Abstract
A hallmark of all solid tumor malignancies is the ability to invade the surrounding tissue and/or metastasize to distant sites. Tumors cells have altered signaling pathways which that to cytoskeleton activation and migration. Myriad studies have attempted to identify specific adhesion molecule(s) expressed in solid tumor cells that correlate with tumor cell migrative and invasive behaviors. Among such candidate molecules is hyaluronan (HA), the major glycosaminoglycan component of extracellular matrix (ECM). HA serves not only as a primary constituent of connective tissue extracellular matrices but also functions as a bio-regulatory molecule. Pertinently, HA is enriched in many types of tumors. HA is capable of binding to CD44 which is a ubiquitous, abundant and functionally important receptor expressed on the surface of many normal cells and tumor cells. Several lines of evidence indicate that CD44 selects its unique downstream effectors and coordinates downstream, intracellular signaling pathways that influence multiple cellular functions. Certain microRNAs [(miRNAs), small RNA molecules with ~20–25 nucleotides] have been shown to play roles in regulating tumor cell migration, invasion, survival and chemotherapy resistance. In this article, a special focus is placed on the role of HA-mediated CD44 interaction with unique signaling molecules in activating intracellular miRNA-signaling and RhoGTPase functions leading to the concomitant onset of tumor cell activities (e.g., tumor cell migration, invasion, survival and chemoresistance) and tumor progression. This new knowledge could serve as groundwork for the future development of new drug targets to inhibit HA/CD44-mediated oncogenic signaling and cancer progression.
Keywords: CD44, cytoskeleton, hyaluronan, miRNAs, RhoGTPases, tumor progression
Tumor invasion and metastasis are the primary causes of morbidity in cancer patients. It is now certain that oncogenic signaling and cytoskeleton function are directly involved in breast tumor cell growth, migration and invasion of surrounding tissue and metastasis1. For example, solid tumor cancers (e.g., breast cancer or head and neck cancer) often develop from epithelial tissues.1,2 The resulting tumors (carcinomas) are very heterogeneous—ranging from very slow growing to highly-aggressive, metastatic forms that are resistant to chemotherapy.2 Both breast carcinomas in situ (either ductal or lobular in origin) and head and neck squamous carcinoma cells (HNSCC) can progress to infiltrating carcinomas in the stroma, and finally metastasize to distant tissues (most often bone, lung, liver or brain) via the extensive lymphatic network.1,2 Diagnosis of whether a lesion is benign or malignant is often difficult since definitive cancer-specific markers are lacking. Furthermore, the basic cellular and molecular processes underlying tumor cell migration, invasion and metastasis are poorly understood. It is quite clear, however, that cell membrane-cytoskeleton interactions are generally involved in tumor cell motility, invasion of surrounding tissue and metastasis.1,2 In particular, it is known that both cytoskeletal proteins and actin-cytoskeleton network are required for tumor cell movement and infiltration of surrounding tissue.1,2 The details of this process, however, remain to be determined.
Hyaluronan (HA) and CD44 in Tumor Progression
A number of studies have been aimed at identifying specific adhesion molecule(s) expressed solid tumor cells that correlate with tumor cell migrative and invasive behaviors. Among such candidate molecules is hyaluronan (HA) which is a non-sulfated, unbranched glycosaminoglycan consisting of repeating disaccharide units, D-glucuronic acid and N-acetyl-D-glucosamine.3 HA, which is detected in the extracellular matrix (ECM) of most mammalian tissues, is synthesized by specific HA synthases3 and digested into various smaller-sized molecules by hyaluroniases.4 Most importantly, HA has been found to accumulate at tumor cell attachment sites and appears to play an important role in promoting tumor cell-specific behaviors.5 In cancer patients, HA concentrations are usually higher in malignant tumors than in corresponding benign or normal tissues, and in some tumor types the level of HA is predictive of malignancy.
