Abstract
Dentin sialoprotein (DSP) and phosphophoryn (PP), acidic proteins critical to dentin mineralization, are translated from a single transcript as a DSP-PP precursor that undergoes specific proteolytic processing to generate DSP and PP. The cleavage mechanism continues to be controversial, in part because of the difficulty of obtaining DSP-PP from mammalian cells and dentin matrix. We have infected Sf9 cells with a recombinant baculovirus to produce large amounts of secreted DSP-PP240, a variant form of rat DSP-PP. Mass spectrometric analysis shows that DSP-PP240 secreted by Sf9 cells undergoes specific cleavage at the site predicted from the N-terminal sequence of PP extracted from dentin matrix: SMQG447↓D448DPN. DSP-PP240 is cleaved after secretion by a zinc-dependent activity secreted by Sf9 cells, generating DSP430 and PP240 products that are stable in the medium. DSP-PP processing activity is constitutively secreted by Sf9 cells, but secretion is diminished 3 days after infection. Using primers corresponding to the highly conserved catalytic domain of Drosophila melanogaster tolloid (a mammalian BMP1 homolog), we isolated a partial cDNA for a Spodopotera frugiperda tolloid-related-1 protein (TLR1) that is 78% identical to Drosophila TLR1 but only 65% identical to Drosophila tolloid. Tlr1 mRNA decreased rapidly in Sf9 cells after baculovirus infection and was undetectable 4d after infection, paralleling the observed decrease in secretion of the DSP-PP240 processing activity after infection. Human BMP1 is more similar to Sf9 and Drosophila TLR1 than to tolloid, and Sf9 TLR1 is more similar to BMP1 than to other mammalian homologs. Recombinant human BMP1 correctly processed baculovirus-expressed DSP-PP240 in a dose-dependent manner. Together, these data suggest that the physiologically accurate cleavage of mammalian DSP-PP240 in the Sf9 cell system represents the action of a conserved processing enzyme and support the proposed role of BMP1 in processing DSP-PP in dentin matrix.
Introduction
Dentin sialoprotein (DSP) and phosphophoryn (PP) are two major noncollagenous dentin proteins derived from a single copy of DSP-PP gene (also referred to as DSPP) whose expression is tightly regulated during dentinogenesis [1]. DSP-PP transcripts are also expressed in other tissues, including inner ear and jaw tissue [2], [3]. The recent demonstration of DSP-PP promoter-driven lacZ expression in multiple tissues, such as bone, kidney [4], hair follicles [5], [6], salivary gland, and lung (Ritchie unpublished data), further suggests that DSP and PP may have physiological roles in several other organs besides teeth.
It is believed that DSP-PP precursor proteins are the immediate translation products of DSP-PP mRNA (here we use “DSP-PP” to refer generically to the precursor secreted in dentin matrix and “DSP-PP240” to refer specifically to the shorter variant form of rat DSP-PP used in these experiments.). DSP-PP undergoes multiple post-translational modifications, including signal peptide cleavage, Asn-linked glycosylation, phosphorylation and proteolytic processing between the DSP and PP domains, to produce mature DSP and PP proteins required for dentin mineralization. Recent research on DSP-PP cleavage has focused on the identification of its initial cleavage site and the protease(s) responsible for cleavage. For example, Qin et al. reported Y438 as the major cleavage site (2001) using tryptic fragments from native, purified DSP [7]. Qin and colleagues proposed that a transmembrane endopeptidase, Phex, was the protease responsible for processing of both DMP1 (e.g., dentin matrix protein 1) and DSP-PP [8], [9]. More recently, Sun et al. reported that a D448A mutation blocked cleavage of recombinant mouse DSP-PP in a cultured human cell system and concluded that the key cleavage site is G447↓D448 [10]. However, the exact cleavage site of the wild type (wt) mouse DSP-PP precursor in cultured cells was not determined by direct sequencing or mass spectrometry (MS); rather identification of cleavage products relied on gel mobility of immuno-stained or Stains-All-stained bands separated by SDS-PAGE. Thus, the D448A mutant also did not provide direct evidence that G447↓D448 was the cleavage site.
Other attempts to localize the initial DSP-PP cleavage site have met with limited success because of the lack of sufficient DSP-PP precursor protein available for quantitative studies. Steiglitz et al. proposed in 2004 bone morphogenetic protein-1 (BMP1) as the candidate enzyme for proteolytic cleavage of both DMP1 and DSP-PP [11]. This work demonstrated that BMP1 was responsible for DMP1 cleavage but did not test DSP-PP cleavage because the DSP-PP precursor protein was not available [11]. Recently, Fisher and co-workers identified a mouse DSP-PP precursor protein by expression in transfected human colon carcinoma LoVo cells which lack furin and therefore should be defective in BMP1 activation [12]. LoVo cells were shown to secrete an intact mouse DSP-PP precursor that could be cleaved by exogenously added BMP1 and by the homologous proteins tolloid-like 1 (TLL1) and tolloid-like 2 (TLL2) to generate a DSP-sized band, as shown by Western blot analysis [12]. However, because of the low amounts of DSP-PP and DSP that were detected, no mass spectrometry or N-terminal sequence data were available to identify the precise cleavage site. A mutant form of mouse DSP-PP (with a substitution equivalent to M445Q446 to I445E446 in rat DSP-PP240) was not cleaved by BMP1, TLL1 or TLL2. Again, analysis of this mutant did not provide direct evidence that G447↓D448 was the cleavage site. Yamakoshi and co-workers [13], showed that a DSP-PP preparation obtained from porcine dentin matrix was cleaved by the matrix metalloproteinases MMP-2 and MMP-20 at multiple sites making them unlikely candidates for processing enzymes. More recently, similar preparations were shown to be cleaved by BMP1 and MEP1A to generate a product similar in mobility to PP [14]. Taken together, despite intensive investigations since 2001, there is no direct evidence proving that G447↓D448 is the DSP-PP cleavage site and only Western blot analysis to substantiate the claim that BMP1 can correctly cleave DSP-PP.
