (yr, mean ± SD) | 55.0 ± 15.5 | 50.1 ± 15.2 | 1.022 (1.008, 1.035) | 0.001 |
| Male gender (%) | 43.2 | 38.4 | 1.22 (0.82, 1.81) | 0.33 |
| Body mass index (kg/m2, mean ± SD) (nm = 5/18) | 32.9 ± 11.9 | 33.4 ± 12.2 | 1.00 (0.98, 1.013) | 0.67 |
| Diabetes mellitus (%) | 29.5 | 20.4 | 1.63 (1.038, 2.55) | 0.034 |
| Dyspnea (%) | 22.6 | 17.7 | 1.36 (0.84, 2.21) | 0.21 |
| ASA class (%) | 1.89 (1.36, 2.64)* | 0.0002 | ||
| 1 | 0 | 6.2 | ||
| 2 | 27.4 | 36.8 | ||
| 3 | 67.1 | 54.5 | ||
| 4 | 4.8 | 1.9 | ||
| 5 | 0.7 | 0.6 | ||
| Disseminated cancer (%) | 3.4 | 3.4 | 1.006 (0.34, 2.96) | 0.99 |
| Chemotherapy (within 30 d, %) | 2.1 | 0.6 | 3.37 (0.55, 20.51) | 0.19 |
| Radiation (within 90 d, %) | 2.1 | 1.2 | 1.67 (0.37, 7.62) | 0.5 |
| Chronic steroid use (%) | 4.8 | 4.0 | 1.20 (0.47, 3.086) | 0.7 |
| Current smoker | 24.0 | 24.8 | 0.96 (0.61, 1.51) | 0.85 |
| Significant alcohol use (%) | 4.8 | 1.2 | 4.016 (1.15, 14.01) | 0.029 |
| Highest preoperative bilirubin level (mg/dL, mean ± SD) (nm = 37/120) | 1.0 ± 0.8 | 1.0 ± 1.2 | 1.018 (0.82, 1.26) | 0.87 |
| Lowest preoperative albumin level (g/dL, mean ± SD) (nm = 34/92) | 3.4 ± 0.6 | 3.5 ± 0.7 | 0.78 (0.55, 1.11) | 0.16 |
Per unit of increasing ASA class.
SD indicates standard deviation; nm, number missing; ASA, American Society of Anesthesiologists; OR, odds ratio; CI, confidence interval.
Surgery characteristics associated with case/control status were also analyzed (Table 3). The percentage of patients who received any antibiotic prophylaxis and/or correct antibiotic prophylaxis was relatively high and did not differ significantly between cases and controls. Although the percentage of patients who underwent emergent procedures was higher in cases compared with controls, this difference was not statistically significant. In contrast, cases were less likely to have undergone gastroesophageal or hepatopancreatobiliary surgery and more likely to have undergone small bowel or colorectal surgery compared with controls. In addition, cases were significantly less likely to have undergone laparoscopic procedures and more likely to have undergone more complex procedures (based on work RVUs). Of note, cases and controls did not differ significantly with respect to wound class. In contrast, estimated blood loss and length of surgery were both significantly higher among cases.
TABLE 3.
