Abstract
Background
Different population studies have reported gender difference in carotid intima-media thickness (CIMT), which is proved to be a risk factor of cardiovascular diseases. However, few longitudinal researches examine this gender difference in type 2 diabetes mellitus (T2DM) patients. Therefore, we prospectively analyzed CIMT in T2DM patients over a 4-year follow-up period.
Methods
355 T2DM patients (mean age 59 years; 54.9% women) were included in the present study. CIMT were measured using Color Doppler ultrasound. CIMT was measured at baseline (CIMT) in 2006 and at follow-up in 2010. Biochemical and clinical measurements were collected at baseline.
Results
Mean value of CIMT1 and CIMT2 were 0.740 ± 0.148 mm and 0.842 ± 0.179 mm, respectively. Men had higher CIMT than women both at baseline and at follow-up (CIMT1: 0.762 ± 0.149 vs 0.723 ± 0.146 mm, P = 0.0149; CIMT2: 0.880 ± 0.189 vs 0.810 ± 0.164 mm, P = 0.0002). Mean annual progression of CIMT (dCIMT) was 0.025 ± 0.022 mm/year. dCIMT was larger in men than in women (0.030 ± 0.025 vs 0.022 ± 0.019 mm, P = 0.0006). In multiple regression analyses, age was an independent risk factor of CIMT in both genders, while dCIMT was associated with age only in men.
Conclusions
Gender difference in CIMT was confirmed in T2DM patients. Moreover, impact of ageing on CIMT progression only existed in men, which might be the reason that gender difference in CIMT increased with age.
Keywords: Type 2 diabetes mellitus, Intima-media thickness, Gender difference
Background
Patients with type 2 diabetes mellitus (T2DM) have 2–6 fold higher risk of cardiovascular disease (CVD) than non-diabetics [1], which accounts for nearly 50% diabetes-related mortality [2]. Carotid intima-media thickness (CIMT) measured by ultrasound has been proved to be an independent risk factor of CVD [3,4]. It is suitable for large population study, because this measurement is non-invasive, low-cost and convenient. Moreover, CIMT has been used in clinical trials to evaluate the efficacy of anti-diabetic, anti-hyperlipidemia and anti-hypertensive treatments [5-8].
Cross-sectional population studies have demonstrated that men usually have higher CIMT than women of similar age [9-11]. However, gender difference in CIMT has been seldom studied in longitudinal studies, especially in T2DM patients [12-17].
Therefore, we prospectively analyzed CIMT in T2DM patients over a 4-year follow-up period in aim to investigate gender difference in CIMT and to track CIMT progression.
Methods
Subjects
The Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine approved the study protocol. From October 2006 to February 2007, 901 T2DM patients (diagnosed according to the 1999 World Health Organization criteria [18]) were recruited via the outpatient clinic of the Department of Endocrine and Metabolism at Ruijin Hospital, Shanghai. All study participants gave written informed consent. Among them, 501 patients underwent carotid ultrasonographic examination both at baseline (2006) and at follow-up (2010). Among them, 68 patients were excluded because of missing clinical information and/or biochemical indices. Both female and male patients were divided into three groups according to their baseline age: ≤55 years, 56–64 years and ≥65 years (at follow-up: ≤59 years, 60–68 years and ≥69 years). We excluded 78 patients in order to make other potential risk factors of CIMT (including age, body mass index [BMI], systolic and diastolic blood pressure, serum concentrations of high density lipoprotein [HDL] and low density lipoprotein [LDL] cholesterol, total cholesterol, triglycerides, plasma glycosylated hemoglobin A1c [HbA1c], smoking and drinking alcohol) comparable between men and women in each age group. Thus, the present analysis included 355 subjects.
Clinical and biochemical assessments
Blood pressure was measured using a standard mercury sphygmomanometer in the sitting position after the patients had rested for at least 10 min. Body mass index was calculated as weight in kilograms divided by height in meter squared. A standardized questionnaire was used to collect information on medical history including the duration of type 2 diabetes mellitus and lifestyle.
