Abstract
Small GTPase proteins regulate cytoskeletal dynamics to orchestrate diverse cellular functions in organismal physiology, development and disease. The Rho GTPase family member Rac1 is central to actin-driven processes in a number of cell types, particularly platelets, where Rac1 serves as an essential mediator of lamellipodia formation and thrombus stability. Despite the importance of Rac1 to platelet function, little is known about how Rac1 activity is regulated in platelets. We recently defined the tyrosine-kinase based signaling cascade that activates mTOR to regulate Rac1 activation downstream of platelet integrin and glycoprotein receptors. We demonstrated a critical role for the mTOR-Rac1 axis in regulating platelet spreading, aggregation and aggregate stability under shear. These studies suggest that in addition to cancer and transplant medicine, intervention of the mTOR system may have implications for hemostatic and thrombotic processes as well as immunotherapies and intravascular stent design.
Keywords: actin, cytoskeleton, GEF, P-Rex1, Rac1, rapamycin, Rheb, S6K1, TIAM1, TSC2
Platelets serve as the primary cellular mediators of hemostasis and thrombosis.1 These anucleate cellular fragments —formed from the proplatelet appendages of megakaryocytic cells in the bone marrow — circulate through blood as guardians of vascular integrity. Upon detecting molecular cues of vessel injury, platelets halt bleeding by adhering to and spreading out on extracellular matrix substrate proteins and aggregating with other platelets to form hemostatic plugs. These events are performed through a carefully regulated change in the shape of platelets from smooth, biconcave disks to spheres with filopodial extensions and lamellipodial structures.
A reorganization of the cytoskeleton is central to the shape changes that drive platelet activation and aggregation, and platelets express abundant Rho GTPase proteins and regulators necessary for actin cytoskeletal remodeling and platelet function.2 Over the past decade, Rho GTPases have been determined to be critical for platelet functions in hemostasis. For instance, activation of RhoA through a Gq-coupled signaling pathway mediates Rho kinase and myosin light chain kinase contractile events important for platelet shape changes.3 RhoA also plays a role in maintaining the integrin-mediated adhesion of platelets to substrates under conditions of physiological shear flow.4,5 Perhaps most notably from a shape change perspective, Rac1 is required to remodel the actin cytoskeleton and drive the spreading and aggregation of platelets.6 Likewise, Rac1 regulators such as the Rac1 guanine nucleotide exchange factor (GEF) Vav1 also have key roles in platelet function and hemostasis.7 Rap1 and its GEF p115RhoGEF have also been shown be critical in platelet shape change and secretion processes.2 More recently, studies from Cdc42-null mice have shown the importance of this small GTPase in platelet PAK kinase activation and subsequent cytoskeletal remodeling events.8
Despite key roles for Rho GTPases such as Rac1 in platelet function, little is known about how small GTPase action is organized and regulated in platelets. The engagement of platelet integrins, glycoprotein receptors or G-protein coupled receptors (GPCRs) triggers a network of tyrosine kinase activity followed by PLC activation and a wave of calcium mobilization.2,9 These signaling events in turn lead to the coordinated activation of MAPKs, PI3Ks, Akt, PKC, and small GTPases such as Rac1. While a system of tyrosine kinases and effectors immediately downstream of platelet integrins and receptors has been extensively investigated, little in known about how more distal signals interact and communicate with one another to regulate the spatial and temporal activation of Rho GTPases as well as their respective GEFs or GTPase activating proteins (GAPs).