CD44, a HA receptor denotes a family of cell-surface glycoproteins which are expressed in a variety of human solid neoplasms, particularly those classified as breast carcinomas and/or head neck squamous cell carcinoma (HNSCC).6,7 Overexpression of CD44 has been shown to be closely associated with breast tumor and HNSCC growth, migration, invasion and metastasis.6,7 Nucleotide sequence analyses reveal that many CD44 isoforms (derived from alternative splicing mechanisms) are variants of the standard form, CD44s. The presence of high levels of CD44 variant (CD44v) isoforms is emerging as an important metastatic tumor marker in a number of cancers including human breast and head and neck cancers.6,7 Recent studies have shown that CD44 is also expressed in tumor stem cells which have the unique ability to initiate tumor cell-specific properties. In fact, CD44 is proposed to be one of the important surface markers for cancer stem cells. All CD44 isoforms contain a HA-binding site in their extracellular domain and thereby serve as a major cell surface receptor for HA.6,7 Both CD44v isoforms and HA are overexpressed at sites of tumor attachment.6,7 It has been determined that HA binding to CD44v isoforms affects both cell adhesion to extracellular matrix (ECM) components, and is involved in the stimulation of a variety of tumor cell-specific functions (e.g., cytoskeleton activation, tumor cell migration/invasion, tumor cell growth/survival and chemoresistance) leading to tumor progression.6,7
Lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) shares at least 43% of sequence homology with CD44. The physiological functions of LYVE-1 include serving as a receptor for HA and facilitating the transport and metabolism of HA in the extracellular matrix. It has also been shown that LYVE-1 is involved in adherence of tumor cells8 and may also promote tumor lymph node metastasis and lymphatic invasion. Another HA receptor, RHAMM (receptor for hyaluronan-mediated motility), has been shown to interact with CD44. Importantly, the binding between RHAMM and CD44 activates ERK1/2 signaling and promotes tumor cell motility.9 The fact that CD44, LYVE1 and RHAMM display a numbers of similarities (e.g., adhesion, motility and signaling) suggests all three HA receptors are involved in HA-associated tumor progression. However, in this short review, I will only focus on HA-regulated CD44 signaling and function in regulating oncogenesis and tumor progression.
Role of HA/CD44 in Activating RhoA/RhoC Signaling and Rho-Kinase (ROK) Function During Tumor Cell Migration and Invasion
Members of the Rho subclass of the Ras superfamily [small molecular weight GTPases (e.g., RhoA, Rac1 and Cdc42)] act as molecular switches that alternate between GTP- and GDP-bound states. The “activated” GTP-bound enzymes preferentially interact with downstream effector molecules that modulate effector activities.10 Previous work has indicated that HA promotes the interaction between CD44 and several Rho-specific guanine nucleotide exchange factors (e.g., p115RhoGEF and LARG),6 thereby upregulating RhoA (a member of the Rho subclass of the Ras superfamily), leading to altered cytoskeleton-mediated cell functions.6 For example, activation of RhoA signaling has been shown to be involved in cytoskeleton-associated functions.6
Activated RhoA has been shown to induce membrane protrusion in epithelial cells.6 RhoC, which shares at least 85% sequence homology with RhoA, also participates in remodeling of the cytoskeleton.11 Accumulating evidence suggests that both RhoA and RhoC are critical for breast tumor invasion and/or metastasis.11 In particular, a correlation between the invasion activity of different tumor cells and the requirement of RhoA/RhoC-activated signaling has been suggested. Several different enzymes have been identified as possible downstream targets for RhoA/RhoC signaling. One such enzyme is Rho-Kinase (ROK-also called Rho-associated kinase) which is a serine-threonine kinase known to interact with Rho in a GTP-dependent manner.6,12 This enzyme has been shown to regulate cytoskeleton function by phosphorylating several important cytoskeletal regulators including the myosin-binding subunit (MBS) of myosin phosphatase, calponin, adducin and LIM kinase. HA-CD44 interaction promotes RhoA-activated ROK phoshorylation of Ins(1,4,5)P3 receptors, Gab-1 and NHE1 leading to cytoskeleton activation, cell proliferation and migration.6
ROK is also involved in the “cross-talk” between Ras and Rho signaling leading to cellular transformation.6 A previous study determined that ROK also is responsible for the phosphorylation of CD44-associated cytoskeletal proteins during actin filament and plasma membrane interaction. When ROK is overexpressed or constitutively activated, changes in actin cytoskeleton organization occur that are similar to those observed during normal Rho-activated conditions.13 ROK is overexpressed in breast tumor cells and is capable of phosphorylating the cytoplasmic domain of CD44.13 Moreover, phosphorylation of the cytoplasmic domain of CD44 by ROK enhances its binding interaction with the cytoskeletal protein, ankyrin.13 Overexpression of the Rho-binding domain (a dominant-negative form) of ROK by transfecting breast tumor cells with RB cDNA induces reversal of tumor cell-specific phenotypes.13 These findings support the notion that ROK plays a pivotal role in CD44-cytoskeleton interaction and the Rho-mediated oncogenic signaling that is required for membrane-cytoskeleton function and metastatic tumor cell migration. However, the cellular and molecular mechanisms involved in the regulation of RhoGTPase and ROK expression in HA/CD44-mediated signaling events remain poorly understood.