Blood samples were obtained after an overnight fasting. Plasma HbA1c was measured by high-performance liquid chromatography (BRO-RAD Company, USA). Serum concentrations of total cholesterol and triglycerides were measured by the enzymatic method, and high density lipoprotein (HDL) cholesterol was measured using a specific precipitation method (Beckman LX-20, Brea, CA, USA). Low density lipoprotein (LDL) cholesterol was calculated using the Friedewald’s formula [19].
Cardiovascular disease was defined as angina, myocardial infarction or revascularization. Microvascular disease was defined as diabetic retinopathy, nephropathy or neuropathy.
CIMT measurement
In 2006 and 2010, the same trained sonographers, who were unaware of the clinical and biochemical information of the subjects, performed CIMT measurements manually using a high-resolution B-mode tomographic ultrasound system (Esaote DU3 and Mylab90, Italy) with a linear 7.5-10 MHz transducer (Figure 1). Precision of the CIMT measurement is 0.01 mm. The sonographers measured CIMT on the fall wall of the right and left common carotid arteries, 1.5 cm proximal to the bifurcation. The transducer was manipulated so that the lumen diameter was maximized in the longitudinal plane. The first and second lines represent the lumen–intimal interface and the collage-contained upper layer of tunic adventitia, respectively [20]. The mean value of the right and left common carotid IMT was used for analysis. The baseline and follow-up CIMT were recorded as CIMT1 and CIMT2, respectively. Progression of CIMT was calculated as the difference between CIMT1 and CIMT2 divided by 4 years and recorded as dCIMT. We performed reproducibility study in 20 subjects. The coefficient of variations were less than 5.1%.
Figure 1.
B-mode tomographic ultrasound figures at CIMT1 and CIMT2. The distance between two cross marks: carotid intima-media thickness (CIMT). (A: using Esaote DU3, Italy in 2006; B: using Esaote Mylab90, Italy in 2010).
Statistical analysis
For database management and statistical analysis, we used SPSS version 13.0. Variables with a skewed distribution including systolic and diastolic blood pressure, HbA1c, TG, and HDL cholesterol were normalized by logarithmic transformation. Comparisons of means and proportions were performed with analyses of variance (ANOVA) and Chi-square test, respectively. Univariate correlations were evaluated by Pearson’s and Spearman’s analyses. We searched for possible covariables of CIMT, using a stepwise regression procedure with the P-values for independent variables to enter and to stay in the model set at 0.05. As covariables, we considered age, BMI, systolic and diastolic blood pressure, HDL and LDL cholesterol, total cholesterol, triglycerides, HbA1c, smoking and alcohol intake.
Results
Characteristics of the patients
355 subjects were included in the present study (Table 1). Clinical and biochemical variables at baseline were not significantly different between men and women. During a 4-year follow-up, 39 patients (women vs men 26 vs 13, P = 0.13) had cardiovascular events and 115 patients (68 vs 47, P = 0.30) developed microvascular diseases.
Table 1.
Characteristics of patients at baseline
| Characteristics | Men | Women | P |
|---|---|---|---|
| Number |
160 |
195 |
|
| Age (years) |
59.1 ± 9.5 |
58.5 ± 7.9 |
0.47 |
| Duration (years) |
7.0 ± 5.9 |
7.8 ± 5.7 |
0.18 |
| Body mass index (kg/m2) |
24.4 ± 2.8 |
24.3 ± 3.4 |
0.73 |
| Systolic blood pressure (mmHg) |
122.2 ± 10.8 |
124.6 ± 14.6 |
0.10 |
| Diastolic blood pressure (mmHg) |
78.2 ± 7.4 |
77.2 ± 8.6 |
0.25 |
| HbA1c (%) |
6.7 ± 1.1 |
6.7 ± 1.2 |
0.99 |
| Total cholesterol (mmol/L) |
5.0 ± 0.9 |
5.1 ± 0.8 |
0.09 |
| Triglycerides (mmol/L) |
1.4 ± 1.0 |
1.5 ± 0.8 |
0.40 |
| HDL cholesterol (mmol/L) |
1.5 ± 0.3 |
1.5 ± 0.2 |
0.05 |
| LDL cholesterol (mmol/L) |
3.0 ± 0.7 |
3.1 ± 0.6 |
0.23 |
| Smoking (%) |
17.2 |
18.5 |
0.76 |
| Alcohol intake (%) |
10.2 |
7.7 |
0.44 |
| History of hypertension (%) |
51.9 |
53.8 |
0.71 |
| History of hyperlipidemia (%) |
40.6 |
43.1 |
0.64 |
| Anti-diabetic treatment (%) |
81.9 |
83.6 |
0.67 |
| Anti-hyperlipidemia treatment (%) |
13.8 |
20.0 |
0.12 |
| Anti-hypertensive treatment (%) |
51.3 |
51.3 |
0.99 |
| Cardiovascular disease (%) |
8.1 |
13.3 |
0.13 |
| Microvascular disease (%) | 29.4 | 34.9 | 0.31 |
Abbreviations: HDL high density lipoprotein, LDL low density lipoprotein.
