Abstract
Background & Aims
Nonalcoholic fatty liver disease (NAFLD) is a cardiovascular risk factor. Although modest alcohol consumption may reduce the risk for cardiovascular mortality, whether patients with NAFLD should be allowed modest alcohol consumption remains an important unaddressed issue. We aimed to evaluate the association between modest alcohol drinking and nonalcoholic steatohepatitis(NASH), among subjects with NAFLD.
Methods
In a Cross-sectional analysis of adult participants in the NIH NASH Clinical Research Network, only modest or non-drinkers were included: participants identified as 1) drinking > 20gm/day, 2) binge drinkers, or 3) non-drinkers with previous alcohol consumption were excluded. The odds of having a histological diagnosis of NASH and other histological features of NAFLD were analyzed using multiple ordinal logistic regression.
Results
The analysis included 251 lifetime non-drinkers and 331 modest drinkers. Modest drinkers compared to nondrinkers had lower odds of having a diagnosis of NASH (Summary odds ratio 0.56, 95%CI 0.39–0.84, p=0.002). The odds of NASH decreased as the frequency of alcohol consumption increased within the range of modest consumption. Modest drinkers also had significantly lower odds for fibrosis (OR 0.56 95%CI 0.41–0.77) and ballooning hepatocellular injury (OR 0.66 95%CI 0.48–0.92) than lifetime non-drinkers.
Conclusions
In a large, well-characterized population with biopsy-proven NAFLD, modest alcohol consumption was associated with lesser degree of severity as determined by lower odds of the key features that comprise a diagnosis of steatohepatitis, as well as fibrosis. These findings demonstrate the need for prospective studies and a coordinated consensus on alcohol consumption recommendations in NAFLD.
Keywords: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, alcohol, liver biopsy
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the United States (U.S.) affecting as many as one third of adults[1]. Only a small subset of patients with NAFLD, namely those with a more severe subtype known as steatohepatitis (NASH), which is characterized by inflammatory infiltrates, ballooning hepatocellular injury, and fibrosis in addition to steatosis, are thought to be at risk for cirrhosis related mortality.
The metabolic risk factors for NAFLD are also closely associated with coronary heart disease (CHD) [2]. Patients with NAFLD[3], and especially those with NASH[4], are at risk for coronary heart disease. Patients with NAFLD are approximately two times more likely to die from coronary heart disease than liver disease[5]. Therefore management of CHD risk in patients with NAFLD is imperative. CHD risk can be modified through lifestyle changes, including diet, exercise and smoking cessation. In addition, modest alcohol consumption has been shown to reduce the risk of coronary heart disease mortality and improve metabolic risk factors related to both coronary heart disease and NAFLD [6, 7]. As many as 50% of the adults in the United States regularly consume a modest amount of alcohol[8]. Excessive alcohol, however, can cause alcoholic liver disease[9]. In the general population, the daily threshold [10–12] of alcohol for liver injury is thought to be between 1–3 drinks per day in women and 2–3 in men. In patients with metabolic risk factors for NAFLD, the threshold may be lower [13]. Despite this, cross-sectional studies have suggested that modest alcohol consumption may protect the liver from NASH and NAFLD [14, 15]. The relationship between modest alcohol consumption and NAFLD severity has not been analyzed in detail. Whether patients with NAFLD should abstain from alcohol or be allowed modest alcohol consumption remains an important question. In practice, physicians often recommend abstinence from alcohol for patients with NAFLD, although the data to support this approach are lacking.
To provide counseling on alcohol consumption for patients with NAFLD, it is important to know whether modest alcohol consumption is associated with NAFLD disease severity. We hypothesize that modest alcohol consumption is associated with lower prevalence of NASH in patients with NAFLD. The primary aim of this study was to investigate a potential association between modest alcohol drinking and steatohepatitis in patients with NAFLD. Secondary aims were to test the association between modest alcohol drinking and the individual histological features of NAFLD including fibrosis.