Previously, we used a baculovirus expression system that was capable of producing high yields of DSP-PP precursor protein. The secreted DSP-PP240 protein could be visualized with Stains-All staining and could be identified unambiguously by mass spectrometry [15]. From MS/MS analysis of isolated tryptic fragments of the PP240 band, we proposed that the DSP-PP cleavage site was G447↓D448 [15]. In this report, we used MS and MS/MS to analyze a smaller, chymotryptic fragment of the PP240 band which has permitted direct determination of the amino acid sequence of this peptide by ion trap/fragmentation MS and firmly establishes that the initial cleavage site in DSP-PP240 is G447↓D448.
We also show that cleavage of DSP-PP240 at this site occurs after secretion into the conditioned medium of Sf9 cells and that cleavage is catalyzed by an endogenous Zn-dependent proteolytic activity secreted by Sf9 cells. Secretion of this activity is suppressed by baculoviral infection. We show, furthermore, that Sf9 cells transcribe a tolloid-related-1 (TLR1) peptidase gene (Spodoptera frugiperda tlr1) and that expression of this gene is similarly suppressed by baculoviral infection. We show that the human homolog of tolloid-related-1, BMP1, can also cleave DSP-PP240 to release DSP430 and PP240.
Materials and Methods
Mass Spectrometric (MS) Analysis of PP240 to Determine the Cleavage Site in Secreted DSP-PP240
PP240 was partially purified by the polyanion extraction procedure as previously described [15]. Briefly, conditioned medium (5 ml) was collected from 1×106 Sf9 cells (Novagen, Madison, WI) 4 days after infection with baculovirus containing the DSP-PP240 cDNA (hereafter termed DSP-PP240 virus) [15]. Trichloroacetic acid (TCA) was added to the medium to a final concentration of 5% (w/v) and incubated at room temperature for 2 min to precipitate the majority of culture medium proteins. The TCA-soluble fraction, collected after centrifugation in an Eppendorf rotor at 12,000 rpm at room temperature for 5 min, was neutralized with one-fifth volume of 3 M Tris-HCl, pH 8.8, and precipitated with one-tenth volume of 1 M CaCl2. The calcium precipitate, collected after centrifugation in an Eppendorf rotor at 12,000 rpm for 5 min at room temperature, was dissolved in 1 ml of 5% trichloroacetic acid, neutralized with 3 M Tris-HCl, pH 8.8, and re-precipitated with 1 M CaCl2. This second CaCl2 precipitate, containing recombinant DSP-PP240 and products of processing, was dissolved in 0.5 ml of 0.1 M EDTA. The dissolved sample (20 µl) was fractionated on a non-denaturing polyacrylamide gel (7.5%). PP240 protein was stained with Stains-All and analyzed by MS and MS/MS analyses as described below.
7.5% non-denaturing polyacrylamide gel samples were stained with Stains-All and then PP240 band was excised, transferred to a 96-well plate, and destained. The gel samples were then subjected to reduction and alkylation and then washed, dehydrated, and digested with chymotrysin. The peptides were extracted from the gel plugs with 2% aceonitrile and 1% formic acid. The extracted peptides (30 µl) were transferred to another 96-well plate, where 5 µl of matrix (α-Cyano) was added to the sample well. The samples were then vaporized to dryness and redissolved in 5 µl of 60% aceonitrile and 0.1% trifuoroacetic acid. Peptide samples were then spotted on a MALDI-TOF/TOF target plate for MS and MS/MS analyses. MS/MS, or tandem mass spectrometry, is a mass spectrometric method in which a peptide is fragmented, and the masses of the resultant fragment ions are recorded in a spectrum. The analyses were performed using the ABI 4800 MALDI-TOF/TOF (Applied Biosystems, Foster City, CA) at the Michigan Protein Consortium. Searches for homologies between the amino acid sequences obtained and those of other known proteins in GenBank™, GenPept, and SwissProt were performed using BLAST software. The Michigan Proteome Consortium provided proteomics data at the University of Michigan.
Preparation of 0-day to 3-day Conditioned Medium (CM0–3d)
Sf9 cells cultured at 28°C in T-25 flasks in 5 ml of Grace’s medium with 10% FBS and 50 µg/ml of Gentamycin were infected with DSP-PP240 virus for 3 days. A mock infection served as a control. Conditioned medium (CM) was harvested by centrifugation (500×g). CM containing DSP-PP240 precursor protein was designated “CM0–3d with viral infection” and CM from uninfected Sf9 cells was designated “CM0–3d without viral infection”.
Preparation of 3-day to 7-day Conditioned Medium (CM3–7d)
Sf9 cells culture and infection with DSP-PP240 virus as described above. After three day viral infection, the conditioned medium was removed and discarded. Cells were then gently washed with 1×PBS and cultured in 5 ml of fresh Grace’s medium with10% FBS and 50 µg/ml Gentamycin for an additional 4 days (from day 3 to day 7 after infections) at 28°C. Then the CM, containing largely uncleaved DSP-PP240 precursor protein, was collected on day 7 by centrifugation (500×g) and designated CM3–7d.
Testing for Proteolytic Activity in Condition Medium of Sf9 Cells
CM3–7d (5 ml) from virus-infected cells was incubated with CM0–3d (5 ml) from uninfected cells for different time periods at 28°C. The CM3–7d from infected cells contained uncleaved DSP-PP240 while the CM0–3d from uninfected cells contained endogenous proteolytic activity secreted by Sf9 cells. In parallel, CM3–7d (5 ml) from virus infected cells was incubated with Grace’s medium containing 10% FBS and 50 µg/ml Gentamycin (5 ml) as a control. At each time point, 1 ml samples of mixture was processed using a standard purification protocol [15]. Briefly, 1 ml medium was adjusted to 5% TCA, in which DSP-PP240, DSP430 and PP240 are soluble but other proteins in conditioned medium are precipitated, incubated at room temperature for 2 min and then centrifuges in an Eppendorf rotor at 12,000 rpm for 5 min at room temperature. The supernatant was neutralized with one-fifth volume of 3 M Tris-HCl pH 8.8 and then precipitated by adding one-tenth volume of 1 M CaCl2 and centrifuging in an Eppendorf rotor at 12,000 rpm for 5 min at room temperature. The pellet from 1 ml medium was resuspended in 100 µl of 0.1 M EDTA. 15 µl of resuspended samples were resolved onto native PAGE as described previously [15], and the gels were fixed and stained with Stains-All to visualize DSP-PP240 and the cleavage products DSP430 and PP240. Stained gels were subjected to quantitative densitometry. Because the PP240 portion of DSP-PP240 is responsible for the majority of staining by the Stains-All reagent, percentage of protein processing was defined as PP240/(PP240+ DSP-PP240).