Carotid intima-media thickness
CIMT
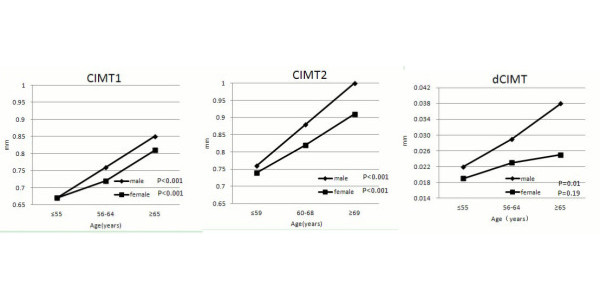
CIMT2 were higher than CIMT1 both in women and in men (Table 2). At baseline and follow-up, men had larger CIMT than women. Figure 2 showed CIMT by gender and age groups. Both female and male patients were divided into three groups according to their baseline age: ≤55 years (follow-up: ≤59 years) (men 46, women 66), 56–64 years (60–68 years) (men 66, women 87) and ≥65 years (≥69 years) (men 48, women 42). In each age group, clinical and biochemical measurements did not have significant differences between women and men. CIMT1 and CIMT2 increased across the age groups in both genders (P < 0.001). Men had higher CIMT2 than women in the group with age ≥69 years at follow-up (P < 0.05, Figure 2). After adjustment for other covariables, age was associated with CIMT1 in both genders, while BMI was associated with CIMT1 in women (Table 3).
Table 2.
Carotid intima-media thickness at baseline and follow-up
| Characteristics | Men | Women | P |
|---|---|---|---|
| CIMT1 (mm) |
0.762 ± 0.149 |
0.723 ± 0.146 |
0.0149 |
| CIMT2 (mm) |
0.880 ± 0.189 |
0.810 ± 0.164 |
0.0002 |
| dCIMT (mm/year) | 0.030 ± 0.025 | 0.022 ± 0.019 | 0.0006 |
Abbreviations: CIMT1 Carotid intima-media thickness at baseline, CIMT2 carotid intima-media thickness at follow-up, dCIMT progression in carotid intima-media thickness, it was calculated as (CIMT2-CIMT1)/4 years.
Figure 2.
Carotid intima media-thickness at baseline (CIMT1) and follow-up (CIMT2), and carotid intima-media thickness progression (dCIMT) by sex and age groups.
Table 3.
Correlates of carotid intima-media thickness
| |
CIMT1 |
dCIMT |
||
|---|---|---|---|---|
| Standardized coefficient Beta | P | Standardized coefficient Beta | P | |
| Women |
|
|
|
|
| Age (years) |
0.373 |
<0.001 |
|
|
| Body mass index (kg/m2) |
0.192 |
0.008 |
|
|
| Men |
|
|
|
|
| Age (years) | 0.508 | <0.001 | 0.194 | 0.031 |
The covariables considered for entry into the multiple regression model were age, body mass index, systolic and diastolic blood pressure, high density and low density lipoprotein cholesterol, total cholesterol, triglycerides, smoking and drinking.
dCIMT
Compared with men, women had lower dCIMT (Table 2). In the same age group, dCIMT was higher in men than in women, but significant difference only existed in the group with age ≥65 years (P < 0.05, Figure 2). The association between age and dCIMT was observed only in men (P = 0.01, Figure 2). After adjustment for other covariables, this association remained significant (P = 0.031, Table 3).