HA-CD44-Regulated miR-10b Signaling and RhoGTPase/ROK Activation
MicroRNAs (miRNAs) are evolutionarily conserved and function as negative regulators of gene expression by inhibiting the translation of mRNAs that contain complementary target sites, referred to as the “seed regions.”14 Previous data have revealed that human miRNAs are processed from capped and polyadenylated transcripts, that are precursors to mature miRNAs.15 In mammalian miRNA biogenesis, primary transcripts of miRNA genes (pri-mRNAs) are subsequently cleaved to produce an intermediate molecule containing a stem loop of ~70 nucleotides (pre-mRNAs) by the nuclear RNase III enzyme DROSHA, and exported from the nucleus by Exportin 5.14 A second RNase III enzyme Dicer then generates mature miRNA which is loaded into the RNA-induced silencing complex (RISC) in association with the argonaute protein (Ago) that induces silencing via the RNA interference pathway. Although Dicer has an important role in the silencing action of miRNAs, recent studies have shown that silencing can still occur in cells that lack Dicer. In addition, the nuclear p68-RNA helicase appears to be required in the uptake of certain miRNAs into the silencing complex.16 p68 belongs to a family of proteins that are involved in RNA metabolism processes such as translation and RNA degradation. Computational predictions of miRNA targets suggest that up to 30% of human protein-coding genes may be regulated by miRNAs.17 This makes miRNAs one of the most abundant classes of post-transcriptional regulators of gene expression.
A number of studies indicate that more than 50% of microRNAs (miRNAs) are located in cancer-associated genomic regions or fragile sites, suggesting that miRNA may be closely associated with the pathogenesis of a variety of cancers.14 In particular, miRNA-10b was found to be overexpressed in malignant glioma in addition to the overexpression of RhoC and uPAR which are contributors to glioma invasion and migration.18 Furthermore, in human esophageal cancer cell lines, Kruppel-like factor 4 (KLF4), a zinc finger protein that has been identified in several human tumors, is also a direct target of miR-10b.19 Moreover, cell invasion and metastasis were both shown to be initiated by miRNA-10b in breast cancer.20,21 Silencing of miR-10b with antagomirs (an anti-miR-10 inhibitor) both in vitro and in vivo significantly decreases the amount of miR-10b and the level of a functionally important miR-10b target, HOXD10 resulting in decreased metastasis.21,22 Thus, the miR-10b inhibitor appears to be a promising candidate for the development of new anti-metastasis agents.