Identification of Tolloid-related-1 Protein mRNA in Sf9 Cells
We aligned tolloid protein sequences from Drosophila melanogaster (GeneBank accession number NM_079763), Culex quinquefasciatus (GeneBank accession number XM_001861486), Aedes aegypti (GeneBank accession number XM_001653501) and Tribolium castaneum (GeneBank accession number XM_965069), and obtained consensus sequences, [i.e., QAMRHWE and IMHYA(R/K)N(T/S)) as shown in Fig. 1. Sense and anti-sense oligonucleotide primers were generated according to these consensus peptide sequences. Total RNA from Sf9 cells infected with DSP-PP240 virus for 3 days was extracted with TRIzol® Reagent (Invitrogen, Carlsbad, CA). Using RT-PCR, tolloid-related-1 cDNA fragments were generated from this RNA preparation. After cloning into a TOPO cloning vector (Invitrogen, San Diego, CA), candidate clones were identified by PCR and confirmed by DNA sequencing. The cDNA sequences were then used to search the NCBI non-redundant protein sequence database for homologous peptide sequences.
Figure 1. Alignment of consensus sequences of tolloid proteins from Culex qunquefasciatus, Aedes aegypti, Tribolium castaneum and Drosophila melanogaster.
Sequences of tolloid protein catalytic domains from the above four species were aligned to identify consensus sequences to be used for designing primer sequences. Consensus peptide sequences are indicated by yellow highlights. Arrows represent the positions of peptide sequences QAMRHWE and IMHYA(R/K)N(T/S) that were used to make sense primer (S) and anti-sense primer (AS). These S and AS primers encompass sequences for the Zn-binding motif (HExxHxxGFxHExxRxDRD) and for another conserved region, the Met-turn (SxMHY).
BMP1 Cleavage of DSP-PP240
CM3–7d from virus infected cells (5 ml) was incubated at 28°C with an equal volume of unconditioned (fresh) Grace’s medium in the presence of 33 ng/ml or 170 ng/ml of recombinant human BMP1 or with no enzyme addition for various times from 1 h to 72 h as indicated in the corresponding figure. At each of the time points, 1 ml of mixed medium was processed as described above. 15 µl of each resuspended sample was analyzed by native PAGE, fixed and stained with Stains-All and the percentage of protein processed was determined as described above.
Quantification of DSP-PP240 and PP240
Stained gels were dried, scanned to produce TIFF files and NIH ImageJ was used to quantify intensity of Stains-All stained bands. The stained protein band images were converted to grayscale and inverted and rectangular areas were used to integrate the intensity of the DSP-PP240 and PP240 bands. These intensity values were then used to calculate the ratio of PP240/(DSP-PP240+PP240). Each experiment was conducted at least 2 times and in most cases 3 or more times with comparable results. Plotted PP240/(DSP-PP240+PP240) ratios expressed as a percentage, represent the mean of at least two experiments and error bars represent the standard deviation of the mean. Data were analyzed and plotted using KaleidaGraph (Synergy Software, Reading, PA).
Results
DSP-PP240 Expressed in Sf9 Cells is Cleaved in Conditioned Medium at the Physiological Processing Site: SMQG447↓D448DPN
Previously, we showed that full-length DSP-PP240 can be expressed in Sf9 cells using a baculovirus vector and that the precursor is partially processed into stable fragments of sizes expected for the physiological products DSP430 and PP240 [15]. Based on mass spectrometric analysis, we identified a 7.7 kDa polyacrylamide gel band from trypsinized recombinant PP240 that likely represented a 76 residue peptide containing the presumed N-terminal sequence of PP240. Due to its large molecular mass, it was not possible to determine the sequence of the peptide directly [15]. To overcome this problem, we used chymotrypsin digestion of baculovirus-expressed PP240, which would be expected to produce a 34 amino acid peptide corresponding to the N-terminus of PP240 (DSP-PP240 residues 448–481) if the precursor was cleaved after G447. MS/MS analysis of this chymotryptic peptide provided the entire expected sequence: DDPNSSDESNGSDGSDDANSESAIENGNHGDASY. This finding demonstrates that baculovirus-produced recombinant PP240 protein starts with DDPN, which corresponds to the N-terminal sequence of phosphophoryn purified from dental matrix (see Fig. 2). Thus cleavage of DSP-PP240 precursor protein in the baculovirus expression system occurs at the physiological site, generating stable fragments that do not undergo further cleavage.
Figure 2. Mass spectrometric analyses of chymotrypsin digested mature PP240.
The location of MS/MS identified peptide sequence for PP240 recombinant protein is labeled in red. The chymotrypsin cleavage site is located C-terminal to the underlined Tyr residue (Y).