Surgery Characteristics of Cases and Controls
| Characteristic (nm = Cases/Controls) | Cases n = 146 | Controls n = 323 | OR (95% CI) | P |
|---|---|---|---|---|
| Any antibiotic prophylaxis (%) (nm = 7/22) | 87.8 | 87 | 1.068 (0.58, 1.97) | 0.83 |
| Correct antibiotic prophylaxis (%) (nm = 7/22) | 74.8 | 76.4 | 0.92 (0.57, 1.47) | 0.72 |
| Emergency procedure (%) | 14.4 | 9.6 | 1.58 (0.87, 2.87) | 0.13 |
| Type of surgery (%) | ||||
| Gastroesophageal | 17.8 | 31.3 | Reference | — |
| Hepatopancreatobiliary | 33.6 | 39.3 | 1.50 (0.87, 2.58) | 0.14 |
| Small bowel | 25.3 | 14.2 | 3.13 (1.69, 5.77) | 0.0003 |
| Colorectal | 23.3 | 15.2 | 2.70 (1.46, 4.99) | 0.0017 |
| Surgical complexity (work RVUs; mean ± SD) (nm = 0/11) | 29.2 ± 12.7 | 24.1 ± 11.3 | 1.036 (1.019, 1.053) | <0.001 |
| Laparoscopic procedure (%) | 14.4 | 50.2 | 0.17 (0.10, 0.28) | <0.001 |
| Wound class (%) | ||||
| 1 | 4.1 | 9 | Reference | — |
| 2 | 78.1 | 76.8 | 2.22 (0.89, 5.52) | 0.085 |
| 3 | 11.6 | 11.8 | 2.16 (0.76, 6.20) | 0.15 |
| 4 | 6.2 | 2.5 | 5.44 (1.48, 19.97) | 0.011 |
| Length of surgery (h, mean ± SD) | 3.4 ± 2.1 | 2.5 ± 1.6 | 1.34 (1.20, 1.50) | <0.001 |
| Estimated blood loss (mL, mean ± SD) (nm = 4/31) | 468.6 ± 740.4 | 286.1 ± 525.5 | 1.001 (1.00, 1.001) | 0.0096 |
| Intraoperative transfusion (%) | 13.7 | 8.7 | 1.67 (0.91, 3.087) | 0.10 |
RVUs indicates relative value units; nm, number missing; SD, standard deviation; CI, confidence interval.
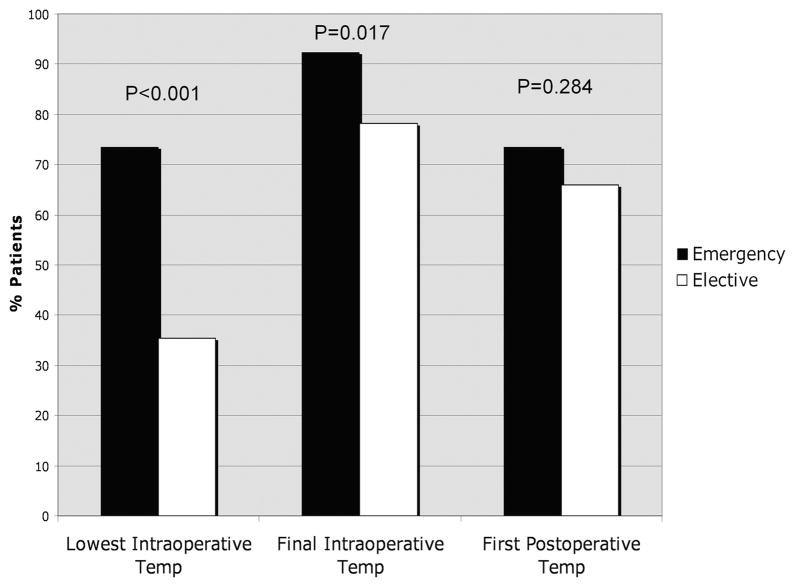
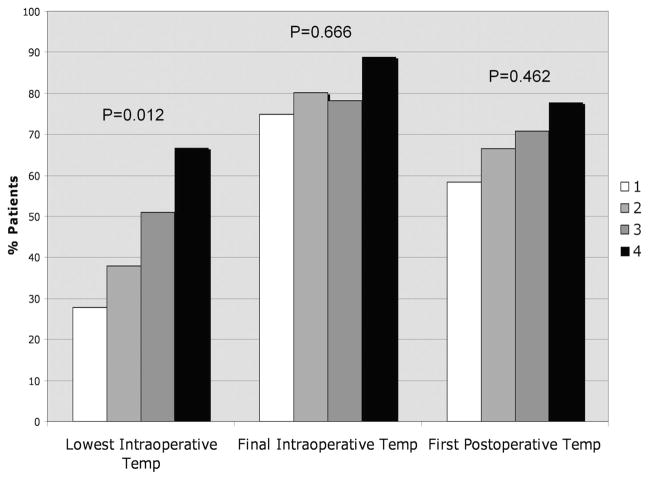
Cases and controls were compared with respect to recorded perioperative core temperatures (Table 4). Of note, median lowest intraoperative temperatures and final intraoperative temperatures were slightly higher in cases compared with controls. To further explore these seemingly paradoxical findings (ie, higher perioperative normothermia rates in cases compared with controls), we analyzed perioperative normothermia rates stratified by emergent surgery status (Fig. 1) and wound class (Fig. 2). Of note, the percentage of patients with intraoperative normothermia was significantly higher among patients who underwent emergency surgery, and the percentage of patients with lowest intraoperative temperature ≥36°C also increased with increasing wound class (ie, patients with more contaminated wounds were more likely to have been normothermic during surgery). Although cases and controls did not differ significantly with respect to median first postoperative temperature, cases were slightly more likely to be normothermic compared with controls (although this difference was not statistically significant).
