Abstract
Objective
Statins have been found to have potent anti-inflammatory and immunomodulating effects, which led to the hypothesis that statins could be neuroprotective agents. However, the beneficial effects of statins could be offset by their unfavorable effects on lowering plasma coenzyme Q10 and urate. We therefore prospectively examined whether use of statins was associated with altered risk of PD.
Design, setting, and participants
A prospective study including 38,191 men and 90,874 women participating in two ongoing US cohorts, the Health Professional Follow-up and the Nurses’ Health Study. Information on regular cholesterol lowering drug use (2+ times/week) was collected in 1994 in both cohorts via questionnaire. Relative risks (RR) and 95% confidence intervals (CI) were computed using Cox proportional hazards models adjusting for age, smoking, caffeine intake, duration of hypercholesterolemia, and other covariates.
Main outcome
Incident PD.
Results
During 12 years of follow-up (1994-2006), we documented 644 incident PD cases (338 women and 306 men). The risk of PD was lower among current statin users (adjusted pooled RR=0.74; 95% CI: 0.54, 1.00; P=0.049), relative to non-users. A significant association was observed in participants who were aged <60 years at baseline (adjusted pooled RR=0.31, 95% CI: 0.11, 0.86; P=0.02), but not among those who were older (adjusted pooled RR=0.83, 95% CI: 0.60, 1.14; P=0.25) (p for interaction=0.03).
Conclusions
We found that regular use of statins was associated with a modest reduction in PD risk. The possibility that some statins may reduce PD risk deserves further consideration.
Statins are one of most prescribed drug classes in the US. Based on NHANES 2003-2004, 11.7% US adults (i.e., 24 million) were taking statins. 1 Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the key enzyme that regulates the synthesis of cholesterol from mevalonic acid. Recently, statins have been found to have potent anti-inflammatory and immunomodulating effects, which led to the hypothesis that statins could be neuroprotective agents. 2-4 However, the beneficial effects of statins could be offset by their unfavorable effects on lowering plasma coenzyme Q10, 5, 6 which may be neuroprotective in individuals with PD.7 This potential for harm makes it all the more important to assess their effects. Several prospective studies have been conducted to examine the association between statin use and PD risk and generated mixed results.2,8, 9 As shown in a recent review, significant associations between statin use and lower PD risk were observed in two out of five prospective studies.2 In two large registry studies published after that review, statin use was not significantly associated with PD risk overall. 8, 9 However, the majority of these studies were based on registry data and failed to control for several important confounders, such as smoking and caffeine intake. We therefore conducted a prospective study to examine whether use of statins was associated with PD risk in two large ongoing US cohorts comprising ~140,000 men and women.
Subjects and Methods
Study population
The Nurses’ Health Study (NHS) cohort comprises 121,700 female registered nurses who were 30 to 55 years of age and resided in one of eleven U.S. states at the time of enrollment in 1976. The cohort has been followed by means of biennial mailed questionnaires, which inquire about lifestyle practices and other exposures of interest, as well as the incidence of disease. The Health Professional Follow-up Study (HPFS) was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40-75 completed a mailed similar questionnaire regarding their medical history and lifestyle. In both cohorts, follow-up questionnaires have been mailed to participants every 2 years to update information on potential risk factors and to ascertain newly diagnosed diseases.
The study was approved by the Human Research Committees at the Harvard School of Public Health and the Brigham and Women's Hospital, with receipt of each questionnaire accepted as participant's consent.
Assessment of statin uses
In the NHS, women were asked to report regular use of cholesterol-lowering drugs (2+ times/week) in 1988, 1994, 1996, and 1998. In the HPFS, information on regular use of cholesterol-lowering drugs was assessed every two years, from 1986 to 1998. Starting in the 2000 HPFS/NHS questionnaires, and then every two years thereafter, participants were asked to report separately use of statins and other classes of cholesterol-lowering drugs. Because previous studies based on national data have shown that statins constituted the majority of the cholesterol-lowering drugs since 1994 10, we used this year as the baseline in our primary analysis and considered use of any cholesterol-lowering drugs as statin use. This assumption is also supported by the fact that approximately 92% of participants who reported use of cholesterol-lowering drugs were statin users based on the 2000 questionnaire. In the current analysis, we excluded participants with PD onset in or prior to 1994 and those missing information on statin use at baseline, leaving 38,192 men and 90,874 women for analyses.