Recently, we discovered a new HA/CD44-mediated signaling mechanism that regulates Twist-associated miR-10b production.21 Specifically, we found that HA binding to CD44 promotes the nuclear translocation of Twist (a stem cell-related transcription factor) and transcriptional activation. Further analyses reveal that miR-10b is controlled by an upstream promoter containing Twist binding site(s), while chromatin immunoprecipitation (ChIP) assays demonstrate that stimulation of miR-10b expression by HA/CD44-specific and Twist-dependent in breast tumor cells. This process results in the reduction of a tumor suppressor protein (HOXD10), RhoA/RhoC upregulation, Rho-Kinase (ROK) activation and breast tumor cell invasion. Treatment of breast tumor cells with Twist-specific small interfering RNAs (siRNAs) effectively blocks HA-mediated Twist signaling events, abrogates miR-10b production and increases HOXD10 expression. Subsequently, this Twist signaling inhibition causes downregulation of RhoA/RhoC expression and impairment of ROK-regulated cytoskeleton function (e.g., tumor cell invasion). To further evaluate the role of miR-10b in RhoGTPase signaling, breast tumor cells were also transfected with a specific anti-miR10b inhibitor in order to silence miR-10b expression and block its target functions. These recent results demonstrate that anti-miR-10b inhibitor not only enhances HOXD10 expression, but also abrogates HA/CD44-mediated tumor cell behaviors in breast tumor cells21. Taken together, these findings indicate that the HA-induced CD44 interaction with Twist plays a pivotal role in miR-10b production leading to the downregulation of tumor suppressor protein (HOXD10), RhoGTPase-ROK activation and tumor cell invasion. All of these events are critical pre-requisite steps for the acquisition of metastatic properties by human breast cancer cells.
HA-CD44-Regulated miR-21 Signaling and Chemotherapy Resistance
A previous study using breast tumor cells found that MDR1 expression and multidrug resistance is linked to a positive feedback circuit involving HA, phosphoinositide 3-kinase (PI3K) and ErbB2.23 In addition, HA-CD44-induced Ca2+ mobilization, Nanog/Stat-3 signaling, EGFR activation and cytoskeletal protein (ankyrin) have been shown to play an important role in regulating drug resistance.7,24 HA-CD44 interaction also influences topoisomerase II activity and Etoposide cytotoxicity in head and neck cancers.7 These findings suggest that the HA-CD44 interaction is definitely involved in multidrug resistance.
The gene networks orchestrated by many oncogenic miRNAs are still largely unknown, although some key targets have been identified as being involved in solid tumor progression.25,26 In particular, miR-21 appears to play a critical role in tumor cell survival, chemoresistance and tumor progression.24,27-29 However, very little is known concerning the regulation of miR-21 and its function in solid tumor cancer. Recently, we found that miR-21 can be activated by HA/CD44-activated stem cell marker (Nanog) signaling in both breast and head and neck cancer cell lines.24,29 For example, our previous work showed that HA-CD44 interaction promotes Nanog interaction with p68 and DROSHA leading to biosynthetic processing and production of miR-21 in breast tumor cells.30 These findings suggest that HA/CD44-mediated Nanog signaling is closely linked to miR-21 production and function during oncogenesis.
Abnormal Stat-3 signaling also appears to play a critical role in oncogenesis. Previous studies showed that the functional link between Nanog and Stat-3 exists in several different tumor cells.24,29,30 In our recent study we observed that HA-CD44 binding activates nuclear localization of Nanog which then forms a complex with Stat-3 in head and neck cancer cells.24 In particular, miR-21 is controlled by an upstream promoter/enhancer containing Stat-3 binding sites in head and neck cancer cells, while chromatin immunoprecipitation (ChIP) assays demonstrate that stimulation of miR-21 production by HA is Nanog/Stat-3 complex-dependent in head and neck cancer cells.24 Most importantly, an anti-miR-21 inhibitor can enhance PDCD4 expression, and block HA/CD44-mediated tumor functions (e.g., survival protein expression, tumor cell growth and survival/chemotherapy resistance) in HNSCC cells. Thus, this newly-discovered HA/CD44-Nanog/Stat-3 signaling pathway and miR-21 production/function are highly innovative and should provide important new drug targets to cause tumor cell apoptosis and overcome chemotherapy resistance in head and neck cancer cells.