Processing of DSP-PP240 into DSP430 and PP240 Occurs after Secretion into Conditioned Medium and is Catalyzed by a Proteolytic Activity Secreted by Sf9 Cells
Continued cleavage of DSP-PP240 in CM0–3d into DSP430 and PP240 during additional 3-day incubation at 28°C
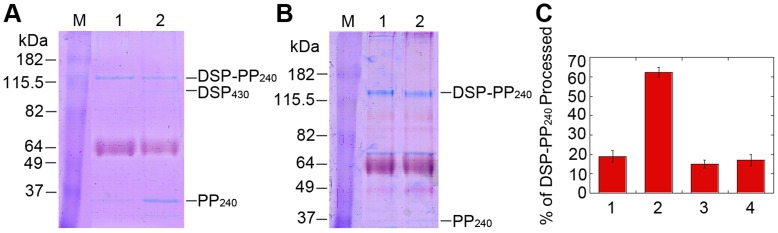
To determine the mechanism of cleavage of DSP-PP240 expressed in Sf9 cells, we first examined the time course of cleavage. In the conditioned medium from Sf9 cells infected with the DSP-PP240 virus for three days (CM0–3d), only a small fraction of DSP-PP240 was cleaved into mature PP240 (Fig. 3A, lane 1, Fig. 3C). After an additional 3 day incubation, DSP-PP240 precursor protein underwent further cleavage, resulting in accumulation of much higher levels of DSP430 and PP240 (Fig. 3A, lane 2). Because DSP430 is weakly stained by Stains-All relative to PP240, the DSP430 band was barely visible, and PP240 alone was therefore used as a quantitative marker for DSP-PP240 protein cleavage. Quantification of DSP-PP240 and PP240 bands showed that 18% of the DSP-PP240 was cleaved to PP240 in the CM0–3d sample and that this increased to 60% after additional 3 day incubation, indicating that significant additional DSP-PP cleavage occurred during the extended 3 day incubation period. Thus DSP-PP240 cleavage largely occurred after secretion into conditioned medium and that the conditioned medium contained a proteolytic activity that was active during the additional 3 day incubation.
Figure 3. Processing of baculovirus-encoded DSP-PP240 in conditioned medium requires an activity that is only secreted by Sf9 cells early but not late after infection.
(A) Processing of DSP-PP240 in conditioned medium of virus-infected Sf9 cells. Sf9 cells were infected with baculovirus containing the DSP-PP240 cDNA. In lane 1, 3 days after infection, CM0–3d was collected and processed for native PAGE and Stains-All staining as described in Materials and Methods. In lane 2, CM0–3d was incubated for an additional 3 days at 28°C before processing for PAGE. M represents protein size markers. (B) Medium conditioned by infected cells 3 to 7 days after infection lacks processing activity. 3 days after infection of Sf9 cells with baculovirus containing DSP-PP240, the medium was replaced with fresh Grace’s medium containing 10% FBS and 50 µg/ml Gentamycin and the cells were cultured for an additional 4 days. In lane 1, the resulting conditioned medium, CM3–7d was collected and processed for native PAGE and Stains-All staining. In lane 2, CM3–7d was incubated for an additional 3 days at 28°C before processing. M represents protein size markers. (C) Quantification of DSP-PP240 precursor processing. Using NIH J image, we measured the image intensity of DSP-PP240 and PP240 bands in gels shown in panels A & B and two other similar gels. Calculation of percent processing was as described in Materials and Methods. Because DSP staining was very weak by Stains-All staining, total density was defined as the sum of the DSP-PP240 and PP240 image densities. Numbers 1–4, respectively, refer to panel A lane 1, panel A lane 2, panel B lane 1 and panel B lane 2. Error bars represent standard deviation of at least duplicate samples.
DSP-PP240 in CM3–7d undergoes little cleavage into DSP430 and PP240 after an additional 3-day incubation at 28°C
To determine whether DSP-PP240 production continued beyond the third day after infection, the culture medium of Sf9 cells infected with the DSP-PP240 virus for 3 days was replaced with fresh medium and incubation was continued for 4 days. When this 3-to-7 day conditioned medium (CM3–7d) was analyzed, even larger amounts of DSP-PP240 were present than that in CM0–3d (compare Fig. 3A, lane 1 to Fig. 3B, lane 1), indicating that DSP-PP240 production continued during the additional 4 day incubation period. However, the PP240 cleavage product band was barely visible, representing only about 15% cleavage of the precursor (Fig. 3C). Even after an additional 3 day incubation in the same medium, the PP240 band was still barely visible (Fig. 3B, lane 2), implying that no significant additional cleavage occurred (Fig. 3C). These results demonstrate that, whereas DSP-PP240 processing was dramatically increased with the extended 3 day incubation of the CM0–3d sample, DSP-PP240 secreted into fresh medium after 3 days of infection (the CM3–7d sample) was largely uncleaved and remained so even when incubated for an additional 3 days (Fig. 3B). Thus the CM3–7d sample appeared to lack the DSP-PP processing activity.
CM0–3d from uninfected Sf9 cells contains a proteolytic activity that promotes DSP-PP240 precursor protein cleavage
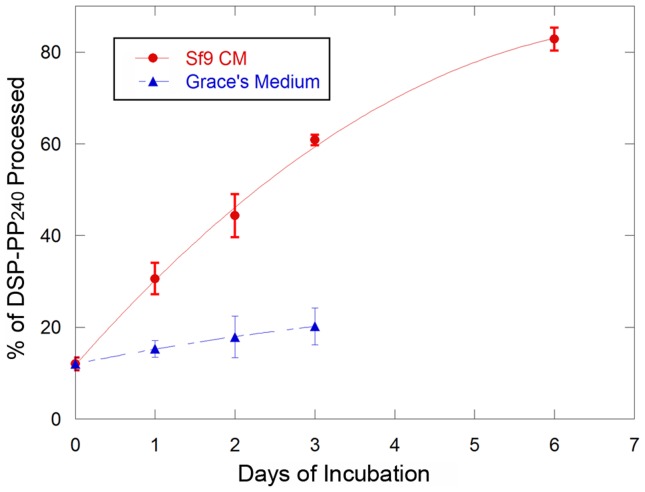
Because DSP-PP240 present in the CM3–7d media did not undergo cleavage even during extended incubation periods, we used this DSP-PP240 sample as substrate to test for the production of a DSP-PP240 processing activity by conditioned medium of uninfected Sf9 cells. To do this, we mixed (1∶1) CM3–7 from infected cells with medium conditioned by incubation for three days with uninfected Sf9 cells and incubated the mixture at 28°C. CM3–7d from infected cells was incubated with Grace’s medium as a control. Fig. 4 shows that, as a function of time, incubation of CM3–7d from infected cells with CM0–3d medium from uninfected Sf9 cells resulted in progressive cleavage of DSP-PP240 to PP240 that was roughly linear for the first 3 days of incubation and reached near completion (∼80% cleavage) after 6 days (Fig. 4, upper curve). Little cleavage was seen in the control containing unconditioned Grace’s medium (Fig. 4, lower curve). Therefore, Sf9 cells secrete an activity that correctly processes DSP-PP240, but secretion of this activity is greatly diminished or suppressed within 3 days after virus infection.