Assessment of potential covariates
Dietary intakes were assessed every four years with validated semi-quantitative food frequency questionnaires in both cohorts. 11 Information on age, weight, height, smoking status, elevated cholesterol, hypertension, diabetes, coronary heart disease, and use of ibuprofen was collected through biennial questionnaires. Body mass index (BMI) was calculated as weight (kg) / height (m) 2. Duration of hypercholesterolemia was estimated by summing use across the 2-year periods encompassed by the biennial questionnaires.
Ascertainment of PD
We identified new PD cases by biennial self-reported questionnaires.12-14 We then asked the treating neurologists to complete a questionnaire to confirm the diagnosis of PD or to send a copy of the medical records. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence of at least two of the three cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. The review of medical records was conducted by the investigators, blind to the exposure status. Overall, the diagnosis was confirmed by the treating neurologist in >90% of the cases.
Statistical analysis
We computed the person-time of follow-up for each participant from the return date of the baseline questionnaire (1994) to the date of the occurrence of the first symptoms of PD, the date of death, or the end of follow up (2006), whichever came first. We categorized participants into regular users versus nonusers of statins at baseline and calculated relative risks (RRs) and 95% confidence intervals (CIs) using a Cox proportional hazards model, controlling for age (in months), smoking status (never smoker, past smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), use of ibuprofen (yes/no), duration of hypercholesterolemia (years) and presence of coronary heart disease, hypertension, and diabetes (yes/no for each), and intake of alcohol(none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), caffeine (quintiles), and lactose (quintiles). Log RR from the two cohorts were pooled by a random-effects model, weighted by the inverse of their variances.
We also examined potential interactions between use of statins and age (continuous), smoking status (never verse ever), caffeine intake (based on median intake), BMI (< vs. ≥ 25 kg/m2), and use of postmenopausal hormones (never verse ever) by adding multiplicative terms in the Cox models, adjusting for other potential confounders.
To take advantage of repeatedly collected information on statin use, in the secondary analysis we considered updated statin use to predict the subsequent incidence of PD. This is practically important because statin use has become increasingly more common during the period of follow-up of our cohorts. To test the robustness of our results, we conducted a sensitivity analysis by restricting to PD cases diagnosed by neurologists. An additional sensitivity analysis was conducted by imputing statin use in 1994 based on the duration of statin use reported in the 2000 HPFS/NHS questionnaires; in this analysis, participants were assumed to be statin users in 1994 if they reported a duration of use ≥ 6 years in 2000, and non-users otherwise.
Results
Individuals who reported statin use had higher BMI, exercised less, were more likely to be past smoker and to use ibuprofen, consumed less caffeine and more lactose, and had a higher prevalence of CHD, diabetes, and hypertension and longer history of hypercholesterolemia relative to nonusers. (Table 1)
Table 1.
Basic characteristics according to use of statins at baseline in the Health Professionals Follow-up Study and the Nurses’ Health Study (1994)