TABLE 4.
Temperature and Normothermia Rates of Cases and Controls at Various Perioperative Time Points
| Temperature or Normothermia Rate (nm = Cases/Controls) | Cases n = 146 | Controls n = 323 | OR (95% CI) | P |
|---|---|---|---|---|
| Lowest intraoperative temperature (mean ± SD) (nm = 1/3) | 35.9 ± 0.8 | 35.7 ± 0.8 | 1.31 (1.01, 1.70) | 0.042 |
| % Patients with lowest intraoperative temperature ≥36°C (nm = 1/3) | 44.8% | 37.8% | 1.34 (0.90, 1.99) | 0.15 |
| Final intraoperative temperature (mean ± SD) (nm = 1/3) | 36.7 ± 0.7 | 36.5 ± 0.8 | 1.57 (1.19, 2.08) | 0.0015 |
| % Patients with final intraoperative temperature ≥36°C (nm = 1/3) | 87.6% | 77.8% | 2.012 (1.15, 3.53) | 0.015 |
| First postoperative temperature (mean ± SD) (nm = 4/5) | 36.4 ± 0.7 | 36.3 ± 0.7 | 1.31 (0.98, 1.73) | 0.065 |
| % Patients with first postoperative temperature ≥36°C (nm = 4/5) | 70.6% | 65.3% | 1.34 (0.87, 2.074) | 0.19 |
CI indicates confidence interval; nm, number missing; SD, standard deviation.
FIGURE 1.
Normothermia (T ≥36.0°C) rates at various perioperative time points according to emergency versus elective surgery status.
FIGURE 2.
Normothermia (T ≥36.0°C) rates at various perioperative time points according to wound class.
We also compared cases and controls with respect to rates of perioperative normothermia (defined as final intraoperative or first postoperative temperature ≥36°C; Fig. 3). Overall, rates of perioperative normothermia were similar between cases and controls (91.5% vs. 86.1%, respectively, P = 0.11). Perioperative normothermia rates did not differ significantly between cases and controls who underwent noncolorectal (90.0% vs. 86.3%, respectively, P = 0.30) or colorectal (93.6% vs. 85.1%, respectively, P = 0.21) gastrointestinal surgery.
FIGURE 3.
Perioperative normothermia (final intraoperative temperature or first postoperative T ≥36.0°C) of cases and controls, stratified by type of surgery. CR indicates colorectal.
Perioperative glucose values associated with case/control status were also analyzed (data not shown). On average, median highest intraoperative and first postoperative glucose levels were slightly higher among cases, although these differences were small and not statistically significant. Similarly, highest postoperative glucose levels (both during the first 48 hours and during the entire length of stay) were slightly higher (although not statistically significant) among cases compared with controls.