Figure 4. DSP-PP240 is cleaved by an activity secreted into conditioned medium by uninfected Sf9 cells.
CM3–7d from virus infected cells (containing most intact DSP-PP240), was mixed with an equal volume of 3d conditioned medium from uninfected Sf9 cells or with an equal volume of unconditioned Grace’s medium as described in Materials and Methods. At the times shown, medium samples were processed for native PAGE and Stains-All staining as described in Materials and Medthods. Percent processing of DSP-PP240 was determined as described in Materials and Methods. Error bars represent standard deviation of at least duplicate samples.
Identification of a Tolloid-related-1 Transcript in Sf9 Cells
Mammalian BMP1 was reported to be a protease capable of cleaving several extracellular matrix proteins, including the pro-α1 precursors of type I, type II, type III, and type VII collagen, the pro-α2 precursors of type I and type V collagen, the human prolysyl oxidase, and DMP1 [11], [16]–[21]. Because the cleavage site of DMP1 exhibits high sequence similarity to the putative cleavage site in DSP-PP [11], we asked whether a BMP1 equivalent protein was present in Sf9 cells.
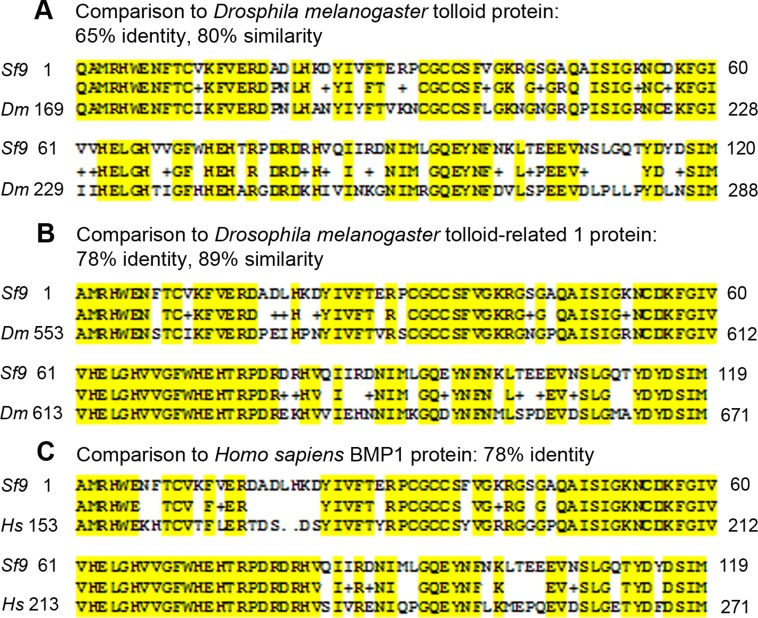
The proteins in Drosophila melanogaster most closely related to human BMP1 are Drosophila tolloid (TLD) and tolloid-related-1 (TLR1). We therefore investigated whether Sf9 cells contain tolloid-related-1 mRNA sequences. We first obtained consensus sequences by aligning tolloid sequences from Drosophila melanogaster, Culex quinquefasciatus, Aedes aegypti and Tribolium castan. Using this alignment, we designed consensus primers corresponding to the most highly conserved regions of the catalytic domains of these enzymes and performed RT-PCR on Sf9 cell mRNA. We obtained a partial cDNA sequence encoding an open reading frame with 65% peptide sequence identity to Drosophila tolloid protein (TLD) and 78% peptide sequence identity to Drosophila tolloid-related-1 protein (TLR1). Because of greater similarity to TLR1, we propose that this clone represents a partial cDNA of a Spodopteria frugiperda tlr1 mRNA. This cDNA encodes part of the astacin protease domain with the known consensus sequence HExxHxxGFxHExxRxDRD containing the zinc-binding motif, and another conserved region, SxMHY, the so-called Met-turn (see Fig. 5).
Figure 5. Partial Sf9 TLR1 sequence aligned with Drosophila TLD/TLR1 protein and Homo sapiens BMP1.
The upper in each case represents the cloned Sf9 TLR1 sequence and lower line the test sequence. (A) The peptide sequence derived from the cloned Sf9 tlr1 cDNA shared 65% sequence identity with Drosophila melanogaster TLD. (B) The peptide sequence derived from the cloned Sf9 tlr1 cDNA shared 78% sequence identity with Drosophila melanogaster TLR1. (C) The peptide sequence derived from the cloned Sf9 tlr1 cDNA shared 78% sequence identity with Homo sapiens BMP1.
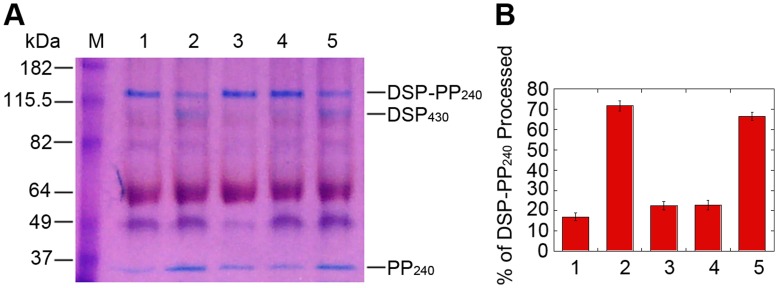
Because proteases in the astacin/TLD/BMP1 family are Zn-metallopeptidases, we tested EGTA and 1,10-phenanthroline [22], [23], both strong Zn++ chelators capable of inhibiting Astacin-type metallopeptidase activities, to determine whether these reagents could inhibit DSP-PP240 cleavage. As shown in Fig. 6, both 22 mM EGTA and 1 mM 1, 10-phenanthroline inhibited DSP-PP240 cleavage to DSP430 and PP240 in Sf9 cell-free medium. Moreover, inclusion of 1 mM ZnCl2 prevented inhibition by 1 mM 1,10-phenanthroline (Fig. 6A&B, compare lanes 4 and 5), demonstrating that DSP-PP240 processing activity secreted by Sf9 cells is a Zn-requiring enzyme.