METHODS
Study Sample
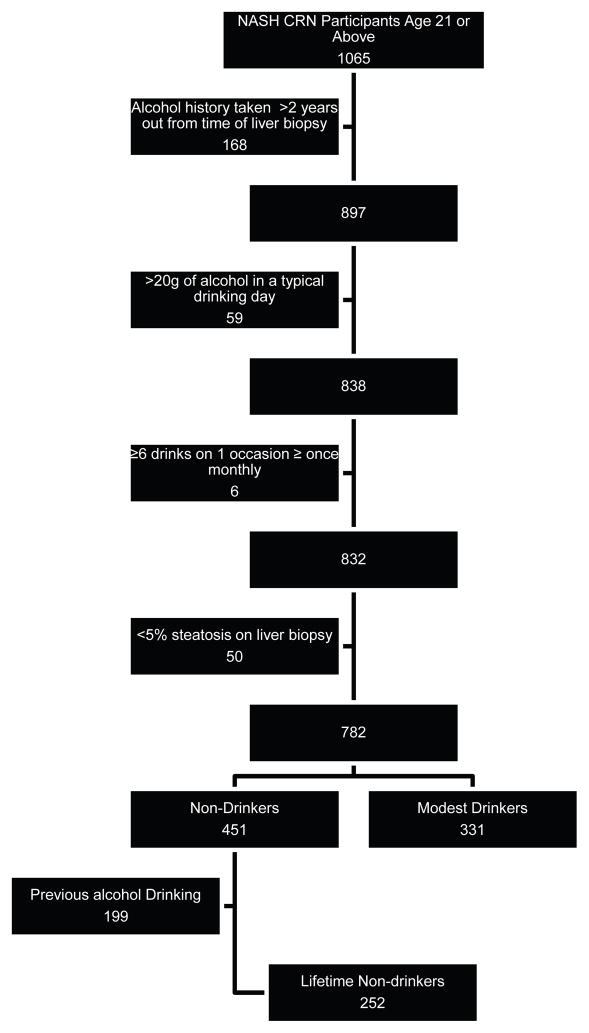
This was a cross-sectional study of the association between modest alcohol consumption and the histological presence and / or severity of recognized lesions in NAFLD. We included baseline data from participants 21 years or older enrolled in two recently published NASH Clinical Research Network (CRN) studies: (1) a cohort study, the NAFLD Database[16]; and (2) a clinical trial, Pioglitazone versus Vitamin E versus Placebo for the Treatment of Non-diabetic Patients with Nonalcoholic Steatohepatitis (PIVENS; Clinical Trial number NCT00063622) [17]. For the NAFLD Database, inclusion required histological diagnosis of NAFLD, imaging suggestive of NAFLD, histological diagnosis of cryptogenic cirrhosis, or clinical evidence of cryptogenic cirrhosis. Patients were excluded if they had other forms of liver diseases, or average alcohol consumption >20gm daily during the 2 years before entry. PIVENS inclusion additionally required patients to have histological evidence of NASH without cirrhosis and the absence of diabetes. Details of the study design can be found elsewhere [16, 17]. The dataset for the current analysis was made up of participants who had central review of pathology completed as of May 2010. The inclusion and exclusion flowchart is illustrated in Figure 1. A minimum age of 21 was chosen for inclusion in this analysis because it is the legal drinking age in the U.S. Participants who had liver biopsy more than 24 months before completing the Alcohol Use Disorders Identification Test (AUDIT) were excluded. Participants consuming on average more than 140g of alcohol per week were already excluded as a part of the enrollment criteria in the NASH CRN. In addition those reporting consumption of more than 2 drinks of alcohol in a typical drinking day and those reporting binge drinking at least once a month were excluded. In order to reduce potential selection bias that subjects may also stop drinking alcohol because of illness related to alcohol, non-drinkers who previously drank alcohol were also excluded. Finally, participants whose biopsy did not have at least 5% steatosis on central reading by the NASH CRN Pathology Committee were not considered to have NAFLD and were excluded from these analyses. Study protocols were approved by all participating center Institutional Review Boards. Each participant provided written informed consent.
Figure 1.
Inclusion and Exclusion Flow Chart.