| Men | Women | |||
|---|---|---|---|---|
| Use of Statins | Use of Statins | |||
| No | Yes | No | Yes | |
| n | 34,886 | 3,306 | 83,145 | 7,729 |
| Age, y | 62.0 | 61.9 | 58.2 | 58.3 |
| Current smokers, % | 6.1 | 5.2 | 13.4 | 13.1 |
| Past smokers, % | 49.8 | 55.5 | 42.3 | 45.9 |
| BMI, kg/m2 | 25.9 | 26.3 | 26.4 | 27.6 |
| Alcohol intake, g/d | 11.1 | 11.6 | 5.0 | 4.0 |
| Caffeine intake, mg/d | 232 | 216 | 240 | 215 |
| Lactose intake, g/d | 14.8 | 15.4 | 14.8 | 15.7 |
| Physical activity, Met/wk | 36.4 | 33.2 | 19.7 | 18.0 |
| Use of ibuprofen, % | 12.9 | 15.4 | 24.8 | 30.2 |
| Duration of hypercholesterolemia, y | 2.1 | 6.5 | 3.0 | 8.4 |
| Coronary heart disease, % | 2.0 | 7.4 | 0.8 | 5.2 |
| Hypertension, % | 31.4 | 49.7 | 36.1 | 60.3 |
| Diabetes, % | 5.3 | 9.4 | 5.9 | 14.4 |
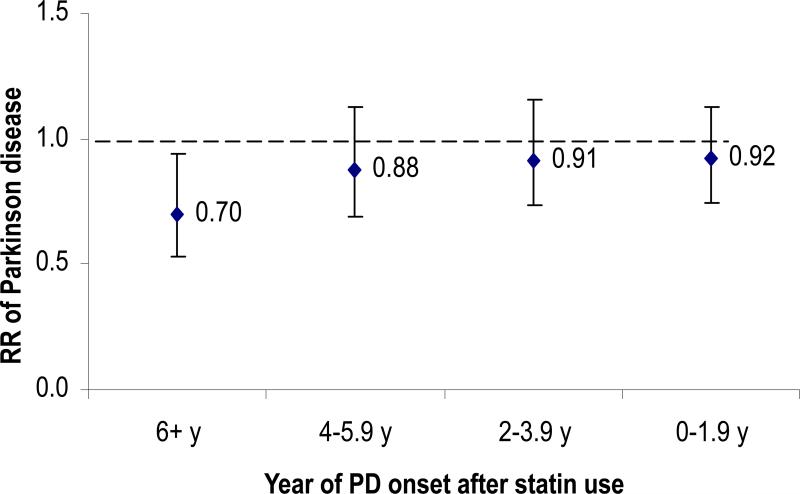
During 12 years of follow-up, we documented 644 incident PD cases (338 women and 306 men). The incidence of PD was lower among statin users relative to non-users. The pooled RR of PD was 0.74 (95% CI: 0.54, 1.00; P = 0.049) comparing statin users to non-users, after adjusting for age, smoking, consumption of caffeine and lactose, use of ibuprofen, duration of hypercholesterolemia, history of major chronic diseases, and other potential confounders (Table 2). Results were similar after restricting the analyses to PD cases confirmed by a neurologist (pooled RR=0.69, 95% CI: 0.48, 0.99) or in analyses in which statin use in 1994 was imputed from duration of statin use reported on the 2000 questionnaire (pooled RR=0.76, 95% CI : 0.38, 1.54). When we used updated statin use to predict incidence of PD, a significant association was seen for PD onset 6 or more years after statin use (pooled RR=0.70, 95%CI: 0.53, 0.93; P=0.02), but not for short term use (P>0.2 for all) (Figure 1). These observations are consistent with the notion that PD has a long pre-clinical stage.15
Table 2.
Use of statins and PD risk in the Health Professionals Follow-up Study and the Nurses’ Health Study (1994-2006)
| Use of statins | |||
|---|---|---|---|
| no | yes | P value | |
| HPFS | |||
| Case # | 282 | 24 | |
| Age- and smoking- adjusted RR | 1(ref) | 0.79(0.52, 1.20) | 0.27 |
| Multivariate RR | 1(ref) | 0.78 (0.50, 1.22) | 0.27 |
| NHS | |||
| Case # | 311 | 27 | |
| Age- and smoking- adjusted RR | 1(ref) | 0.74 (0.50, 1.11) | 0.14 |
| Multivariate RR | 1(ref) | 0.70 (0.46, 1.07) | 0.10 |
| Pooled RR | 1(ref) | 0.74 (0.54, 1.00) | 0.049 |
1Adjusted for age (in months), smoking status (never smoker, past smoker, current smoker with 1-14 cigarettes/d, or current smoker with ≥ 15 cigarettes/d), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), intake of caffeine (quintiles), lactose (quintiles), and alcohol (none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), physical activity (quintiles), use of ibuprofen (yes/no), duration of hypercholesterolemia (years) and presence of coronary heart disease, hypertension, and diabetes (yes/no for each).
Figure 1.
Lag analysis of updated statin use and risk of developing PD, adjusted for age (in months), smoking status (never smoker, past smoker, current smoker with 1-14 cigarettes/d, or current smoker with ≥ 15 cigarettes/d), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), intake of caffeine (quintiles), lactose (quintiles), and alcohol (none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), physical activity (quintiles), use of ibuprofen (yes/no), duration of hypercholesterolemia (years) and presence of coronary heart disease, hypertension, and diabetes (yes/no for each) .