Over the past several years, studies in nucleated cell systems have shown that Rac1 activity is influenced by the mammalian target of rapamycin, or mTOR, system in a variety of cellular contexts.10 mTOR is a serine/threonine protein kinase well known for regulating cell growth and differentiation at the level of protein translation.11 Mitogenic signals of nutrient availability lead mTOR to phosphorylate the 70 kD ribosomal protein S6 kinase (S6K1) as well as the eukaryotic translation initiation factor 4E-BP1 to integrate protein translation processes at ribosomes with cell growth and proliferation.12 Pharmacological inhibitors of mTOR such as rapamycin (Sirolimus) are potent antiproliferative agents and commonly used to prevent organ rejection in transplant medicine. mTOR inhibitors also serve as antineoplastic agents and are in development for the treatment of several malignancies.13 While mTOR inhibitors potently block cell proliferation, mTOR inhibition also has effects on chemotaxis, cell migration and invasion processes that drive cancer metastasis.10 Accordingly, mTOR inhibitors such as rapamycin block the growth factor- and nutrient-directed migration of intestinal cells14 and smooth muscle cells.15 While the exact mechanisms by which mTOR contributes to cell migration are not understood, mTOR inhibitors have been shown to block the migration of ovarian cancer and colon cancer cells in part by inhibiting Rho GTPase activation in these cells.16,17
These studies, together with others showing an association of S6K1 with Rac1 and actin in nucleated cells,18,19 led us to hypothesize that mTOR controls Rac1 activation in platelets. While earlier studies had shown that mTOR in platelets has specific functions in clot retraction and thrombus remodeling,20 the role of mTOR in platelet activation and aggregation pathways had remained largely ignored. Pioneering work by the Weyrich group demonstrates that platelets synthesize proteins in an mTOR-dependent manner upon activation by fibrinogen and other substrate ligands.20 Such studies notably showed that platelets synthesize Bcl-3, a B-cell lymphoma oncoprotein with ties to tyrosine kinase activity, in an mTOR-dependent manner to regulate clot retraction and thrombus consolidation.21 Additional clues pointing to a role for mTOR in platelet activities come from studies of intravascular stents which allude that rapamycin may have antiplatelet and antithrombotic properties. Rapamycin is commonly added to the coating of intravascular stents as an antiproliferative agent to prevent restenosis in coronary arteries associated with intravascular stent insertion. Interestingly, some studies of rapamycin-eluting stents have noted that the surfaces of such stents may have antiplatelet properties, as platelets do not aggregate on the surfaces of such stents as they do in non-coated stents.22 While this work shows that mTOR inhibitors such as rapamycin may have antiplatelet properties, they do not offer a mechanism of how mTOR relates to platelet thrombotic functions.
To better understand how Rac1 is activated in platelets in response to integrin activation, we examined the coordinated activation of Src family tyrosine kinases (SFKs), the mTOR pathway effector S6K1 and Rac1 in platelets in response to binding to fibrinogen.23 We determined that platelet SFKs activate mTOR and its effector S6K1 upstream of Rac1 as platelets form lamellipodia and spread on a surface of fibrinogen (Fig. 1). Imaging and biochemical analyses revealed that S6K1, Rac1 and the Rac1 GEF TIAM1 colocalize and interact at the leading lamellipodial edge of platelets, suggesting that the mTOR system can work with Rac1 and Rac1 GEFs to drive platelet spreading. Inhibition of mTOR with a variety of pharmacological agents blocked platelet Rac1 activation, spreading and aggregation. Interestingly, Rac1 inhibition markedly increased S6K1 phosphorylation in platelets, suggesting that Rac1 may also mediate a negative feedback system that limits S6K1 activation. Continuous Rac1 and mTOR activity was also required to keep platelet aggregates stable under shear flow conditions.
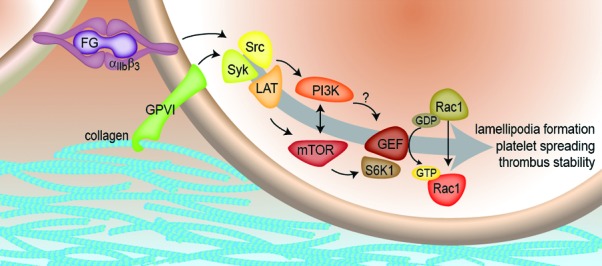
Figure 1. The adhesive proteins fibrinogen (FG) or collagen engage their respective platelet receptors (integrin αIIbβ3 or glycoprotein GPVI) to activate mTOR and S6K1 in a signaling step downstream of SFK activation but before Rac1 activation. Platelet stimulation first triggers the activation of tyrosine kinases Src and Syk to then activate PI3K and ultimately guanine nucleotide exchange factors (GEF) which catalyze the exchange of GDP for GTP on Rac1. In turn, Rac1 activation drives platelet lamellipodia formation and platelet spreading and aggregation. The specific mechanisms by which mTOR/S6K1 or PI3K pathways may activate Rac1 GEFs such as TIAM1, P-Rex1 or Vav1 to help drive Rac1 have yet to be determined.