Discussion
The present study found that T2DM men had higher CIMT than T2DM women, especially in those with age ≥ 69 years. Age was the main risk factors of CIMT in both genders. However, the impact of ageing on CIMT progression only existed in male patients.
The gender difference in CIMT in T2DM patients was in line with other population researches. In a study of healthy Taiwan population, CIMT was significantly higher in men than in women (0.558 vs 0.527 mm, P = 0.012) [21]. Kablak-Ziembicka et al confirmed this result in subjects without CVD (men vs women 1.05 vs 0.93 mm, P < 0.001) [22]. CIMT in our study was higher than that in Taiwan healthy population, while was lower than that in population without CVD [22] or with normal glucose tolerance [23]. This might be due to the differences in age, ethnicity and physical condition of study populations.
We found that gender difference in CIMT only existed in the group with age ≥ 69 years. This finding was consistent with the Tromsø study [24], which found that gender difference in CIMT was not significant in young population and gradually increased with age [24]. Dalla Pozza et al reported that there CIMT was not different between male and female children with type 1 diabetes mellitus [25]. However, contrary results were found in subjects without CVD risk factors studied by Sinning et al [26]. In their study, gender difference in CIMT only exists in young population (mean age: 35 years) but not in elderly population (age range: 55–72 years) [26]. The possible reason might be that the estrogen, which suppressed the progression of arteriosclerosis, decreased in postmenopausal women. Thus, gender difference in CIMT was weakened in elderly population as observed by Sinning et al. However, our subjects were patients with diabetics, which is an important and independent risk factor of CVD. Hayashi et al reported gender difference in CVD risk factors [27], which may cause gender difference in CIMT. Moreover, men are more susceptible to CVD risk factor than women [28-30]. Therefore, gender difference in CIMT was significant in our study due to diabetic status.
Another main finding of our study was that CIMT progression was faster in men than women, which was in keeping with other studies [24,31]. Stensland-Bugge et al reported that the average progression per year of CIMT was greater in men than in women (0.010 mm versus 0.009 mm, P < 0.01) [24]. In the present study, these value were 0.030 mm for men and 0.022 mm for women (P < 0.01). The progression of CIMT in our study was higher than those reported from healthy population (0.007 mm/year in Germen, 0.008 mm/year in Japanese) [32,33] and similar to those reported from subjects with impaired glucose tolerance [34] and T2DM [35]. In the current study, after adjustment for other covariables, age was an independent risk factors of CIMT progression only in men. This might be the reason that men had higher CIMT than women and this gender difference was more significant in patients with old age and more significant after a 4-year follow-up.
The present study must be interpreted within the context of its limitations. First, we did not find gender difference in the incidence of cardiovascular diseases or microvascular diseases after a 4-year follow-up, although men had higher CIMT than women at baseline. This might be due to the small sample size and the relatively short follow-up period. Second, we did not categorize anti-diabetic treatment, which might have effect on the results.
Conclusion
Our study showed that age was the most important and independent risk factor of CIMT in diabetics. However, impact of ageing on CIMT progression only existed in T2DM men, which might lead to the phenomenon that gender difference in CIMT increased with age.
Abbreviations
CIMT: Carotid intima-media thickness; dCIMT: Progression of carotid intima-media thickness; T2DM: Type 2 diabetes mellitus; CVD: Cardiovascular disease; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TG: Triglycerides; TC: Total cholesterol; HDL: High density lipoprotein; LDL: Low density lipoprotein; HbA1C: Glycosylated hemoglobin A1C; BMI: Body mass index.
Competing interests
The authors declared that they have no conflicts of interests.
Authors’ contributions
BZ and WZ participated in the design of the study. BZ and YL drafted the manuscript. BZ and YZ analysed the data. BZ, WZ, YZ, YC, ZY, and YZ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Bo Zhao, Email: zhaobolcyx@163.com.
Yanping Liu, Email: maoweitaoliu@126.com.
Yifei Zhang, Email: feifei-a@163.com.