To analyze the independent association of perioperative normothermia on postoperative SSI, perioperative normothermia was forced into multivariate models controlling for age, diabetes, ASA class, significant alcohol use, surgery status (elective vs. emergency), type of surgery, surgical approach (laparoscopic vs. nonlaparoscopic), surgical complexity, wound class, length of surgery, and estimated blood loss (Table 5). Of note, perioperative normothermia was not independently associated with SSI, even when accounting for potential negative confounders (elective vs. emergency surgery status and wound class). In contrast, diabetes and small bowel surgery were associated with approximately a 2- and 3-fold risk of SSI (respectively), whereas laparoscopic surgery was associated with almost 4-fold reduction in risk. Surgical complexity was also associated with an increasing risk of SSI per each increasing unit of work RVUs. Of note, there were also no associations between final intraoperative normothermia (final intraoperative temperature ≥36°C) or immediate postoperative normothermia (first postoperative temperature ≥36°C) and SSI on additional multivariate analyses (data not shown).
TABLE 5.
Independent Risk Factors for Incisional Surgical Site Infection
| Risk Factor | Adjusted OR (95% CI)* | P |
|---|---|---|
| Diabetes | 1.87 (1.06, 3.30) | 0.031 |
| Type of surgery | ||
| Gastroesophageal | Reference | |
| Hepatopancreatobiliary | 0.73 (0.36, 1.50) | 0.35 |
| Small bowel surgery | 2.97 (1.30, 6.74) | 0.011 |
| Colorectal | 2.13 (0.92, 4.96) | 0.085 |
| Laparoscopic approach | 0.27 (0.14, 0.52) | <0.001 |
| Increasing surgical complexity (per work RVU) | 1.034 (1.005, 1.064) | 0.018 |
Controlling for age, diabetes, ASA class, significant alcohol use, surgery status (elective vs. emergency), wound class, length of surgery, estimated blood loss, and perioperative normothermia.
OR indicates odds ratio; CI, confidence interval; RVU, relative value unit.
DISCUSSION
Using a nested, case-control study design, we analyzed the association between perioperative normothermia and incisional SSIs after GI surgery. Cases and controls were similar with respect to percentage of patients with perioperative normothermia (91.5% vs. 86.1%, respectively, P = 0.11), even when the analysis was stratified by colorectal versus noncolorectal surgery. Emergent surgery and higher wound class were associated with higher rates of intraoperative normothermia. The independent risk factors for SSIs were diabetes, increasing surgical complexity, small bowel surgery, and nonlaparoscopic surgery. There was no association between perioperative normothermia and SSIs, even when controlling for patient-or surgery-risk factors or wound class (adjusted odds ratio, 1.05; 95% confidence interval, 0.48–2.33; P = 0.90).
There is persistent controversy regarding the beneficial effects of active warming during colorectal surgery (and by extension, of postoperative normothermia after colorectal surgery) on postoperative, incisional SSIs. Although the trial of active warming versus routine intraoperative thermal care reported by Kurz et al was prospective, randomized, and double-blinded, serious concerns remain about some of the methods used in the study.14,21 Specifically, patients in the “control” group were purposely rendered hypothermic, patients in both groups received almost 4 days of postoperative systemic antibiotics, 35% of patients in the hypothermia group received intraoperative transfusions (a known risk factor for SSIs) compared with only 22% of patients in the normothermia group, and length of hospital stay was atypically long (approximately 2 weeks) in both groups.26–28 Furthermore, SSI (the primary outcome of interest) was defined by positive aerobic or anaerobic cultures from pus that was aspirated or “expressed from the surgical incision” during the first 15 days after surgery (rather than on the more standard definitions of SSI used by the Centers for Disease Control and Prevention on which the ACS NSQIP definitions are based [Table 1]).29
Other groups have investigated the association between normothermia and wound infections after GI surgery. Barone et al retrospectively reviewed the records of 150 consecutive patients who underwent colorectal surgery over a 30-month period.21 Although at least one or more warming modalities were used in all patients, approximately 33% of patients were hypothermic (defined as T < 34.3°C) during the intraoperative or immediate postoperative period. Despite the rather extreme definition of hypothermia used in this study (even lower then the mean final intraoperative temperature [T < 34.7°C] of the hypothermia group in the trial by Kurz et al), the rates of wound infections (defined as “suppuration requiring removal of sutures”) was identical at 6% in both the normothermic and hypothermic patient populations.