Summary
HA/CD44-mediated tumor cell-specific phenotypes are closely linked to the small GTP-binding proteins such as RhoA and RhoC. Activation of RhoGTPases (e.g., RhoA and RhoC) and Rho-kinase (ROK) has been shown to produce specific structural changes in actin assembly, cytoskeleton reorganization, transcriptional activation, tumor cell growth, survival, migration and invasion. Our current models for illustrating HA-dependent and CD44-specific microRNA (e.g., miR-10b and miR-21) signaling pathways are described as follows:
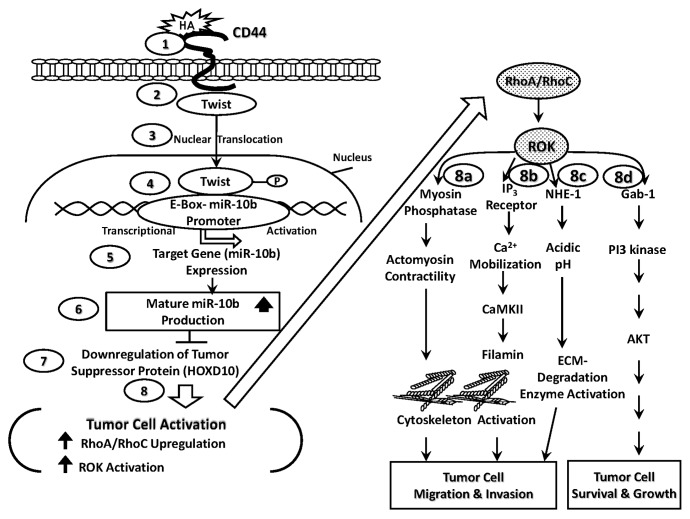
(1) HA-CD44-regulated miR-10b signaling and RhoGTPase/ROK activation pathways. Specifically, HA binding to tumor cell surface(s) (Fig. 1, Step 1) promotes CD44 association with a transcription factor, Twist (Fig. 1, Step 2). Twist then translocates from the cytosol to the nucleus (Fig. 1, Step 3) and interacts with the E-box region of miR-10b promoter (Fig. 1, Step 4), resulting in miR-10b gene expression (Fig. 1, Step 5) and mature miR-10b production (Fig. 1, Step 6). The resultant miR-10b then functions to downregulate the tumor suppressor protein (HOXD10) (Fig. 1, Step 7) and promotes tumor cell activation (e.g., RhoA/RhoC upregulation, ROK activation and cytoskeleton reorganization) (Fig. 1, Step 8). Subsequently, HA/CD44-activated RhoA-ROK stimulates myosin phosphatase activity, thereby activating myosin adenosine triphosphatase (ATPase) and generating actomyosin-mediated tumor cell migration and invasion (Fig. 1, Step 8a). HA/CD44-activated RhoA-ROK also induces Ins(1,4,5)P3 receptor phosphorylation, Ins(1,4,5)P3 production and intracellular Ca2+ mobilization resulting in CaMKII activation. CaMKII then phosphorylates the cytoskeletal protein, filamin, leading to cytoskeleton reorganization and tumor cell migration/invasion (Fig. 1, Step 8b). In addition, HA/CD44-activated RhoA-ROK promotes NHE1 phosphorylation. Most importantly, the phosphorylation of NHE1 by RhoA-ROK promotes Na+-H+ exchange activity and extracellular acidification leading to an activation of low pH-dependent extracellular matrix (ECM) degradation enzymes required for ECM modification and tumor cell migration and invasion (Fig. 1, Step 8c). Finally, HA-CD44 activation of RhoA-ROK appears to phosphorylate certain cellular proteins including the linker molecule, Gab-1. Most importantly, phosphorylation of Gab-1 by RhoA-ROK promotes Gab-1 interaction with Ins(1,4,5)P3 kinase leading to AKT activation and tumor cell survival and growth (Fig. 1, Step 8d). Thus, the results of these studies elucidating the HA/CD44 signaling pathway-specific mechanisms involved in microRNA production and RhoGTPase signaling are significant for the formulation of future intervention strategies in the treatment of solid tumor invasion and/or metastasis.