These results have led us to hypothesize potential mechanisms by which mTOR could regulate Rac1 activity in platelets. These proposed models of Rac1 activation in platelets are currently a topic of investigation by our group and others. As a first possibility, Rho GTPase activities, actin remodeling and lamellipodia formation may require mTOR-dependent protein translation and synthesis processes. As platelets are known to continuously synthesize proteins, it is possible that protein translation and protein synthesis are coupled to platelet spreading and aggregation processes. Indeed, Rosenwald et al. demonstrated that continuous protein synthesis is needed for ADP-stimulated platelet aggregation.24 We found that in the presence of the ADP scavenger apyrase, collagen-triggered platelet aggregation is abrogated by mTOR inhibitors but not effected by puromycin, a translational inhibitor that globally blocks protein synthesis. Future studies utilizing pharmacological agents which block protein translation more specifically at the level of mTOR and its effectors S6K1 and 4E-BP1 may provide further insight into the role of mTOR-dependent translation in platelet function.
Second, mTOR signaling systems may be tied to specific Rac GEFs or GAPs. Surprising, little is known regarding how the mTOR system affects Rac1 GEFs such as Vav1. Work in neurons and cancer cells shows that S6K1 associates with the Rac GEF T-cell lymphoma invasion and metastasis protein TIAM1.25 We similarly found that S6K1 and TIAM1 associate in a protein complex and colocalize at the leading lamellipodial edge in platelets.23 Whether or not TIAM1 has a major role in platelet Rac1 function, however, remains to be determined. Intriguingly, studies of G-protein-mediated activation of Rac1 have shown that mTOR is tied to the activities of the phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein P-Rex1, an mTOR-associated Rac GEF important for immune cell function and ErbB2 signaling in breast cancer cells.26-28 Accordingly, P-Rex1 could link platelet GPCR activities of PAR and puronergic P2Y receptors to Rac activation, implicating mTOR as an intermediary. In the course of our studies, we found that Rac1 associates P-Rex1 in platelets; however, data from P-Rex1-null mice suggests that P-Rex1 plays at most a minor role in platelet cytoskeletal remodeling.29
Finally, mTOR may work directly with Rac1 to control actin-mediated processes in platelets. Indeed, recent work by Saci et al. demonstrates that mTOR and Rac1 interact and that their activities are tied in the regulation of cell growth.30 This work suggests that rather than working directly with S6K1, Rac1 may partner with mTOR itself or the mTOR complex 1 and 2 (mTORC1 and mTORC2) components Raptor and Rictor, respectively. Our results show for the first time that mTORC1 and mTORC2 protein complexes are present and intact in platelets;23 however, more work is needed to determine the specific functions of mTORC1 and mTORC2 complexes in platelets. Such studies will likely take advantage of pharmacological agents that specifically target mTORC1 or both mTORC1 and mTORC2.31 Intriguingly, Moore et al. have recently shown that mTORC2 is a key regulator of Akt serine 473 phosphorylation in platelets.32 mTORC2 was also recently shown to play a role in the RhoA-dependent chemotaxis of neutrophils.33 How mTOR-dependent mechanisms of immune cell modulation overlap with actin remodeling and directed cell migration process in platelets vs. other peripheral blood cells such as neutrophils remains to be determined. Interestingly, mTOR activity is in large part directed by the small GTPase Rheb. The tuberous sclerosis protein TSC2, a Rheb GAP that regulates the GTP bound state of Rheb to control mTOR activity,34 also coordinates Rac1 and Cdc42 activities through mTOR to mediate the migration of colon cancer cells and fibroblasts.34 As TSC2 is at the center of a number of signaling networks, TSC2 may serve as a master regulator of platelet function through the concerted effects of PI3K, mTOR, PKC and PDK systems.34 Future work will determine how a TSC2-Rheb-mTOR network may influence Rac1 and platelet activation.
Acknowledgments
We thank Tiffani Howard for illustration services. This work was supported by the National Institute of Health grants T32HL007781 (J.E.A.) and R01HL101972 (O.J.T.M.) and the American Heart Association 09GRNT2150003 (O.J.T.M.).