Our nested, matched, case-control study design allowed us to analyze the independent association between perioperative normothermia and postoperative SSIs using a methodologically more rigorous (and statistically more efficient) study design. The cases in our cohort consisted of all GI surgery patients operated on at our institution during a 3-year period who were entered into our ACS NSQIP database and developed an incisional SSI. The predefined sampling algorithm by which patients were selected for inclusion into the ACS NSQIP database during each consecutive 8-day cycle minimized inclusion/exclusion bias and nonrandom sampling. Controls in our cohort were matched by month/year of surgery to control for temporal trends in intraoperative and/or postoperative care during the study period. Data on SSIs during the 30-day postoperative period were obtained by trained nurse reviewers using standardized definitions, thereby minimizing observer and misclassification bias. The fact that data on our primary risk factor of interest (perioperative normothermia) were based on data which were objectively recorded in the medical record before development and knowledge of our outcome of interest (postoperative SSI) allowed for a nested, case-control design, further minimizing information (ie, reporting) bias. Furthermore, our study explored the association between both intra- and postoperative normothermia on SSIs (after both colorectal and noncolorectal surgery) explicitly controlling for potential patient- and surgery-related risk factors for SSI and accounting for timing and type of antibiotic prophylaxis and perioperative glucose levels.
Previous studies have shown that elective surgery induces an increase in circulating interleukin-6 within 1 to 3 hours, and that the magnitude of the elevation is related directly to tissue injury and postoperative septic morbidity.30–32 Elevated interleukin-6 levels are associated with epidural fever (even in the absence of maternal or fetal infection) and have been linked to postoperative sepsis after major cancer surgery and during the early postoperative period.33,34 Although emergency surgery and increasing wound class were not associated with SSI in our cohort, it is interesting to note that they were associated with higher rates of intraoperative normothermia. These differences were most pronounced at the lowest intraoperative time points, and less pronounced (and/or no longer significant) at the final intraoperative and/or first postoperative time points. The observed associations between normothermia, emergency surgery, and advanced wound class in our study suggest that the higher rates of normothermia in our cases may have been the result of higher rates of subclinical systemic inflammatory response syndrome in these patients. If so, one might expect that higher rates of apparent hospital “compliance” (ie, perioperative normothermia) with SCIP INF 10 could paradoxically be associated with higher rates of SSI (and other postoperative infections), rather than the converse.
Our study has several potential limitations. Although data on the various ACS NSQIP variables were fairly complete, data on some of the variables collected from the medical record (eg, type of antibiotic prophylaxis) were occasionally missing or illegible. Only 18% of the GI surgery patients in our cohort underwent colorectal surgery, a reflection of our referral patterns and status as a tertiary center specializing in esophageal, bariatric, and hepatopancreatobiliary surgery. As such, we may have been underpowered to detect associations between perioperative normothermia and SSI in our stratified (ie, colorectal vs. noncolorectal GI surgery) analyses. Finally, because the use of forced-air warmers and fluid warmers was not routinely documented in the nursing and/or anesthesia records, we could not control for selective use of these warming modalities (and potential confounding) in our study, although matching of cases and controls by month/year may have partially controlled for temporal trends in their use in our cohort during the study period. The fact that the observed rates of perioperative normothermia were relatively high in both cases and controls in our cohort may have limited our ability to detect its “protective effect” on SSI (ie, case status), if it existed. Finally, our study focused solely on the potential association between perioperative normothermia and SSI after GI surgery, and did not explore any potential correlations with adverse cardiac events, altered drug metabolism, or coagulopathy.
