Abstract
The treatment of metastatic colorectal cancer (mCRC) remains one of the largest hurdles in cancer therapeutics to date. The most advanced treatment option for mCRC patients are anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) that bind to and inhibit the activity of EGFR. While the use of anti-EGFR mABs has had great impact in the treatment of mCRC, it has now been widely accepted that mCRC tumors with a mutation in the small GTPase KRAS do not respond to these therapies. KRAS mutations allow for EGFR independent activation of various oncogenic signaling cascades. In attempts to inhibit KRAS mutant tumor growth, BRAF, MEK and farsenyltransferase inhibitors have been used, however, their clinical efficacy is still accruing in the setting of CRC. Recent data suggests that various other inhibitors, including inhibitors of Src family kinases (SFK) and hepatocyte growth factor receptor (MET), may have potential preclinical and clinical success in KRAS mutant tumors. Additionally, it is becoming increasingly clear that different KRAS missense mutations may have varied biological responses to cetuximab, suggesting that cetuximab may still be a potential therapeutic option in some KRAS mutant tumors. In this review, we highlight the importance for both improved multimodality approaches for treating KRAS mutant mCRC tumors and stratification of KRAS mutations in response to different treatment regimes in order to optimize the best possible care for mCRC patients.
Keywords: EGFR, GTPase, KRAS, cetuximab, metastatic colorectal cancer, resistance
Introduction
Of all human cancers, metastatic colorectal cancer (mCRC) remains one of the deadliest in the United States.1 Upon diagnosis with CRC, 40–50% of patients demonstrate secondary metastases with an overall five-year survival period of just 11%.2 With increasing need to treat mCRC patients with new therapeutic regimes, anti-epidermal growth factor receptor (EGFR) therapy, a target that is frequently overexpressed in mCRC tumors, has become a leading treatment. EGFR is a member of the HER family of growth factor receptor tyrosine kinases (RTKs). Stimulation of this receptor by various cognate ligands induces a conformational change in EGFR’s extracellular domain that promotes either homo- or hetero- dimerization with other RTKs.3 Dimerization activates EGFR’s intrinsic kinase activity, leading to the auto-phosphorylation of tyrosine residues on its C-terminal tail. Phospho-tyrosine residues on EGFR serve as docking sites for various adaptor and kinase proteins, many of which are known to stimulate oncogenic signaling cascades resulting in cellular survival, proliferation, migration and angiogenesis.3 To date, inappropriate EGFR activation has been linked to the development, progression and metastatic spread of various cancers.4,5
Due to the high percentage of solid tumors overexpressing the EGFR, the FDA has approved five molecular targeting agents directed to block EGFR function. Of these five drugs, the anti-EGFR monoclonal antibodies (mAbs) cetuximab (ICM-225, Erbitux: ImClone Systems) and panitumumab (Vectibix: Amgen) have been FDA approved for treatment of mCRC. Cetuximab is a chimeric human:murine mAB that blocks EGFR regulated signaling events by binding to EGFR’s ligand binding domain preventing both ligand binding and sterically hindering dimerization with other RTKs.6 Additionally, cetuximab can induce EGFR degradation and antibody dependent cellular cytotoxicity (ADCC).7,8 Panitumumab functions similarly, however, it is a fully humanized mAb and thus may induce less ADCC response.9,10
Initial trials of chemo-refractory and chemo-naïve mCRC patients treated with anti-EGFR mABs in addition to chemotherapy demonstrated a 10–30% response rate with a 0.9-mo increase in progression free survival time.11,12 Additionally, treatment with anti-EGFR mABs in the first line setting has demonstrated increased response rates, and progression free survival times over chemotherapy alone.13 Various studies have also demonstrated that tumors with a lack of significant EGFR expression (quantified via immunohistochemistry, IHC) may still respond to anti-EGFR mAbs.14 Thus, predicting subsets of patients that will respond positively to anti-EGFR mAbs based on EGFR expression levels has been challenging.