Figure 1. HA/CD44-mediated Twist signaling and miR-10b production leading to RhoGTPase upregulation and ROK activation leading to tumor cell migration and invasion as well as tumor cell survival and growth. The proposed signaling events are described as follows: Step 1: HA binds to tumor cell surface receptor, CD44. Step 2: HA induces CD44-Twist complex formation. Step 3: Twist translocates from the cytosol to the nucleus. Step 4: Twist then interacts with the E-box region of miR-10b promoter. Steps 5 and 6: Twist binds to the E-box and induces miR-10b gene expression and miR-10 production. Steps 7 and 8: miR-10b then downregulates the tumor suppressor protein (HOXD10) and promotes tumor cell activation (e.g., RhoA/RhoC upregulation, ROK activation and cytoskeleton reorganization). Step 8a: HA/CD44/miR-10b-activated RhoA-ROK enhances myosin phosphatase activity, thereby generating actomyosin-mediated tumor cell migration and invasion. Step 8b: HA/CD44/miR-10b-activated RhoA-ROK stimulates IP3 receptor phosphorylation, IP3 production and intracellular Ca2+ mobilization resulting in CaMKII activation. CaMKII then phosphorylates the cytoskeletal protein, filamin, leading to cytoskeleton reorganization and tumor cell migration/invasion. Step 8c: HA/CD44/miR-10b-activated RhoA-ROK induces NHE1-mediated extracellular acidification leading to an activation of low pH-dependent extracellular matrix (ECM) degradation enzymes required for ECM modification and tumor cell migration and invasion. Step 8d: HA/CD44/miR-10 activated RhoA-ROK promotes certain cellular proteins including the linker molecule, Gab-1 which then interacts with PI3 kinase leading to AKT activation and tumor cell survival and growth.
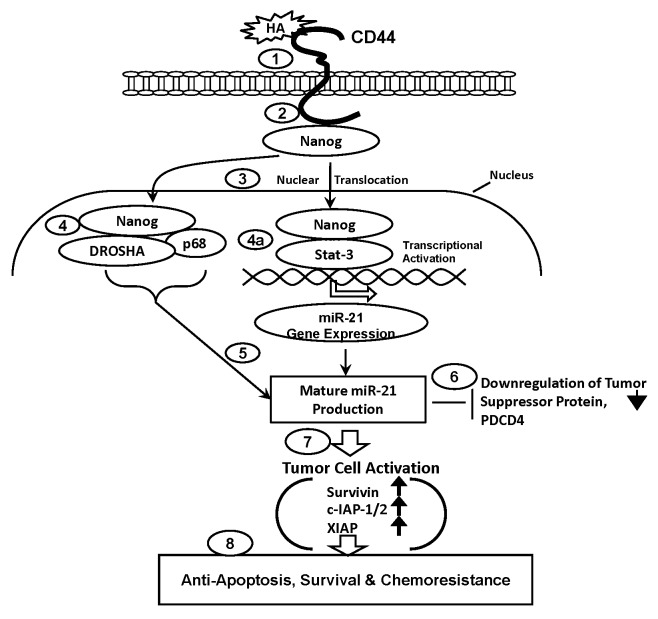
(2) HA-CD44-regulated miR-21 signaling and chemotherapy resistance pathways. HA binding to tumor cell surface(s) (Fig. 2, Step 1) also promotes CD44 association with the stem cell marker, Nanog (Fig. 2, Step 2) which then becomes translocated from the cytosol to the nucleus (Fig. 2, Step 3) and interacts with (a) the microprocessor complex containing the RNAase III (DROSHA) and the RNA helicase (p68) (Fig. 2, Step 4) and/or (b) an upstream/enhancer region (containing Stat-3 binding sites) of the miR-21 promoter (Fig. 2, Step 4a), resulting in miR-21 gene expression and mature miR-21 production (Fig. 2, Step 5). The resultant miR-21 then functions to downregulate the tumor suppressor protein (PDCD4) (Fig. 2, Step 6). Subsequently, these changes result in the expression of survival proteins (e.g., survivin, c-IAP1/2 and XIAP) (Fig. 2, Step 7) and stimulation of anti-apoptosis, survival and chemoresistance (Fig. 2, Step 8) in tumor cells. Taken together, these findings suggest that targeting HA/CD44-mediated Nanog signaling pathways and miR-21 function may provide a new drug target to sensitize tumor cell apoptosis/death and overcome chemotherapy resistance in tumor cells.