The evaluation of health care quality has traditionally followed the Donabedian model that focuses on structure, process, and outcomes.35 Unlike process measures, which are usually evidence-based, easy to measure, and largely viewed as nonthreatening, outcomes are often difficult to define, costly to measure, and often viewed as threatening to provider and hospital reputations.36 Not surprisingly, regulatory agencies have traditionally used “evidence-based” process measures as proxies for outcomes and health care quality. Our study suggests that perioperative normothermia process measures, such as SCIP INF 7 and SCIP INF 10, may not be supported by the current evidence, and moreover, may not be widely applicable to other surgical populations (ie, patients undergoing noncolorectal, GI surgery). Moreover, apparent “compliance” with these measures (ie, perioperative normothermia) may, in fact be paradoxically associated with higher rates of the main adverse outcome the measures were designed to prevent (ie, incisional SSI). Indeed, a recent study showed that hospital-level compliance with various SCIP process measures focusing on prevention of SSI and venous thromboembolism was poorly correlated with risk-adjusted outcomes.36
In summary, our results suggest that although active warming during colorectal surgery may reduce the rate of postoperative SSIs, perioperative normothermia in and of itself is not independently associated with a decreased risk of SSI after colorectal or noncolorectal GI surgery. As such, hospital- and provider-level compliance with pay-for-reporting process measures focused on perioperative normothermia of limited clinical value in preventing SSIs after GI surgery. Trials to determine the benefit of active warming during noncolorectal GI surgery are warranted, and confirmatory studies may be needed to further define the association of perioperative normothermia on SSI after colorectal and noncolorectal GI surgery. In the interim, providers and hospitals seeking to reduce SSI rates after GI surgery are advised to move beyond simple compliance with processes measures (particularly those centered around perioperative normothermia), channel personnel and institutional resources toward implementation of other evidence-based best practices to prevent SSI, and focus their efforts on tracking and improving health outcomes, the ultimate measures of health care quality.
Acknowledgments
Supported partially by grants NIH/NIDDK 5T35DK007431–25 (to S.J.L.), 1P30CA138313–01 (to G.O.), and NIH 1UL1RR029882 (REDCap).
The authors thank John Clark (MUSC SUCCESS Center) for his technical assistance in the creation of Research Electronic Data Capture (REDCap) database and Stacy Miers for her expert administrative support.
Footnotes
Presented at the 130th Annual Meeting of the American Surgical Association, April 8–10, 2010, Chicago, IL.
References
- 1.National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992–June 2001, issued August 2001. Am J Infect Control. 2001;29:404–421. doi: 10.1067/mic.2001.119952. [DOI] [PubMed] [Google Scholar]
- 2.Engemann JJ, Carmeli Y, Cosgrove SE, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592–598. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- 3.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 4.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 5.Scott R. The Direct Medical Costs of Healthcare-Associated Infections in US Hospitals and the Benefits of Prevention. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Cited March 26, 2010.