Figure 6. Processing of DSP-PP240 in conditioned medium is Zn-dependent.
(A) Stains-All staining of DSP-PP240 cleavage. Lane 1: CM0–3d of virus-infected cells without further incubation. Lane 2: CM0–3d from virus-infected cells incubated for an additional 3days. Lane 3: Same reaction as lane 2 except with addition of EGTA (22 mM). Lane 4: Same reaction as lane 2 except with addition of 1mM 1,10-phenanthroline. Lane 5: Same reaction as lane 2 except with addition of 1 mM 1,10-phenanthroline and 1 mM ZnCl2. M represents size marker. (B) Quantification of DSP-PP240 processing shown in panel A and two similar gels. Numbers 1–5 correspond to lanes 1–5. Error bars represent standard deviation of the mean.
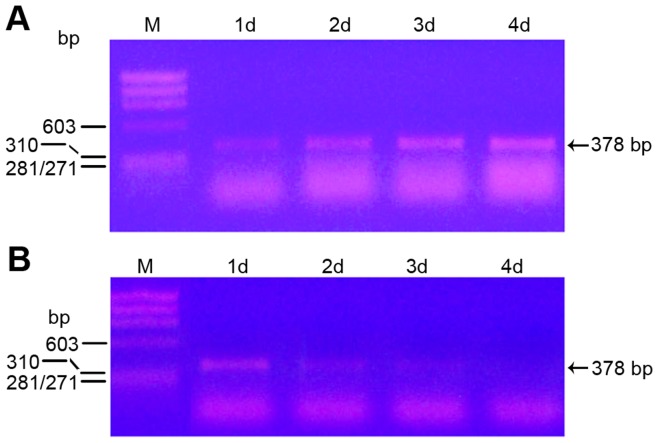
We next tested whether tlr1 mRNA was expressed by uninfected and DSP-PP240 viral infected Sf9 cells. Using the sense and antisense primers specific for Sf9 tlr1 cDNA, we performed RT-PCR on RNA from uninfected and DSP-PP240 viral infected Sf9 cells. In Sf9 cells without viral infection, tlr1 mRNA expression increased steadily from day 1 to day 4 (Fig. 7A). In Sf9 cells infected with DSP-PP240 virus, tlr1 mRNA expression was highest at 1 day post-infection but dropped dramatically from day 2 to day 4 post-infection (Fig. 7B). The decrease seen in expression of Sf9 tlr1 mRNA after virus infection is consistent with the lack of secretion of DSP-PP240 processing activity by 3 days after viral infection (Fig. 3B).
Figure 7. Tlr1 mRNA expression.
At the indicated time point, medium was removed, Sf9 cells were washed with PBS, and total RNA was extracted with RNAzole. Then reverse transcription was performed to generate a cDNA pool. Tlr1 sense and antisense primers were used to detect the presence of tlr1 mRNA at various time points. (A) cDNA pools (100 µl) were generated from 2 µg total RNA from uninfected Sf9 cells and 1 µl was used for PCR analyses. Lane 1: ϕx174 Hae III size marker. Lane2: 1d Sf9 cells. Lane3: 2d Sf9 cells. Lane 4: 3d Sf9 cells. Lane 5: 4d Sf9 cells. (B) cDNA pools (100 µl) were generated from 2 µg total RNA from Sf9 cells infected with baculovirus containing DSP-PP240 cDNA and 2 µl was used for PCR analyses. Lane 1: ϕ×174 Hae III size marker. Lane 2: 1d viral infection. Lane 3: 2d viral infection. Lane 4: 3d viral infection. Lane 5: 4d viral infection. The arrow indicates the position of the 378 bp partial tlr1 PCR fragment.
Cleavage of DSP-PP240 Precursor Protein by BMP1
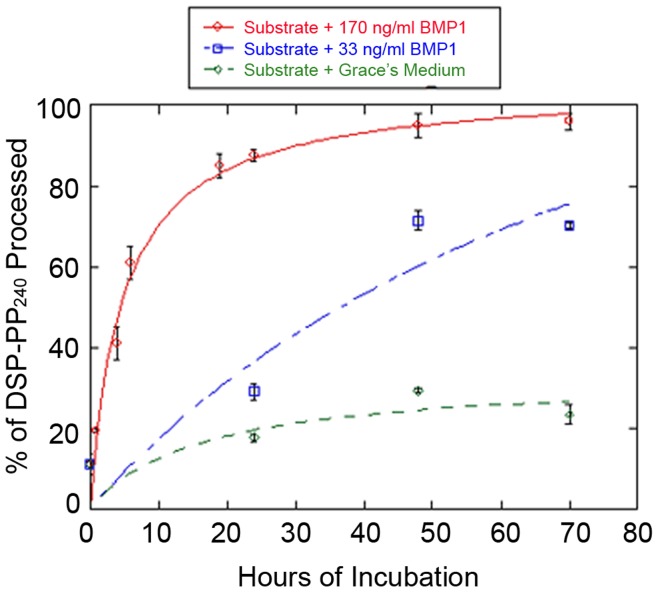
We next tested whether purified BMP1 could cleave DSP-PP240. For these studies we utilized CM3–7d medium samples, which contained significant quantities of stable DSP-PP240 precursor protein as substrate. Fig. 8 shows the kinetics of DSP-PP240 cleavage by BMP1 added to the CM3–7d substrate at concentrations of 33 ng/ml or 170 ng/ml. BMP1 cleaved DSP-PP240 precursor protein in a concentration-dependent manner yielding mature PP240, while control incubation with Grace’s medium yielded minimal cleavage.
Figure 8. DSP-PP240 is accurately cleaved by human BMP1.