The RAS Family of Small Protein GTPases
One of the most powerful predictive markers for resistance to anti-EGFR mABs are mutations in the KRAS gene.15 KRAS is a small protein GTPase that is part of a superfamily of small GTPases that contains over 154 members, all of which have been organized into five subfamilies based on their DNA sequence and function.16 The five subfamilies are: Ras, Rho, Rab, Arf and Ran. KRAS is a member of the Ras subfamily that consists of four 21 kD proteins that differ in sequence at their c-terminus: HRAS, NRAS, KRAS4A and KRAS4B. KRAS4A and KRAS4B are different splice variants produced by alternative splicing at the c-terminus of the KRAS gene; KRAS4B is the most common splice variant and is denoted in most literature as KRAS.16 All Ras proteins are activated when bound to guanosine triphosphate (GTP), a reaction that is increased by guanine nucleotide exchange factors (GEFs) that serve to open up the GTP binding site.17 When bound to GTP, Ras proteins have increased affinity for specific downstream effector molecules, many of which are kinases that initiate various intracellular signaling cascades. Ras proteins are subsequently deactivated through the use of their intrinsic GTPase activity, which hydrolyzes GTP.17 GTPase activating proteins (GAPs) are essential for this hydrolysis process to be complete due to their ability to stabilize the high-energy transition state of this reaction.17
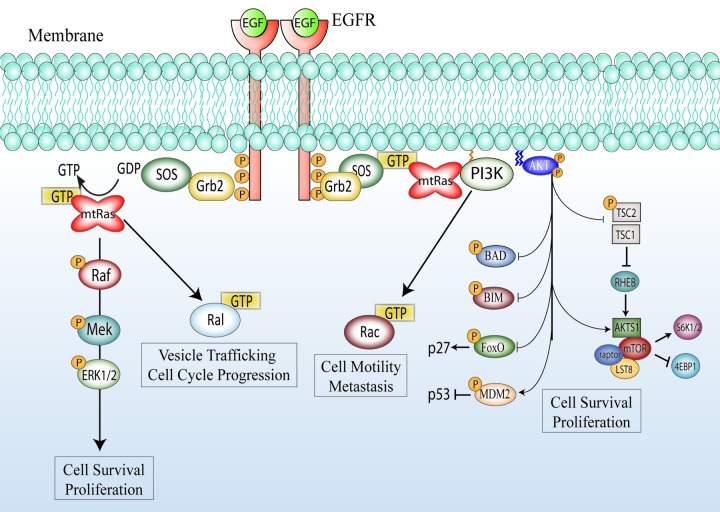
KRAS functions downstream of the EGFR and serves to activate critical oncogenic signaling cascades. Upon activation of EGFR, adaptor proteins such as SH-2 containing protein (SHC) and growth-factor-receptor bound protein 2 (GRB2) recruit specific GEFs like son of sevenless homolog 1 (SOS1) to the cell membrane.4,16 KRAS is intrinsically targeted to the cell membrane through farnesylation and geranylgeranylation of its c-terminal tail.16 Upon association with SOS1, KRAS becomes activated and serves to further activate both phosphatidylinositol 3-kinase (PI3K) and RAF kinase, resulting in signaling through the PI3-K/AKT and mitogen-activated protein kinase (MAPK, also known as ERK) pathways.4,16 Additionally, KRAS-GTP activates exchange factors for the small GTPases Rac and Ral leading to the modulation of cytoskeletal dynamics and vesicular trafficking.16,18 Thus, KRAS is a critical mediator of EGFR induced signaling cascades (Fig. 1).
Figure 1.
KRAS mutation and activation of down stream signaling cascades. In the KRAS wild-type setting EGFR activation recruits SOS to its C-terminal tail via the adaptor protein Grb2.4 SOS is a GEF that can activate KRAS by promoting the exchange of GDP for GTP.16 Activation of KRAS leads to a conformational change in RAF kinase, which will ultimately lead to the activation and nuclear transport of MAPK. Activated KRAS can also directly recruit PI3K to the cell surface, leading to the activation of the serine/threonine kinase AKT, resulting in the further activation of MTOR and inhibition of various pro-apoptotic signals.4 Additionally, KRAS can recruit various GEF proteins responsible for the activation of the small GTPases RAL and RAC.16 Through the activation of these various intracellular proteins, KRAS serves to initiate a broad oncogenic expression program that results in cellular proliferation, survival, and migration.4 In the KRAS mutant setting (mtRAS), KRAS is constitutively bound to GTP, and cannot hydrolyze GTP due to mutations in the GAP binding domain. In this setting, the signals emanating from KRAS are hyper-activated, and EGFR has lost its ability to control KRAS activation deeming EGFR inhibitors ineffective.