- 6.Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S51–S61. doi: 10.1086/591064. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki M, Sessler DI, Suzuki H, et al. Nitrous oxide decreases the threshold for vasoconstriction less than sevoflurane or isoflurane. Anesth Analg. 1995;80:1212–1216. doi: 10.1097/00000539-199506000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Sessler DI, Rubinstein EH, Moayeri A. Physiologic responses to mild peri-anesthetic hypothermia in humans. Anesthesiology. 1991;75:594–610. doi: 10.1097/00000542-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Chang N, Mathes SJ. Comparison of the effect of bacterial inoculation in musculocutaneous and random-pattern flaps. Plast Reconstr Surg. 1982;70:1–10. doi: 10.1097/00006534-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson K, Hunt TK, Mathes SJ. Oxygen as an isolated variable influences resistance to infection. Ann Surg. 1988;208:783–787. doi: 10.1097/00000658-198812000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohn DC, MacKay RD, Halliday B, et al. Effect of O2 tension on microbicidal function of leukocytes in wounds and in vitro. Surg Forum. 1976;27:18–20. [PubMed] [Google Scholar]
- 12.Hunt TK, Pai MP. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135:561–567. [PubMed] [Google Scholar]
- 13.Jonsson K, Hunt TK, Brennan SS, et al. Tissue oxygen measurements in delayed skin flaps: a reconsideration of the mechanisms of the delay phenomenon. Plast Reconstr Surg. 1988;82:328–336. doi: 10.1097/00006534-198808000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 15.QualityNet: National Hospital Quality Measures, Specifications Manual, Discharges 07/01/2006 to 9/30/2006. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier3&cid=1142976368240. Cited March 26, 2010.
- 16.QualityNet: National Hospital Quality Measures, Specifications Manual, Discharges 10/01/2009 to 03/31/2010. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228695698425. Cited March 26, 2010.
- 17.Kurz A. Thermal care in the perioperative period. Best Pract Res Clin Anaesthesiol. 2008;22:39–62. doi: 10.1016/j.bpa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Smith CE, Desai R, Glorioso V, et al. Preventing hypothermia: convective and intravenous fluid warming versus convective warming alone. J Clin Anesth. 1998;10:380–385. doi: 10.1016/s0952-8180(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 19.Weirich TL. Hypothermia/warming protocols: why are they not widely used in the OR? AORN J. 2008;87:333–344. doi: 10.1016/j.aorn.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Hedrick TL, Heckman JA, Smith RL, et al. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg. 2007;205:432–438. doi: 10.1016/j.jamcollsurg.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Barone JE, Tucker JB, Cecere J, et al. Hypothermia does not result in more complications after colon surgery. Am Surg. 1999;65:356–359. [PubMed] [Google Scholar]
- 22.Hall BL, Hamilton BH, Richards K, et al. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 23.Neumayer L, Hosokawa P, Itani K, et al. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert DN, Moellering RC, Jr, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 39. Sperryville, VA: Antimicrobial Therapy, Inc; 2009. [Google Scholar]
- 26.Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33:659–661. doi: 10.1097/00005373-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal N, Murphy JG, Cayten CG, et al. Blood transfusion increases the risk of infection after trauma. Arch Surg. 1993;128:171–176. doi: 10.1001/archsurg.1993.01420140048008. discussion 176–177. [DOI] [PubMed] [Google Scholar]
- 28.Ford CD, Van Moorleghem G, Menlove RL. Blood transfusions and postoperative wound infection. Surgery. 1993;113:603–607. [PubMed] [Google Scholar]
- 29.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Biffl WL, Moore EE, Moore FA, et al. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baigrie RJ, Lamont PM, Kwiatkowski D, et al. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757–760. doi: 10.1002/bjs.1800790813. [DOI] [PubMed] [Google Scholar]
- 32.Martin C, Boisson C, Haccoun M, et al. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Goetzl L, Evans T, Rivers J, et al. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am J Obstet Gynecol. 2002;187:834–838. doi: 10.1067/mob.2002.127135. [DOI] [PubMed] [Google Scholar]
- 34.Mokart D, Capo C, Blache JL, et al. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg. 2002;89:1450–1456. doi: 10.1046/j.1365-2168.2002.02218.x. [DOI] [PubMed] [Google Scholar]
- 35.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198:626–632. doi: 10.1016/j.jamcollsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Hall BL, et al. New approaches to the National Surgical Quality Improvement Program: the American College of Surgeons experience. Am J Surg. 2009;198(suppl 5):S56–S62. doi: 10.1016/j.amjsurg.2009.07.026. [DOI] [PubMed] [Google Scholar]