CM3–7d from virus infected cells containing mostly unprocessed DSP-PP240 precursor protein was incubated with an equal volume of Grace’s medium alone or Grace’s medium containing 33 ng/ml or 170 ng/ml of human BMP1 for the indicated times at 28°C. At each time point, 1 ml samples were processed for native PAGE and Stains-All staining and gels quantified as describe in Materials and Methods. The data were fit to the Michaelis-Menton equation using KaleidaGraph. Error bars represent standard deviation of at least duplicate samples.
Taken together, the results presented here show clearly that DSP-PP240 precursor secreted by viral infected Sf9 cells is cleaved after secretion, at the known physiological cleavage site, by an endogenous Zn-metalloproteinase activity secreted by Sf9 cells to yield DSP430 and PP240 proteins. We have shown that BMP1 is also capable of correct cleavage of DSP-PP240 produced in the baculovirus system. We have shown further that Sf9 cells express an mRNA encoding the BMP1 homolog, TLR1. A reasonable interpretation of these results is that the specific cleavage of DSP-PP240 in the Sf9 cells expression system represents the action of a highly conserved processing enzyme, TLR1, on the mammalian substrate.
Discussion
Expression of the DSP-PP precursor protein is essential for normal tooth development due to requirement for PP in dental matrix mineralization. As a result, elucidating the mechanism and regulation of DSP-PP maturation is fundamental to understanding matrix formation. As shown here, baculovirus expression of DSP-PP240 can provide a valuable model with which to address key questions about the maturation of DSP-PP. The baculovirus expression system permits the purification of substantial quantities of DSP-PP240 precursor for biochemical analysis of processing. Like the precursor produced by odontoblasts, DSP-PP240 produced in Sf9 cells is extensively glycosylated and phosphorylated (Ritchie HH, unpublished results).
Previously [15], we found that recombinant DSP-PP240, isolated using TCA precipitation, neutralization, calcium precipitation, and EDTA resuspension followed by preparative native PAGE, underwent spontaneous cleavage into DSP430 and PP240 when incubated at 37°C. We considered it unlikely that a contaminating protease in gel-purified DSP-PP240 was responsible for cleavage because of the harsh, denaturing conditions used in the purification. Indeed, the low pH and chelation steps should have inactivated Zn-dependent peptidases. Regardless of these results, we have shown here that DSP-PP240 is secreted into the conditioned medium intact, where it undergoes accurate and specific processing at the physiological cleavage site by a Zn-metalloproteinase secreted by Sf9 cells to generate stable fragments that correspond to the physiological cleavage products DSP430 and PP240.
In this study, we compared the fate of DSP-PP240 secreted into conditioned medium in the first 3 days after viral infection (CM0–3d) to that of DSP-PP240 secreted into freshly added medium during days 3–7 after infection (CM3–7d). Surprisingly, while significant amounts of mature DSP430 and PP240 were generated by incubation of the CM0–3d sample, little, if any cleavage of DSP-PP240 occurred in the CM3–7d sample on extended incubation, suggesting that a processing enzyme secreted by Sf9 cells within the first 3 days after infection, was no longer secreted after the first three days. Using CM3–7d media, which contained stable DSP-PP240, we could test whether a processing enzyme was secreted by uninfected Sf9 cells. We were able to demonstrate that CM0–3d media from uninfected Sf9 cells contained a Zn-dependent proteolytic activity that cleaved the DSP-PP240 into DSP430 and PP240 (Fig. 4 and Fig. 6).
Sf9 cells are routinely used for expression of recombinant proteins encoded by baculovirus vectors. Our identification of a partial tlr1 cDNA from Sf9 cells is a new finding that may prove useful to others interested in examining the cleavage of Sf9-produced recombinant proteins. Although we do not have definitive evidence that the DSP-PP240 processing activity is Sf9 TLR1, the fact that the activity is secreted and Zn-dependent and that it cleaves DSP-PP240 selectively at the physiological cleavage site makes it likely that this is the case. Moreover, the decline in expression of tlr1 mRNA after infection (Fig. 7B), which may be due to baculovirus inhibition of the endogenous gene expression, paralleled the decline seen in DSP-PP240 cleavage activity in the conditioned medium (Fig. 3B).
As we have shown here, the decrease in endogenous processing activity after infection can be used to produce unprocessed DSP-PP240 that can be used as substrate in studies using exogenously added protease. Using this approach, we have shown that BMP1 can accurately process baculovirus-expressed DSP-PP240 to produce stable PP240 and DSP430. These data are consistent with BMP1, or one of the related enzymes TLL1 or TLL2, being the physiological processing enzyme for DSP-PP. BMP1 belongs to the astacin family of metalloproteinases [24], [25] and is present in many tissues [26]. BMP1 was reported to be involved in the cleavage of several extracellular matrix proteins such as the pro-α1 precursors of type I, type II, type III, and type VII collagen, the pro-α2 precursors of type I and type V collagen [16]–[20], and the human prolysyl oxidase [21]. In bone and teeth, as well as in non-mineralized tissues, BMP1 is required for cleavage of such extracellular matrix proteins as procollagen, to provide mature proteins that can then go on to effect both hard and soft tissue maturation. DSP-PP is also found in non-mineralized tissues (i.e., kidney, hair follicle, salivary gland and lung) that also contain BMP1, suggesting a role for BMP1 cleavage in precursor maturation in these tissues as well.
Taken together, our results suggest that the specific and physiologically accurate cleavage of DSP-PP240 in the Sf9 cell system represents the action of a highly conserved processing enzyme.
Based on our results with the Sf9 cell system, we favor a model in which DSP-PP is cleaved after secretion into dentin matrix by BMP1 (or TLL1 or TLL2). This is suggested by the observation that although DSP-PP240 and the Zn-dependent processing activity are co-expressed and co-secreted by Sf9 cells, the majority of DSP-PP240 processing cannot be occurring until after secretion. Key questions raised by this model that can be addressed in the baculovirus system are why processing is delayed until secretion and how cleavage is prevented until after secretion if DSP-PP and BMP1 are co-secreted. Delay of processing may be necessary if the DSP portion of the precursor is required to prevent premature oligomerization of PP, which has been found to form large paracrystalline aggregates when expressed without the DSP portion [27]. Intracellular processing may be prevented if DSP-PP240 undergoes a conformational change upon secretion, possibly due to high extracellular calcium that promotes efficient cleavage by BMP1 or a related enzyme. Finally, it is of interest to know whether interaction with extracellular calcium protects mature DSP and PP from further proteolysis.