KRAS Mutations and Clinical Outcome
KRAS mutation has been documented in 35–40% of colorectal tumors, while NRAS and HRAS mutations are less common in this cancer (1–3%).15,16,19 The most common mutations found in KRAS are missense mutations leading to amino acid substitutions at codons 12, and 13 of exon 2, with mutation at codon 12 being most prevalent and tumorigenic in colon cancer.15,20 The most common amino acid substitution at both codon 12 and 13 is a glycine to an aspartate residue.15,21 Additionally, mutations in codons 61, 146 and 154 have been documented but are rare.15 All of these mutations promote the oncogenic potential of KRAS by (1) disabling the intrinsic GTPase activity of KRAS, and (2) preventing GAPs from associating with KRAS.15 Thus, mutant KRAS cannot hydrolyze GTP to GDP, and therefore cannot be shut down readily leading to EGFR independent increases in the activation of both PI3K/AKT and MAPK pathways (see Fig. 1). Additionally, there has been a high prevalence of activating mutations in the BRAF gene (~15% of mCRC patients) and PIK3CA p110 PI3K subunit (~13%), along with loss of the PTEN phosphatase (~20%) in colorectal tumors.2 BRAF is a serine threonine kinase that is directly activated by KRAS. Mutations in BRAF are considered mutually exclusive to those of KRAS mutations since they both constitutively activate the MAPK pathway.2
Due to the EGFR independent activation of KRAS upon its mutation, it is not surprising that anti-EGFR mAbs have provided little clinical benefit in this setting. In over 10 clinical studies, mCRC patients treated with anti-EGFR antibodies responded better if they harbored a wild type KRAS allele.15 Mutant KRAS patients treated with anti-EGFR antibody therapy had little to no response (< 10%), and one study reported detrimental effects to some patients.15,22 Thus, in January of 2009, the American Society for Clinical Oncology (ASCO) published a series of guidelines that strongly suggested that anti-EGFR antibody therapy be used only in the setting of wild-type KRAS.23
Targeting KRAS Mutant Tumors: Past and Present
KRAS mutant mCRC patients have little option post chemotherapy failure. To better combat KRAS mutant tumors, researchers have tried to inhibit alternative downstream kinases. BRAF inhibitors have been in clinical trials since 2004. Initial studies with the partial RAF inhibitor sorafenib (nexavar) and more selective RAF inhibitor PLX4032 (vemurafenib) demonstrated little advantage in open trials of mCRC patients.24,25 Soon after, it was shown that antitumor effects were seen in a select group of mCRC patients with a V600E mutation in the BRAF gene.26,27 Hatzivassiliou et al. further demonstrated that the tumorigenicity of BRAF wild type, KRAS wild type, and KRAS mutant mCRC cell lines can actually be enhanced by BRAF inhibition due to the activation of various oncogenic feedback loops, and thus patients should be highly selected for BRAF mutation V600E in order to receive this treatment.28 Overall, BRAF inhibitors may only be beneficial for BRAF mutant mCRC patients and may explain the low response rates in past trials due to the heterogeneity of the patient population treated.
In addition to BRAF inhibition, MEK 1 and 2 inhibition has also been considered. MEK kinases are activated by BRAF. MEK kinases phosphorylate and activate MAPK. Laboratory research has shown positive outcomes in both KRAS and BRAF mutant cell lines treated with MEK inhibitors. In an in vitro and mouse xenograft model, Solit et al. demonstrated that sensitivity to MEK inhibitors was specific for cell lines with the single BRAF mutation V600E.29 Yoon et al. further showed in a KRAS mutant isogenic mCRC cell and xenograft model that the MEK inhibitors AS703026 and AZD6244 could inhibit tumor cell growth.30 In another preclinical study, the very specific MEK inhibitor CI-1040 (PD 184352) demonstrated a broad range of anti-tumorigenic effects in vitro and in vivo models, especially in the setting of pancreatic cancer.31,32 Currently, various MEK inhibitors are being evaluated for their clinical efficacy. A phase II study by Rinehart et al. demonstrated that the MEK inhibitor CI-1040 was well tolerated by patients, however, had very little antitumor effect in the patients treated.33 A subsequent Phase II clinical trial in mCRC patients showed that the MEK inhibitor AZD6224 (selumetinib) had similar outcomes to treatment with the chemotherapeutic capecitabine, demonstrating possible antitumor effects of this drug, however this still needs to be further validated.34 The potential for using these small molecule MEK inhibitors have also been hindered by studies modeling primary resistance to AZD6244, which lead to amplification of the mutant BRAF600E and KRASG13D genes, leading to increased signaling through the MAPK pathway.35 Overall, it seems that MAPK pathway inhibition in the setting of mutant KRAS mCRC tumors remains elusive.