Histological Features
The primary outcome for this analysis was the diagnosis of steatohepatitis, reported as none, borderline or definite, by the central review by the Pathology Committee. The assignment of a diagnostic category was based on consensus recognition of the global histological features including those characteristic of steatohepatitis including steatosis and ballooning hepatocellular injury with a zone 3 predominance as well as lobular inflammation.[18]. Secondary outcomes included the following histological variables: fibrosis (stage 0, 1, 2, 3, 4), steatosis (5–33%, 34–66%, >66%), lobular inflammation (<2, 2–4 and >4 under 20X magnification), portal inflammation (none, mild, more than mild), ballooning hepatocellular injury(none, few, many), microvesicular steatosis (absent, present), Mallory-Denk bodies (absent or rare, many), megamitochondria (absent or rare, many), acidophil bodies (absent or rare, many), large lipogranulomas (absent, present).
Alcohol Consumption
The primary exposure was modest alcohol consumption compared to lifetime abstinence from alcohol. Current alcohol consumption was assessed using the AUDIT[19]. Participants were asked “how often do you have a drink containing alcohol?” Those who responded “never” were considered non-drinkers. Participants were subsequently asked “how many drinks containing alcohol do you have on a typical day when you are drinking?” Those who reported drinking more than 2 drinks on a drinking day were excluded. Participants were asked “how often do you have six or more drinks on one occasion”. Those who reported binge drinking once monthly or more were excluded because previous publications have indicated that episodic heavy drinking as little as once a month is associated with fibrosis progression in patients with NAFLD[20].
Prior alcohol consumption was measured using the Lifetime Drinking History questionnaire[21]. Participants were asked “Over the course of your lifetime have you ever had at least one drink of alcohol, beer, liquor, wine, or wine coolers, per month during a 12-month time period, or at least three drinks per day for at least three consecutive days?” Non-drinkers who responded “yes” to this lifetime drinking history question were also excluded as being previous drinkers.
Social, Demographic and Lifestyle Confounders
Social, demographic and lifestyle confounders including age, gender, race, income, education and physical activity level were collected using standardized questionnaires. Race and ethnicity were categorized into Asian, Hispanic, non-Hispanic white, Pacific Islander and others. Annual household income was categorized into <$30,000, $30,000 to $50,000, or more than $50,000. Education was categorized into less than high school graduation, high school graduation, some college, and bachelor’s degree or higher. Smoking history was categorized as never, previous and current smoker. Dietary variables include total calories per day, percent calories from carbohydrates and percent calories from fat. Number of METS per week for non-recreational activity and recreational activity was calculated from self-reported physical activity assessed using the Physical Activity Questionnaire[22]. Height and weight were measured in a standardized fashion and body mass index (BMI) was calculated as weight[kg]/height[m]2.
Patatin-like Phospholipase Domain-containing Protein 3 (PNPLA3) Genotyping
Recently a nonsynonymous sequence variation (rs738409) that substitutes methionine for isoleucine at codon 148 in the gene encoding patatin-like phospholipase domain-containing (PNPLA3) has been shown to be associated with hepatic steatosis [23], as well as the severity of histological injury in both NAFLD[24] and in alcoholic liver disease[25]. Therefore, we included these data to determine whether the relationship between modest alcohol consumption and the odds of having NASH differed by rs738409 genotype (CC, CG, GG). Genotyping for rs738409 was done using the Sequenom MassARRAY iPLEX Gold platform (Sequenom, Inc., San Diego, CA) and recently published by the NASH CRN[24].
Statistical Analysis
Most histological features were ordinally graded. To account for the ordinal nature of histological features, we used multiple ordinal logistic regression to address the association between the histological features and modest alcohol drinking. Under the proportional odds assumption, the odds ratio is the same regardless of how the histological features are dichotomized. The proportional odds assumption was verified using the score test. Multiple regression analyses adjusted for the social, demographic and lifestyle confounders listed above. Income, non-recreational and recreational activity data were missing in 37 participants. Rather than excluding these participants from statistical analysis, multiple imputation was used to replace each missing value with five imputed values in five complete datasets. Multiple regression analysis was performed on each of the five datasets and summary statistics were generated using the SAS procedure MIANALYSE. The dose response association of alcohol frequency and steatohepatitis was tested using Jonckheere-Terpstra trend test, which is similar to the Cochran-Armitage trend test but allows the response to be ordinal rather that binomial. While there was no evidence of interaction, we performed a gender-based sub-analysis because there is a considerable body of literature describing gender-based differences as well as threshold differences in risk for liver injury between men and women. A PNPLA3 genotyped based sub-analysis was also performed to measure the effect of modest alcohol consumption in the CC, GC and GG genotype. SAS 9.1 (SAS Institute Inc., Cary. NC) was used for statistical analysis.