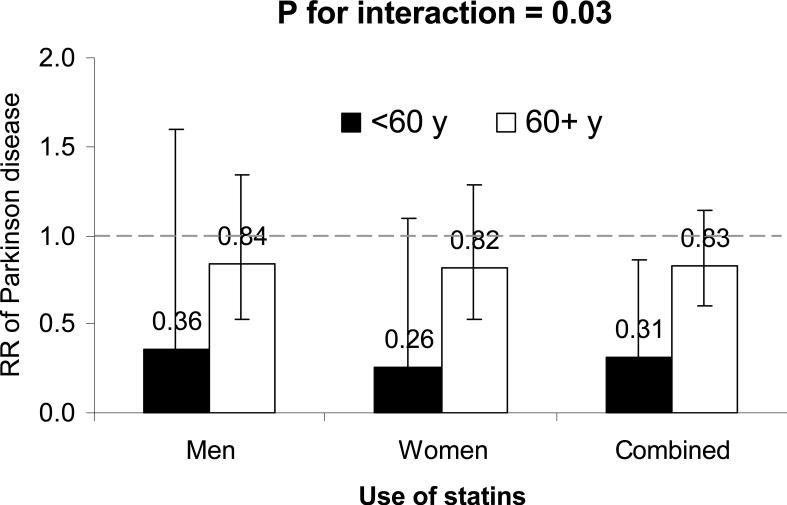
We observed a significant interaction between statin use and age in relation to PD risk (P for interaction = 0.03). The significant association was observed in participants who were aged <60 years at the beginning of follow-up (adjusted pooled RR=0.31, 95% CI: 0.11, 0.86; P=0.02), but not among those who were older (adjusted pooled RR=0.83, 95% CI: 0.60, 1.14; P=0.25) (Figure 2) The interactions between smoking status, caffeine intake, or BMI and statin use on PD risk were not significant (P-interaction > 0.2 for all).
Figure 2. Relative risks and 95% confidence intervals of Parkinson's disease according to statin use, stratified by age.
Adjusted for age (in months), smoking status (never smoker, past smoker, current smoker with 1-14 cigarettes/d, or current smoker with ≥ 15 cigarettes/d), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), intake of caffeine (quintiles), lactose (quintiles), and alcohol (none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), physical activity (quintiles), use of ibuprofen (yes/no), duration of hypercholesterolemia (years) and presence of coronary heart disease, hypertension, and diabetes (yes/no for each). Case # was 172 in participants aged <60 years, and 471 in participants aged 60+ years in 1994.
Discussion
In this prospective study, self-reported statin use was associated with a lower PD risk. Adjustment for smoking, caffeine intake, history of heart disease and hypercholesterolemia, and other potential confounders did not materially change the results.
The observed association between regular use of statins and lower PD risk is consistent with the results of in vivo and in vitro experimental studies in models of PD, which suggest that statins could reduce alpha-synuclein accumulation and oxidative stress, suppress COX-2 expression, reduce release of TNF-α and NFκB activation, activate PPAR-γ, and upregulate dopamine D1/D2 receptors in the brain. 2-4, 16, 17 These effects would be expected to alleviate neuroinflammation and reduce PD risk.
Epidemiologic studies have generated mixed results regarding statin use and PD risk. Significant protective effects of statins were observed in two retrospective case-control studies.18, 19 However, recall and selection biases cannot be ruled out in these studies. In a prospective study based on the Rotterdam cohort (incident PD case number =87), use of any statins was associated with a non-significant 67% of lower risk of PD (RR=0.33, 95% CI: 0.08, 1.35), after adjusting for age, smoking and sex.20 A significantly inverse association between use of overall or certain subclasses of statins and PD risk was observed in some 21, 22 but not all prospective studies 23-25 using registry data. However, these studies were limited by residual confounding and potential misclassification of PD diagnosis.
We observed that the association between statin use and PD was modified by age -- the protective effects of statins appeared only among adults younger than 60 years. In a previous case-control study, a slightly stronger association was also seen in younger (<60 years) relative to older participants.18 However, such difference was not observed in another Denmark based prospective study.23 Because we used cholesterol lowering drug as a surrogate of statin use in the current study, we cannot exclude the possibility that the significant statin-age interaction is confounded by the indications that younger participants were more likely to receive the newer, and more expensive cholesterol lowering drugs such as statins, relative to older participants.
Our results should be interpreted in the context of several limitations. Because we classified use of any cholesterol-lowering drugs before 2000 as statin use, misclassification was inevitably introduced. However, based on the US retail prescription data 10, statins accounted for 72% of total cholesterol-lowering drug use in 1994 and, 80% in 1996. In the HPFS/NHS, statins accounted for more than 90% of all cholesterol-lowering drugs used in 2000. Further, the sensitivity analysis based on the information on duration of statin use on the 2000 questionnaire generated similar results.