Other methods to inhibit KRAS activation have been to prevent its association with the plasma membrane where it becomes activated. Farnesyltransferase inhibitors block the ability for Ras proteins to be farnesylated, a posttranslational modification necessary for plasma membrane association. Unfortunately, KRAS can alternatively become geranylgeranylated, which has proven to provide the same function as farnesylation; NRAS cannot be geranylgeranylated and thus tumors harboring NRAS mutations have proven sensitive to these inhibitors.36 Laboratory studies of dual treatment farnesyl- and geranylgeranylase inhibitors in a model of KRAS mutant pancreatic cancer proved successful by promoting a greater level of apoptosis, however geranylgeranylase inhibitors were toxic in mouse models suggesting that it may be inapplicable for human treatment.37 Later, it was reported that these inhibitors induced apoptosis not through inhibition of KRAS activity, but partially through inhibition of RhoB GTPase.36
Currently, there have been efforts to treat mCRC KRAS mutant patients with inhibitors of various other RTKs and kinases. Both insulin like growth factor receptor type 1 (IGF-1R) and the hepatocyte-growth factor receptor (MET) have been considered as potential targets due to their overexpression in mCRC tumors.38,39 The use of these inhibitors in KRAS mutant tumors present similar challenges as EGFR inhibitors because both IGF-1R and MET signal through KRAS. While early clinical trials with anti-IGF-1R mAbs were unsuccessful in mCRC, mABs directed toward c-MET in addition to panitumumab demonstrated potential in the KRAS wild-type setting with a 31% response rate (compared with 21% with panitumumab alone).40 In vitro results have suggested, however, that both IGF-1R and MET inhibitors can overcome resistance to anti-EGFR mAB therapy.41-43 Interestingly, an in vitro study using the BRAF mutant mCRC cell line line (Colo205) demonstrated that the combinatory use of both IGF-1R and MEK inhibitors proved to be effective.44 This combination prevented EGFR and IGF-1R crosstalk, and effectively shut down both the PI3K/AKT and MAPK signaling cascades.
Another potential target in KRAS mutant tumors are the Src family kinases (SFKs), which are overexpressed, overactive and a marker for poor clinical outcome in mCRC.45 Preclinical data has demonstrated that targeting SFKs with the pan SFK inhibitor dasatinib (Sprycel, Bristol-Myers Squibb) concomitantly with cetuximab in KRAS mutant cell lines and xenograft models resulted in growth inhibitory effects. Through the use of a human phospho-kinase array, researchers demonstrated that dual drug treatment lead to a decrease in components of the MAPK and β-catenin pathways and a decrease in expression of various STAT family transcription factors. In xenograft models, dual drug treatment decreased proliferation and enhanced apoptosis, suggesting that dasatinib could sensitize KRAS mutant mCRC tumors to cetuximab.46 Overall, these studies demonstrate the importance for dual kinase inhibition in the setting of mCRC, and also indicate that EGFR may still be a drugable target in the KRAS mutant setting.
Finally, it is becoming increasingly clear that cetuximab may actually increase both overall and progression-free survival in patients that have a mutation in KRAS at codon 13.47 In a study by De Roock et al., a pooled data set of 579 mCRC patients across various clinical trials treated with cetuximab plus/minus chemotherapy demonstrated that overall and progression-free survival was significantly longer in patients with G13D KRAS mutant tumors. Patients with G13D KRAS mutant tumors and treated with cetuximab/chemotherapy regimes had overall survival and progression free survival of average 7.6 and 4.0 mo vs. 5.7 and 1.9 mo in other KRAS mutant tumor subtypes. This year at ASCO, Tejpar et al. further supported this finding by presenting retrospective analyses of two large phase III multicenter trials (CRYSTAL and OPUS) representing 83 patients with G13D KRAS mutant mCRC tumors who had longer overall survival and progression free survival on average post cetuximab treatment then other KRAS mutant subtypes.21 Overall, these data suggest that anti-EGFR mAbs should still be considered as treatment options for patients with a G13D KRAS mutation, and that stratification of different KRAS mutant subtypes should now be documented.