Sensitivity Analyses
In order to address potential biases, 5 sets of sensitivity analysis were performed. Participants might change their alcohol consumption over time. While the main analysis excluded participants with liver biopsy more than 24 months before completing the alcohol history, sensitivity analysis #1 further excluded participants with liver biopsy more than 12 months old. Current nondrinkers who previously stopped drinking may have different histology than lifetime non-drinkers. The main analysis excluded non-drinkers who previously had higher alcohol consumption. Sensitivity analysis #2 included the non-drinkers who were previous drinkers. The main analysis used multiple imputation to handle missing confounder variables. Sensitivity analysis #3 excluded participants with missing values on confounders rather than using multiple imputation. A person may discontinue drinking after a diagnosis of diabetes. The main analysis did not include the history of diabetes as a confounder because diabetes can potentially be in the causal pathway. Sensitivity analysis #4 additionally controlled for a history of diabetes, while sensitivity analysis #5 excluded participants with a history of diabetes.
RESULTS
Study Sample
The study sample included 252 lifetime non-drinkers and 331 modest drinkers enrolled in the NASH CRN studies with central pathology readings. The social, demographical, lifestyle and metabolic characteristics of the two groups are presented in Table 1. As compared to lifetime non-drinkers, modest drinkers were more likely to be male, have higher income and education, have higher insulin sensitivity and HDL, and less likely to have diabetes.
Table 1.
Social, Demographic, Lifestyle and metabolic characteristics of modest drinkers and lifetime non-drinkers
| Life time non-drinkers (N= 251) | Modest Drinkers (N = 331) | p | |
|---|---|---|---|
| Age, mean (SD), year | 49.0(12.0) | 47.7(11.7) | 0.16 |
| Gender, n (%) | 0.007 | ||
| Male | 70(27.9) | 128(38.7) | |
| Female | 181(72.1) | 203(61.3) | |
| Race, n (%) | 0.12 | ||
| White | 179(71.3) | 261(78.9) | |
| Hispanic | 36(14.3) | 34(10.3) | |
| Asians or Pacific Islander | 15(6.0) | 20(6.0) | |
| Other | 21(8.4) | 16(4.8) | |
| Income, n (%) | 0.001 | ||
| <30K | 68(27.4) | 48(14.8) | |
| 30–50K | 58(23.4) | 64(19.7) | |
| >50K | 122(49.2) | 213(65.5) | |
| Education, n (%) | <0.001 | ||
| <High School | 39(15.6) | 11(3.3) | |
| High School Graduation | 73(29.2) | 61(18.4) | |
| Some College | 84(33.6) | 117(35.4) | |
| Bachelor or Higher | 54(21.6) | 142(42.9) | |
| Diabetes, n (%) | 94(37.5%) | 73(22.1%) | <0.001 |
| BMI, mean (SD), kg/m2 | 35.1(7.0) | 33.8(6.2) | 0.04 |
| Waist, mean (SD), cm | 0.17 | ||
| Male | 113(15) | 110(12) | |
| female | 107(14) | 106(14) | |
| Hip, mean (SD), cm | 0.23 | ||
| Male | 113(12) | 112(10) | |
| Female | 120(16) | 118(15) | |
| WHR,, mean (SD) | 0.77 | ||
| Male | 1.00(0.06) | 0.98(0.06) | |
| female | 0.90(0.07) | 0.90(0.07) | |
| SBP, mean (SD), mmHg | 133(17) | 132(14) | 0.55 |
| DBP, mean (SD), mmHg | 76(11) | 77(10) | 0.24 |
| triglyceride, mean (SD), mg/dl | 187(155) | 178(119) | 0.51 |