We did not collect information on use of specific statins, which could have different effects on central nervous system due to difference in synthetic origins and structure (e.g., lipophilicity vs. hydrophility)4 and thus differ in blood-brain barrier penetrability.4 For example lovastatin and simvastain have been shown to have more potent to cross blood-brain barrier, relative to atorvastatin. Further, hypouricemic effects differ among various statins. Previous studies have reported that atorvastatin, but not simvastatin, can lower serum urate,26 a powerful antioxidant associated with lower PD risk or slower disease progression among individual with PD. 27-30 However, based on the national retail data, lovastatin and simvastain were the two most commonly used statins during 1994-96, comprising ~60-70% of total statins.31 Our results, therefore, could be largely driven by these two subclasses. Interestingly, simvastatin use was associated with a lower PD risk in two previous prospective studies. 8, 21 Although this has been shown to reduce alpha-synuclein accumulation and nitric oxide production in animal studies, 32 human studies regarding lovastatin and PD risk generated inconsistent results.18, 21
Another limitation is that we cannot exclude a possibility of residual confounding, because of the observational study design. Among participants in these cohorts, we have previously reported a significant association between ibuprofen use and lower PD risk. 33 Further, indication bias cannot be excluded because elevated cholesterol has been found to be associated with lower PD risk in some, but not all, previous prospective studies, as reviewed elsewhere.34
It is important to note that statins may have unfavorable effects on central nervous system. Because HMG-CoA involves the synthesis of coenzyme Q10, inhibition of this enzyme by statins leads to reduction of serum coenzyme Q10 concentrations, 5, 6, 35 which is an essential carrier in the mitochondrial respiratory chain and is under clinical investigation for the treatment of PD. Further, as discussed above, certain statins could lower plasma urate concentrations.26 A case report found that use of lovastatin was associated with onset of PD symptom. 36 However, this has not been confirmed by large scaled prospective studies.
In summary, we observed an association between regular use of statins and lower risk of developing PD, particularly among younger participants. However, our results should be interpreted with caution because only approximately ~ 70% of cholesterol lowering drug users at baseline were actual statin users . Further the results were only marginally significant and could be due to chance. In contrast with use of ibuprofen, which has been consistently found to be inversely associated with PD risk in these cohorts (pooled RR = 0.62; 95% CI: 0.42-0.93 ) as well as in other longitudinal studies33, the overall epidemiological evidence relating statin use to PD risk remains unconvincing. Given the potential adverse effects of statins, further prospective observational studies are needed to explore the potential effects of different subtype of statins on risk of PD and other neurodegenerative diseases.
Acknowledgement
The study was supported by NIH/NINDS grant R01 NS048517, NIH R01 NS061858, P01 CA055075, P01 CA87969 and NIH K24NS060991. None of the sponsors participated in the design of this study or in the collection, analysis, or interpretation of the data.
Footnotes
Author Contributions:
Study concept and design: Gao and Ascherio.
Acquisition of data: Gao, Schwarzschild, and Ascherio.
Analysis and interpretation of data: Gao, Simon, Schwarzschild, and Ascherio.
Drafting of the manuscript: Gao.
Critical revision of the manuscript for important intellectual content: Gao, Simon, Schwarzschild, and Ascherio.
Obtained funding: Schwarzschild, and Ascherio.
Study supervision: Ascherio.
Financial Disclosure: Dr Gao has reported consultant relationship with Teva. The other authors report no conflicts of interest.