Figure 2. HA/CD44-mediated Nanog signaling and miR-21 production leading to oncogenesis and chemoresistance in tumor cells. The proposed signaling events are described as follows: Step 1: HA binds to tumor cell surface receptor, CD44. Step 2: HA promotes CD44 association with Nanog. Step 3: Nanog translocates from the cytosol to the nucleus. Step 4: Nanog interacts with the microprocessor complex containing the RNAase III (DROSHA) and the RNA helicase (p68). Steps 4a and 5: Nanog also binds to Stat-3 and becomes associated with an upstream/enhancer region (containing Stat-3 binding sites) of the miR-21 promoter, resulting in miR-21 gene expression and mature miR-21 production. Step 6: HA/CD44/activated miR-21 downregulates the tumor suppressor protein (PDCD4). Step 7: HA/CD44/activated miR-21 promotes the expression of inhibition of survival proteins (survivin, c-IAP1/2 and XIAP). Step 8: HA/CD44/activated miR-21 causes anti-apoptosis, survival and chemotherapy resistance in tumor cells.
Our current model suggests that the close interactions between CD44 and its selected binding partners play a pivotal roles in promoting “cross-talk” among various intracellular signaling pathways (e.g., miRNAs and RhoGTPases) leading to the concomitant onset of multiple functions. The coordinated HA/CD44 activation of miRNAs (e.g., miR-10b and miR-21) and RhoGTPase signaling as well as the cytoskeleton as described in both models (Figs. 1 and 2) may be a possible mechanism underlying various tumor cell-specific behaviors, transcriptional activation, tumor cell growth, survival, migration and invasion-all obvious prerequisites for tumor metastasis and progression.
Acknowledgments
We gratefully acknowledge the assistance of Drs Gerard J. Bourguignon and Walter M. Holleran in the preparation and review of this manuscript. This work was supported by Veterans Affairs (VA) Merit Review grant, United States Public Health grants (R01 CA66163) and DOD grant. L.Y.W.B. is a VA Senior Research Career Scientist.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/19110
References
- 1.Parker B, Sukumar S. Distant metastasis in breast cancer: molecular mechanisms and therapeutic targets. Cancer Biol Ther. 2003;2:14–21. doi: 10.4161/cbt.188. [DOI] [PubMed] [Google Scholar]
- 2.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer Metastasis Rev. 2005;24:35–45. doi: 10.1007/s10555-005-5046-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Spicer AP. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–6. doi: 10.1016/S0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 4.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–6. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 6.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–9. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SJ, Bourguignon LY. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am J Pathol. 2011;178:956–63. doi: 10.1016/j.ajpath.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–21. doi: 10.1016/S1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci. 2008;121:925–32. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 10.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 11.Wu D, Asiedu M, Wei Q. Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC. Oncogene. 2009;28:2219–30. doi: 10.1038/onc.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 13.Bourguignon LYW, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43:269–87. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Cowland JB, Hother C, Grønbaek K. MicroRNAs and cancer. APMIS. 2007;115:1090–106. doi: 10.1111/j.1600-0463.2007.apm_775.xml.x. [DOI] [PubMed] [Google Scholar]
- 15.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–66. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salzman DW, Shubert-Coleman J, Furneaux H. P68 RNA helicase unwinds the human let-7 microRNA precursor duplex and is required for let-7-directed silencing of gene expression. J Biol Chem. 2007;282:32773–9. doi: 10.1074/jbc.M705054200. [DOI] [PubMed] [Google Scholar]
- 17.Cullen BR. Derivation and function of small interfering RNAs and microRNAs. Virus Res. 2004;102:3–9. doi: 10.1016/j.virusres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Sasayama T, Nishihara M, Kondoh T, Hosoda K, Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int J Cancer. 2009;125:1407–13. doi: 10.1002/ijc.24522. [DOI] [PubMed] [Google Scholar]
- 19.Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H, et al. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem. 2010;285:7986–94. doi: 10.1074/jbc.M109.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–8. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 21.Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem. 2010;285:36721–35. doi: 10.1074/jbc.M110.162305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–7. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–5. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- 24.Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. 2012;31:149–60. doi: 10.1038/onc.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010;12:201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baffa R, Fassan M, Volinia S, O’Hara B, Liu CG, Palazzo JP, et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214–21. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]
- 27.Si M-L, Zhu S, Wu H, Lu Z, Wu F, Mo Y-Y. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 28.Asangani IA, Rasheed SAK, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 29.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–46. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–51. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]