| HDL, mean (SD), mg/dl | 0.007 | ||
| Male | 37(10) | 41(13) | |
| Female | 47(11) | 48(13) | |
| QUICKI, mean (SD) | 0.31(0.03) | 0.31(0.03) | 0.03 |
| Work Related Activity, mean (SD), METS /wk | 80(43) | 83(39) | 0.52 |
| Non-work Related Activity, mean (SD), METS / wk | 35(29) | 39(39) | 0.12 |
| Smoking | 0.02 | ||
| Never | 160(63.7) | 171(52.1%) | |
| Past | 69(27.5%) | 121(36.9%) | |
| Current | 22(8.8%) | 36(11.0%) | |
| Total Calorie | 1756(923) | 1898(869) | 0.57 |
| %Carbohydrate | 49.3(9.5) | 46.0(8.3) | <0.001 |
| %fat | 37.6(7.9) | 39.4(7.3) | 0.006 |
| %protein | 15.1(3.6) | 15.9(2.9) | 0.004 |
Prevalence of Histological Features in Lifetime Non-drinkers and Modest Drinkers
The frequency and adjusted odds ratio for each histological feature are presented in Table 2. The primary outcome, steatohepatitis, was present in 69.8% of lifetime non-drinkers and 53.2% of modest drinkers. Compared to nondrinkers, modest drinkers had 0.49 (95% CI 0.33 – 0.72) times the adjusted odds of having steatohepatitis, and 0.64 (95% CI 0.40 – 1.03) times the adjusted odds of having steatohepatitis or borderline steatohepatitis. The proportional odds assumption was verified using the Score test. The adjusted summary odds ratio was 0.52 (95% CI 0.36 – 0.76). Refer to table 3 for the univariable and multivariable summary odds ratios adjusting for each confounder. In addition, the association was stronger in those who drank more frequently. For modest drinkers who drank ≤ once weekly vs. ≥ twice a week, the adjusted summary odds were 0.54 (95% CI 0.38 – 0.79) and 0.24 (95% CI 0.10 – 0.55) respectively. The Jonckheere-Terpstra Test for dose response was highly significant (p < 0.0001). In gender based sub-group analysis, male modest drinkers had 0.47 (95% CI 0.24 – 0.91) time the adjusted summary odds of having steatohepatitis, while female had 0.57 (95%CI 0.36 – 0.90) times the odds. For male modest drinkers who drank ≤ once weekly vs. ≥ twice a week, the adjusted summary odds were 0.49 (95% CI 0.25 – 0.97) and 0.24 (95% CI 0.07 – 0.79) respectively. For female modest drinkers, the odds were 0.55 (0.37 – 0.79) and 0.24 (0.1 – 0.55) respectively.
Table 2.
Prevalence and Adjusted* Odds ratio for Histological Features in Lifetime non-drinkers and Modest Drinkers
| Life Time Non Drinker | Modest Drinker | Adjusted Summary Odds Ratio (95% Confidence Interval) | p | ||
|---|---|---|---|---|---|
| Steatohepatitis | None | 16.3% | 22.7% | 0.52(0.36 – 0.76) | 0.0006 |
| Borderline | 13.9% | 24.2% | |||
| Definite | 69.8% | 53.2% | |||
| Macrovesicular Steatosis | 5–33% | 44.8% | 41.7% | 1.11(0.79 – 1.55) | 0.56 |
| 34–66% | 32.9% | 35.6% | |||
| >66% | 22.2% | 22.7% | |||
| Microvesicular Steatosis | Absent | 86.1% | 92.7% | 0.57(0.31 – 1.06) | 0.57 |
| Present | 13.9% | 7.3% | |||
| Lobular Inflammation* | <2 foci | 52.2% | 51.1% | 1.09(0.77 – 1.55) | 0.63 |
| 2–4 foci | 37.3% | 36.0% | |||
| >4 foci | 10.7% | 13.0% | |||
| Portal Inflammation | None | 9.5% | 18.7% | 0.69(0.48 – 1.00) | 0.052 |
| mild | 64.3% | 62.8% | |||
| >mild | 26.2% | 18.4% | |||