Future Directions
Key questions remain unanswered in the field of mCRC therapy and the role of KRAS in this setting. First and foremost, we must identify the key factors that influence the oncogenicty of KRAS mutant tumors. The apparent lack of response to MAPK pathway inhibitors in mCRC suggests that KRAS signals through multiple oncogenic pathways to influence tumorigenicity, and that MAPK pathway inhibition may not be sufficient to inhibit tumor growth. Thus, it is essential to focus on multiple pathway inhibition, and to continue discovery of other unrelated activated pathways in this setting. Second, we must continue to stratify the important predictive factors for response to different targeted therapies. Identification of mutations in the KRAS and BRAF genes as negative predicative markers for response to anti-EGFR mAbs has made a great impact in the field, and has prevented patients from receiving useless therapy. However, recent data suggests that EGFR may still be a target in this setting. It is becoming more apparent that different missense mutations (codon 12 vs. 13) in the KRAS gene are not equal in their transforming potential. Thus, further preclinical and clinical trials are necessary in order to devise the best treatment options for specific mutant KRAS tumor subtypes. At present, mCRC patients with a mutant KRAS gene are in dire need for better treatment options, and thus both clinicians and research scientists alike must work together to make this a reality.
Acknowledgments
This project was supported in part by grant P30CA014520 from the National Cancer Institute, grant 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources and the National Institutes of Health (D.L.W.), by grant RSG-10-193-01-TBG from the American Cancer Society (D.L.W.), and by NIH grant 1T32GM081061-0IA2 and Molecular Pathogenesis of Human Diseases (T.M.B.).
Glossary
Abbreviations:
- ADCC
antibody dependent cellular cytotoxicity
- ASCO
America Society for Clinical Oncology
- BRAF
v-Raf murine sarcoma viral oncogene homolog B1
- CRC
colorectal cancer
- EGFR
epidermal growth factor receptor
- FDA
Food and Drug Administration
- GAPs
GTPase activating proteins
- GEFs
guanosine nucleotide exchange factors
- GRB2
growth-factor-receptor bound protein 2
- GTP
guanosine triphosphate
- HGFR
hepatocyte growth factor receptor
- IGF-1R
insulin-like growth factor receptor type 1
- IHC
immunohistochemistry
- KRAS
V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- MAPK
mitogen-activated protein kinase
- mABs
monoclonal antibodies
- mCRC
metastatic colon cancer
- MEK
mitogen-activated protein kinase kinase
- MET
hepatocyte growth factor receptor
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homologue deleted in chromosome 10
- RTKs
receptor tyrosine kinases
- SFKs
Src family kinases
- SHC
SH-2 containing protein
- SOS
son of sevenless homolog 1
- STAT
signal transducer and activator of transcription
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/18751
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Di Fiore F, Sesboue R, Michel P, Sabourin JC, Frebourg T. Molecular determinants of anti-EGFR sensitivity and resistance in metastatic colorectal cancer. Br J Cancer. 2010;103:1765–72. doi: 10.1038/sj.bjc.6606008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 4.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–15. doi: 10.1016/S0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98:1275–80. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–61. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 9.Saltz L, Easley C, Kirkpatrick P. Panitumumab. Nat Rev Drug Discov. 2006;5:987–8. doi: 10.1038/nrd2204. [DOI] [PubMed] [Google Scholar]
- 10.Yang XD, Jia XC, Corvalan JRF, Wang P, Davis CG. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17–23. doi: 10.1016/S1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 13.Borner M, Koeberle D, Von Moos R, Saletti P, Rauch D, Hess V, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288–92. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 14.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–10. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–27. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 16.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 17.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–54. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara K, Kishida S, Matsuura Y, Kitayama H, Noda M, Kikuchi A. Plasma membrane recruitment of RalGDS is critical for Ras-dependent Ral activation. Oncogene. 1999;18:1303–12. doi: 10.1038/sj.onc.1202425. [DOI] [PubMed] [Google Scholar]