Yuhong Chen, Email: chenyh70@yahoo.com.cn.
Zhifang Yang, Email: fangni0514@163.com.
Ying Zhu, Email: zhuying_rj@126.com.
Weiwei Zhan, Email: shanghairuijin@126.com.
Acknowledgments
The present study would not have been possible without the participation of the patients. We are sincerely indebted to Shanghai Clinical Center for Endocrine and Metabolic Diseases for providing clinical and laboratory results of the study participants.
References
- Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.1979.03290450033020. [DOI] [PubMed] [Google Scholar]
- Sicree R, Shaw J, Zimmet P. In: Diabetes atlas. 3. Gan D, editor. International Diabetes Federation, Belgium; 2006. Diabetes and impaired glucose tolerance; pp. 15–103. [Google Scholar]
- O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- Nomura K, Hamamoto Y, Takahara S, Kikuchi O, Honjo S, Ikeda H, Wada Y, Nabe K, Okumra R, Koshiyama H. Relationship between carotid intima-media thickness and silent cerebral infarction in Japanese subjects with type 2 diabetes. Diabetes Care. 2010;33:168–170. doi: 10.2337/dc09-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo S, Visconti CL, Amoroso GR. et al. Asymptomatic carotid lesions add to cardiovascular risk prediction. Eur J Cardiovasc Prev Rehabil. 2010;17(5):514–518. doi: 10.1097/HJR.0b013e328337ccbd. [DOI] [PubMed] [Google Scholar]
- Costanzo P, Perrone-Filardi P, Vassallo E. et al. Does carotid intima-media thickness regression predict reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J Am Coll Cardiol. 2010;56(24):2006–2020. doi: 10.1016/j.jacc.2010.05.059. [DOI] [PubMed] [Google Scholar]
- Polak JF, Backlund JY, Cleary PA. et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60(2):607–613. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu M, Ishii H, Watarai M. et al. Impact of acarbose on carotid intima-media thickness in patients with newly diagnosed impaired glucose tolerance or mild type 2 diabetes mellitus: A one-year, prospective, randomized, open-label, parallel-group study in Japanese adults with established coronary artery disease. Clin Ther. 2010;32(9):1610–1617. doi: 10.1016/j.clinthera.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.STR.30.4.841. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lin CH, Lu CJ, Yip PK, Chen RC. Carotid atherosclerosis, intima media thickness and risk factors–an analysis of 1781 asymptomatic subjects in Taiwan. Atherosclerosis. 2002;164:89–94. doi: 10.1016/S0021-9150(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.CIR.96.5.1432. [DOI] [PubMed] [Google Scholar]
- Takato T, Yamada N, Ashida T. Effects of ageing and sex on progression of carotid intima-media thickness: a retrospective 6-year follow-up study. Geriatr Gerontol Int. 2008;8:172–179. doi: 10.1111/j.1447-0594.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- vail der Meer IM, Iglesias del Sol A, Hak AE, Bets ML, Hofinan A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the medal tree: the Rotterdam Study. Stroke. 2003;34:2374–2379. doi: 10.1161/01.STR.0000088643.07108.19. [DOI] [PubMed] [Google Scholar]
- Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: The Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195:e191–e196. doi: 10.1016/j.atherosclerosis.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Sander K, Horn CS, Briesenick C, Sander D. INVADE Study Group; INVADE Study Group. High-sensitivity C-reactive protein is independently associated with early carotid artery progression in women but not in men: the INVADE Study. Stroke. 2007;38:2881–2886. doi: 10.1161/STROKEAHA.106.481531. [DOI] [PubMed] [Google Scholar]
- Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, Szklo M, Howard G, Evans GW. Risk Factors for Progression of Common Carotid Atherosclerosis: The Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Chambless L, Jacobs DR, Nieto FJ, Szklo M. Socioeconomic Differences in Progression of Carotid Intima-Media Thickness in the Atherosclerosis Risk in Communities Study. Arterioseler Thromb Vasc Biol. 2006;26:411–416. doi: 10.1161/01.ATV.0000198245.16342.3d. [DOI] [PubMed] [Google Scholar]
- Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. The American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 1997;23:1108–1112. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Liu YP, Zhan WW, Zhang YF, Chen YH, Lin YY, Zhu Y, Ren XP, Li XY, Ning G. Carotid intima-media thickness and stiffness in relation to type 2 diabetes in Chinese. Endocrine. 2007;31:289–293. doi: 10.1007/s12020-007-0035-6. [DOI] [PubMed] [Google Scholar]
- Tan TY, Lu CH, Lin TK, Liou CW, Chuang YC, Schminke U. Factors associated with gender difference in the intima-media thickness of the common carotid artery. Clin Radiol. 2009;64:1097–1103. doi: 10.1016/j.crad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Kablak-Ziembicka A, Przewlocki T, Tracz W, Pieniazek P, Musialek P, Sokolowski A. Gender differences in carotid intima-media thickness in patients with suspected coronary artery disease. Am J Cardiol. 2005;96:1217–1222. doi: 10.1016/j.amjcard.2005.06.059. [DOI] [PubMed] [Google Scholar]
- Yoo HJ. et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol. 2011;10:103. doi: 10.1186/1475-2840-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensland-Bugge E, Bønaa KH, Joakimsen O. Age and sex differences in the relationship between inherited and lifestyle risk factors and subclinical carotid atherosclerosis: the Tromsø study. Atherosclerosis. 2001;154:437–448. doi: 10.1016/S0021-9150(00)00486-X. [DOI] [PubMed] [Google Scholar]
- Dalla Pozza R. et al. The effect of cardiovascular risk factors on the longitudinal evolution of the carotid intima medial thickness in children with type 1 diabetes mellitus. Cardiovasc Diabetol. 2011;10:53. doi: 10.1186/1475-2840-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinning C, Wild PS, Echevarria FM, Wilde S, Schnabel R, Lubos E, Herkenhoff S, Bickel C, Klimpe S, Gori T, Münzel TF, Blankenberg S, Espinola-Klein C. Sex differences in early carotid atherosclerosis (from the community-based Gutenberg-Heart Study) Am J Cardiol. 2011;107:1841–1847. doi: 10.1016/j.amjcard.2011.02.318. [DOI] [PubMed] [Google Scholar]
- Hayashi T. et al. Age, gender, insulin and blood glucose control status alter the risk of ischemic heart disease and stroke among elderly diabetic patients. Cardiovasc Diabetol. 2011;10:86. doi: 10.1186/1475-2840-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- Bhuiyan AR, Srinivasan SR, Chen W, Paul TK, Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189:1–7. doi: 10.1016/j.atherosclerosis.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:72. doi: 10.1186/1475-2840-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Burke GL, Evans GW, Crouse JR, Riley W, Arnett D, de Lacy R, Heiss G. Relations of intimal-medial thickness among sites within the carotid artery as evaluated by B-mode ultrasound. ARIC Investigators. Atherosclerosis Risk in Communities. Stroke. 1994;25:1581–1587. doi: 10.1161/01.STR.25.8.1581. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Kraft K, Rucker W, Huther AM. Diagnosis of very early arteriosclerotic vascular wall changes using duplex sonography. Klin Wochenschr. 1989;67:442–446. doi: 10.1007/BF01725140. [DOI] [PubMed] [Google Scholar]
- Handa N, Matsumoto M, Maeda H, Hougaku H, Ogawa S, Fukunaga R, Yoneda S, Kimura K, Kamada T. Ultrasonic evaluation of early carotid atherosclerosis. Stroke. 1990;21:1567–1572. doi: 10.1161/01.STR.21.11.1567. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose Slows Progression of Intima-Media Thickness of the Carotid Arteries in Subjects With Impaired Glucose Tolerance. Stroke. 2004;35:1073–1078. doi: 10.1161/01.STR.0000125864.01546.f2. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Katakami N, Yamasaki Y. Recent Advances of Intervention to Inhibit Progression of Carotid Intima-Media Thickness in Patients With Type 2 Diabetes Mellitus. Stroke. 2006;37:2420–2427. doi: 10.1161/01.STR.0000236632.58323.cd. [DOI] [PubMed] [Google Scholar]