References
- 1.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008 Sep;42(9):1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 2.Becker C, Meier CR. Statins and the risk of Parkinson disease: an update on the controversy. Expert Opin Drug Saf. 2009 May;8(3):261–271. doi: 10.1517/14740330902859956. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Yan J, Chen X, et al. Statins: Multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol. Apr 18; doi: 10.1016/j.expneurol.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Wood WG, Eckert GP, Igbavboa U, Muller WE. Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci. Jun;1199:69–76. doi: 10.1111/j.1749-6632.2009.05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med. 1997;18(Suppl):S137–144. doi: 10.1016/s0098-2997(97)00014-9. [DOI] [PubMed] [Google Scholar]
- 6.De Pinieux G, Chariot P, Ammi-Said M, et al. Lipid-lowering drugs and mitochondrial function: effects of HMG-CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br J Clin Pharmacol. 1996 Sep;42(3):333–337. doi: 10.1046/j.1365-2125.1996.04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002 Oct;59(10):1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 8.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. Bmj. 340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritz B, Manthripragada AD, Qian L, et al. Statin use and Parkinson's disease in Denmark. Mov Disord. Jul 15;25(9):1210–1216. doi: 10.1002/mds.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel D, Lopez J, Meier J. Use of Cholesterol-lowering Medications in the United States from 1991 to 1997. The American Journal of Medicine. 2000;108:496–499. doi: 10.1016/s0002-9343(00)00319-3. [DOI] [PubMed] [Google Scholar]
- 11.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993 Jul;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 12.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001 Jul;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Genetic determinants of hair color and Parkinson's disease risk. Ann Neurol. 2009 Jan;65(1):76–82. doi: 10.1002/ana.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Family history of melanoma and Parkinson disease risk. Neurology. 2009 Oct 20;73(16):1286–1291. doi: 10.1212/WNL.0b013e3181bd13a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003 Mar-Apr;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Presa MA, Martin-Ventura JL, Ortego M, et al. Atorvastatin reduces the expression of cyclooxygenase-2 in a rabbit model of atherosclerosis and in cultured vascular smooth muscle cells. Atherosclerosis. 2002 Jan;160(1):49–58. doi: 10.1016/s0021-9150(01)00547-0. [DOI] [PubMed] [Google Scholar]
- 17.Zelvyte I, Dominaitiene R, Crisby M, Janciauskiene S. Modulation of inflammatory mediators and PPARgamma and NFkappaB expression by pravastatin in response to lipoproteins in human monocytes in vitro. Pharmacol Res. 2002 Feb;45(2):147–154. doi: 10.1006/phrs.2001.0922. [DOI] [PubMed] [Google Scholar]
- 18.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Statin use and the risk of Parkinson disease. Neurology. 2008 Apr 15;70(16 Pt 2):1418–1422. doi: 10.1212/01.wnl.0000286942.14552.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Chen H, Miller WC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson's disease. Mov Disord. 2007 Feb 15;22(3):377–381. doi: 10.1002/mds.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lau LM, Stricker BH, Breteler MM. Serum cholesterol, use of lipid-lowering drugs, and risk of Parkinson disease. Mov Disord. 2007 Oct 15;22(13):1985. doi: 10.1002/mds.21582. [DOI] [PubMed] [Google Scholar]
- 21.Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. Bmj. 2011;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritz B, Manthripragada AD, Qian L, et al. Statin use and Parkinson's disease in Denmark. Mov Disord. 2011 Jul 15;25(9):1210–1216. doi: 10.1002/mds.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samii A, Carleton BC, Etminan M. Statin use and the risk of Parkinson disease: a nested case control study. J Clin Neurosci. 2008 Nov;15(11):1272–1273. doi: 10.1016/j.jocn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Becker C, Jick SS, Meier CR. Use of statins and the risk of Parkinson's disease: a retrospective case-control study in the UK. Drug Saf. 2008;31(5):399–407. doi: 10.2165/00002018-200831050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Ogata N, Fujimori S, Oka Y, Kaneko K. Effects of three strong statins (atorvastatin, pitavastatin, and rosuvastatin) on serum uric acid levels in dyslipidemic patients. Nucleosides Nucleotides Nucleic Acids. 2011 Jun;29(4-6):321–324. doi: 10.1080/15257771003741323. [DOI] [PubMed] [Google Scholar]
- 27.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007 Sep 1;166(5):561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol. 2008 Apr 1;167(7):831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009 Dec;66(12):1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008 Jun;65(6):716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The National Institute for Health Care Management Research and Educational Foundation [Nov 8, 2010];Prescription drug expenditures in 2000. 2010 http://www.nihcm.org/pdf/spending2000.pdf.
- 32.Koob AO, Ubhi K, Paulsson JF, et al. Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Exp Neurol. 2011 Feb;221(2):267–274. doi: 10.1016/j.expneurol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011 Mar 8;76(10):863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu G. Total cholesterol and the risk of Parkinson's disease: a review for some new findings. Parkinsons Dis. 2010;2010:836962. doi: 10.4061/2010/836962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Human JA, Ubbink JB, Jerling JJ, et al. The effect of Simvastatin on the plasma antioxidant concentrations in patients with hypercholesterolaemia. Clin Chim Acta. 1997 Jul 4;263(1):67–77. doi: 10.1016/s0009-8981(97)06557-1. [DOI] [PubMed] [Google Scholar]
- 36.Muller T, Kuhn W, Pohlau D, Przuntek H. Parkinsonism unmasked by lovastatin. Ann Neurol. 1995 May;37(5):685–686. doi: 10.1002/ana.410370527. [DOI] [PubMed] [Google Scholar]