- 19.Bos JL, Fearon ER, Hamilton SR, Verlaandevries M, Vanboom JH, Vandereb AJ, et al. Prevalence of Ras gene-mutations in human colorectal cancers. Nature. 1987;327:293–7. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 20.Guerrero S, Casanova I, Farre L, Mazo A, Capella G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6. [PubMed] [Google Scholar]
- 21.Tejpar S, Bokemeyer C, Celik I, Schlichting M, Sartorius U, Van Cutsem E. Influence of KRAS G13D mutations on outcome in patients with metastatic colorectal cancer (mCRC) treated with first-line chemotherapy with or without cetuximab. ASCO Annual Meeting. Chicago, Illinois, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 23.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–6. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 24.Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677–85. doi: 10.1158/1535-7163.MCT-04-0297. [DOI] [PubMed] [Google Scholar]
- 25.Sartore-Bianchi A, Bencardino K, Cassingena A, Venturini F, Funaioli C, Cipani T, et al. Therapeutic implications of resistance to molecular therapies in metastatic colorectal cancer. Cancer Treat Rev. 2010;36:S1–5. doi: 10.1016/S0305-7372(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 26.Flaherty K, Puzanov I, Sosman J, Kim K, Ribas A, McArthur G, et al. Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 2009;27(suppl; abstr 9000):15s. [Google Scholar]
- 27.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 29.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon J, Koo KH, Choi KY. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res. 2011;71:445–53. doi: 10.1158/0008-5472.CAN-10-3058. [DOI] [PubMed] [Google Scholar]
- 31.Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–6. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 32.Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Semin Oncol. 2003;30:105–16. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Rinehart J, Adjei AA, LoRusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–62. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 34.Bennouna J, Lang I, Valladares-Ayerbes M, Boer K, Adenis A, Escudero P, et al. A phase II, open-label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. 2011;29:1021–8. doi: 10.1007/s10637-010-9392-8. [DOI] [PubMed] [Google Scholar]
- 35.Little AS, Balmanno K, Sale MJ, Newman S, Dry JR, Hampson M, et al. Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal. 2011;4:ra17. doi: 10.1126/scisignal.2001752. [DOI] [PubMed] [Google Scholar]
- 36.Brunner TB, Hahn SM, Gupta AK, Muschel RJ, McKenna WG, Bernhard EJ. Farnesyltransferase inhibitors: an overview of the results of preclinical and clinical investigations. Cancer Res. 2003;63:5656–68. [PubMed] [Google Scholar]
- 37.Lobell RB, Omer CA, Abrams MT, Bhimnathwala HG, Brucker MJ, Buser CA, et al. Evaluation of farnesyl:protein transferase and geranylgeranyl:protein transferase inhibitor combinations in preclinical models. Cancer Res. 2001;61:8758–68. [PubMed] [Google Scholar]
- 38.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–33. doi: 10.1016/S0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi H, Bilchik A, Saha S, Turner R, Wiese D, Tanaka M, et al. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res. 2003;9:1480–8. [PubMed] [Google Scholar]
- 40.Eng C, Van Cutsem E, Nowara E, Swieboda-Sadlej A, Tebbutt NC, Mitchell P, et al. A randomized, phase Ib/II trial of rilotumumab (AMG 102; ril) or ganitumab (AMG 479; gan) with panitumumab (pmab) versus pmab alone in patients (pts) with wild-type (WT) KRAS metastatic colorectal cancer (mCRC): Primary and biomarker analyses. ASCO Annual Meeting. Chicago, Illinois, 2011 [Google Scholar]
- 41.Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman-Davis N, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without Cetuximab, in patients with Cetuximab- or Panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–6. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG. Targeting MET as a strategy to overcome crosstalk-related resistance to EGFR inhibitors. Lancet Oncol. 2009;10:709–17. doi: 10.1016/S1470-2045(09)70137-8. [DOI] [PubMed] [Google Scholar]
- 43.Liska D, Chen CT, Bachleitner-Hofmann T, Christensen JG, Weiser MR. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res. 2011;17:472–82. doi: 10.1158/1078-0432.CCR-10-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–32. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 45.Lieu C, Kopetz S. The SRC family of protein tyrosine kinases: a new and promising target for colorectal cancer therapy. Clin Colorectal Cancer. 2010;9:89–94. doi: 10.3816/CCC.2010.n.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn EF, Iida M, Myers RA, Campbell DA, Hintz KA, Armstrong EA, et al. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. 2011;30:561–74. doi: 10.1038/onc.2010.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–20. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]