Acknowledgments
We want to thank Dr. David Ritchie for helpful discussions in experimental design and help with editing the manuscript. We also want to thank Dr. Philip Andrew for suggesting the use chymotrypsin to obtain the PP240 N-terminal sequence.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health NIDCR DE18901 to HHR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Butler W, Ritchie H. The nature and functional significance of dentin extracellular matrix proteins. Int Dev Biol. 1995;39:169–179. [PubMed] [Google Scholar]
- 2.Xiao S, Yu C, Chou X, Yuan W, Wang Y, et al. Dentinogenesis imperfecta 1 with or without progressive hearing loss associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Zhao J, Li C, Gao S, Qiu C, et al. DSPP mutation in dentinogenesis imperfecta shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 4.Godovikova V, Li XR, Saunders TL, Ritchie HH. A rat 8 kb DSP-PP promoter directs cell-specific lacZ activity in multiple mouse tissues. Development Biology. 2006;289:507–516. doi: 10.1016/j.ydbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Alvares K, Kanwar YS, Veis A. Expression and potential role of dentin phosphophoryn (DPP) in mouse embryonic tissues involved in epithelial-mesenchymal interactions and branching morphogenesis. Dev Dyn. 2006;235:2980–2990. doi: 10.1002/dvdy.20935. [DOI] [PubMed] [Google Scholar]
- 6.Tang X, Zhu Y, Marcelo C, Ritchie H. Expression of mineralized tissue associated proteins: Dentin sialoprotein and phosphophoryn in rodent hair follicles. J Dermat Sci. 2011;64:92–98. doi: 10.1016/j.jdermsci.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin C, Cook RG, Orkiszewski RS, Butler WT. Identification and characterization of the carboxyl-terminal region of rat dentin sialoprotein. J Biol Chem. 2001;276:904–909. doi: 10.1074/jbc.M006271200. [DOI] [PubMed] [Google Scholar]
- 8.Qin C, Baba O, Butler W. Post-translational Modifications of SIBLING Proteins and Their Roles in Osteogenesis and Dentinogenesis. Critical Reviews in Oral Biology & Medicine. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 9.Qin C, Brunn J, Cook R, Orkiszewski R, Malone J, et al. Evidence for the Proteolytic Processing of Dentin Matrix Protein 1. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Lu Y, Chen S, Prasad M, Wang X, et al. Key proteolytic cleavage site and full-length form of DSPP. J Dent Res. 2010;89:498–503. doi: 10.1177/0022034510363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiglitz B, Auala M, Narayanan K, George A, Greenspan D. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 12.von Marschall Z, Fisher L. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix 2 products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biology. 2010;29:295–303. doi: 10.1016/j.matbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamakoshi Y, Hu J, Iwata T, Kobayashi K, Fukae M, et al. Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem. 2006;281:38235–38243. doi: 10.1074/jbc.M607767200. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya S, Simmer J, Hu J, Richardson A, Yamakoshi F, et al. Astacin Proteases Cleave Dentin Sialophosphoprotein (Dspp) to Generate Dentin Phosphoprotein (Dpp). J Bone Mineral Res. 2011;26:220–228. doi: 10.1002/jbmr.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godovikova V, Ritchie HH. Dynamic Processing of Recombinant Dentin Sialoprotein-Phosphophoryn Precursor Protein. Journal of Biological Chemistry. 2007;282:31341–31348. doi: 10.1074/jbc.M702605200. [DOI] [PubMed] [Google Scholar]
- 16.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan D. Bone Morphogenetic Protein-1: The Type I Procollagen C-Proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Sieron A, Fertala A, Hojima Y, Arnold W, et al. The C-Proteinase that Processes Procollagens to Fibrillar Collagens is Identical to the Protein Previously Identified as Bone Morphogenic Protein-1. Proc Natl Acad Sci U S A. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappano W, Steiglitz B, Scott I, Keene D, Greenspan D. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol. 2003;23:4428–4438. doi: 10.1128/MCB.23.13.4428-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott I, Blitz I, Pappano W, Imamura Y, Clark T, et al. Mammalian BMP-1/Tolloid-Related Metalloproteinases, Including Novel Family Member Mammalian Tolloid-Like 2, Have Differential Enzymatic Activities and Distributions of Expression Relevant to Patterning and Skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 20.Unsold C, Pappano W, Imamura Y, Steiglitz B, Greenspan D. Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. J Biol Chem. 2002;277:5596–5602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- 21.Uzel M, Scott I, Babakhanlou-Chase H, Palamakumbura A, Pappano W, et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276:22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 22.Felber J, Coombs T, Vallee B. The Mechanism of Inhibition of Carboxypeptidase A by 1,l0-Phenanthroline. Biochemistry. 1962;1:231–238. doi: 10.1021/bi00908a006. [DOI] [PubMed] [Google Scholar]
- 23.Salvesen G, Nagase H. Inhibition of proteolytic enzymes in Proteolytic enzymes: a practical approach (Beynon, RJ and Bond, JS) 2nd Eds. 2001;1:105–130. [Google Scholar]
- 24.Bond J, Beynon R. The Astacin Family of Metalloendopeptidases. Protein Science. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterchi E, Stöcker W, Bond J. Meprins, Membrane-bound and Secreted Astacin Metalloproteinases. Molecular Aspects of Medicine. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su A, Wiltshire T, Batalov S, Lapp H, Ching K, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. PNAS USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Olton D, Lee D, Kumta P, Sfeir C. Cell Derived Hierarchical Assembly of a Novel Phosphophoryn-Based Biomaterial. Cells Tissues Organs 2009. 2009;189:252–255. doi: 10.1159/000158571. [DOI] [PMC free article] [PubMed] [Google Scholar]