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/19137
References
- 1.Wei AH, Schoenwaelder SM, Andrews RK, Jackson SP. New insights into the haemostatic function of platelets. Br J Haematol. 2009;147:415–30. doi: 10.1111/j.1365-2141.2009.07819.x. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–9. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–54. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenwaelder SM, Hughan SC, Boniface K, Fernando S, Holdsworth M, Thompson PE, et al. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. J Biol Chem. 2002;277:14738–46. doi: 10.1074/jbc.M200661200. [DOI] [PubMed] [Google Scholar]
- 5.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2011 doi: 10.1182/blood-2011-08-372193. In press. [DOI] [PubMed] [Google Scholar]
- 6.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–84. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce AC, Wilde JI, Doody GM, Best D, Inoue O, Vigorito E, et al. Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood. 2002;100:3561–9. doi: 10.1182/blood.V100.10.3561. [DOI] [PubMed] [Google Scholar]
- 8.Akbar H, Shang X, Perveen R, Berryman M, Funk K, Johnson JF, et al. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS One. 2011;6:e22117. doi: 10.1371/journal.pone.0022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Huang S. mTOR signaling in cancer cell motility and tumor metastasis. Crit Rev Eukaryot Gene Expr. 2010;20:1–16. doi: 10.1615/critreveukargeneexpr.v20.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–9. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–41. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhoads JM, Niu X, Odle J, Graves LM. Role of mTOR signaling in intestinal cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;291:G510–7. doi: 10.1152/ajpgi.00189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakakibara K, Liu B, Hollenbeck S, Kent KC. Rapamycin inhibits fibronectin-induced migration of the human arterial smooth muscle line (E47) through the mammalian target of rapamycin. Am J Physiol Heart Circ Physiol. 2005;288:H2861–8. doi: 10.1152/ajpheart.00561.2004. [DOI] [PubMed] [Google Scholar]
- 16.Ip CK, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene. 2011;30:2420–32. doi: 10.1038/onc.2010.615. [DOI] [PubMed] [Google Scholar]
- 17.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou MM, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–83. doi: 10.1016/S0092-8674(00)81257-X. [DOI] [PubMed] [Google Scholar]
- 19.Crouch MF. Regulation of thrombin-induced stress fibre formation in Swiss 3T3 cells by the 70-kDa S6 kinase. Biochem Biophys Res Commun. 1997;233:193–9. doi: 10.1006/bbrc.1997.6419. [DOI] [PubMed] [Google Scholar]
- 20.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109:1975–83. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95:5556–61. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan CJ, Tang JJ, Weng YJ, Wang J, Huang N. Preparation and characterization of rapamycin-loaded PLGA coating stent. J Mater Sci Mater Med. 2007;18:2193–8. doi: 10.1007/s10856-007-3075-9. [DOI] [PubMed] [Google Scholar]
- 23.Aslan JE, Tormoen GW, Loren CP, Pang J, McCarty OJ. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood. 2011;118:3129–36. doi: 10.1182/blood-2011-02-331579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenwald IB, Pechet L, Han A, Lu L, Pihan G, Woda B, et al. Expression of translation initiation factors elF-4E and elF-2alpha and a potential physiologic role of continuous protein synthesis in human platelets. Thromb Haemost. 2001;85:142–51. [PubMed] [Google Scholar]
- 25.Buchsbaum RJ, Connolly BA, Feig LA. Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J Biol Chem. 2003;278:18833–41. doi: 10.1074/jbc.M207876200. [DOI] [PubMed] [Google Scholar]
- 26.Herńndez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Herńndez-García R, Calderón-Salinas JV, Reyes-Cruz G, et al. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708–15. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 27.Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40:877–92. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, et al. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–21. doi: 10.1016/S0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 29.Aslan JE, Spencer AM, Loren CP, Pang J, Welch HC, Greenberg DL, et al. Characterization of the Rac guanine nucleotide exchange factor P-Rex1 in platelets. J Mol Signal. 2011;6:11. doi: 10.1186/1750-2187-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saci A, Cantley LC, Carpenter CL. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell. 2011;42:50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 32.Moore SF, Hunter RW, Hers I. mTORC2 protein complex-mediated Akt (Protein Kinase B) Serine 473 Phosphorylation is not required for Akt1 activity in human platelets [corrected] J Biol Chem. 2011;286:24553–60. doi: 10.1074/jbc.M110.202341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–57. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–22. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]