| Fibrosis Stage† | 0 | 19.9% | 30.0% | 0.56(0.41 – 0.78) | 0.0005 |
| 1 | 24.7% | 33.6% | |||
| 2 | 22.3% | 15.6% | |||
| 3 | 20.3% | 14.7% | |||
| 4 | 12.7% | 6.1% | |||
| Ballooning Degeneration | None | 25.8% | 37.8% | 0.62(0.45 – 0.87) | 0.006 |
| Few | 26.2% | 26.3% | |||
| Many | 48.0% | 36.0% | |||
| Mallory-Denk bodies | Rare/absent | 65.1% | 74.9% | 0.65(0.43 – 0.97) | 0.04 |
| Many | 34.9% | 25.1% | |||
| Acidophil Bodies | Rare/absent | 71.0% | 68.3% | 1.16(0.79 – 1.72) | 0.46 |
| Many | 29.0% | 31.7% | |||
| Large Lipogranulomas | Absent | 57.5% | 61.3% | 0.73(0.50 – 1.07) | 0.11 |
| Present | 42.5% | 38.7% | |||
| Megamitochondria | Rare/absent | 81.7% | 87.0% | 0.71(0.43 – 1.18) | 0.19 |
| Many | 18.3% | 13.0% |
Multivariate models adjusted for gender, age, race, income, education BMI, recreational and non-recreational physical activity, smoking, total calories per day, percent calories from carbohydrates and percent calories from fat
Foci per 200x field
Fibrosis stage 0 = none, stage 1 = perisinusoidal or periportal, stage 2 = perisinusoidal and portal / periportal, stage 3 = bridging fibrosis, stage 4 = cirrhosis
Table 3.
Summary Odds Ratio for Steatohepatitis in Modest Drinkers compared to Lifetime non-drinkers, adjusting for each confounders
| Model | Summary Odds Ratio |
|---|---|
| Univariable | 0.53(0.45 – 0.61) |
| Adjusted for | |
| Gender | 0.55(0.47 – 0.64) |
| Age | 0.53(0.46 – 0.62) |
| Race / Ethnicity | 0.53(0.45 – 0.61) |
| Income | 0.54(0.47 – 0.63) |
| Education | 0.54(0.46 – 0.63) |
| Body Mass Index | 0.53(0.45 – 0.61) |
| Work & non-work related physical activity | 0.53(0.46 – 0.62) |
| Smoking | 0.51(0.44 – 0.60) |
| Total Calories, % carbohydrate, % fat | 0.52(0.44 – 0.60) |
| All of the above confounders | 0.52(0.36 – 0.76) |
In addition, modest drinkers had significantly lower summary odds ratio than lifetime nondrinkers for fibrosis (OR 0.56 95% CI 0.41 – 0.78), ballooning hepatocellular injury (OR 0.62 95%CI 0.45 – 0.87) and Mallory-Denk bodies (OR 0.65, 95%CI 0.43 – 0.97). In contrast, there were no significant differences in degree of macrovesicular steatosis (p=0.35) or lobular inflammation (p=0.46) and minimal differences in degree of portal inflammation (p=0.052).
In the five sets of sensitivity analysis, the odds ratios for all histological features were similar, except the results for Mallory-Denk bodies were not statistically significant in 3 of the sensitivity analysis (#1, 3 and 5). For example, adjustment for diabetes (sensitivity analysis #4) changed the odds ratio for steatohepatitis slightly from 0.52 (95% CI 0.36 – 0.76) to 0.58 (95% CI 0.41 – 0.84).
Data for the rs738409 SNP were available for 184 lifetime non-drinkers and 252 modest drinkers. The genotype distribution was not different ( p = 0.82) based upon classification of drinking habits: lifetime non-drinkers ( CC 27.2%, CG 42.4%, GG 30.4%) and modest drinkers (CC 29.0%, CG 43.2%, GG 27.8%). In sub-group analysis based on PNPLA3 genotype, the adjusted summary odds of having steatohepatitis in modest drinker compared to non-drinker was 0.28 (95% CI 0.10 – 0.72) in CC, 0.40 (95% CI 0.19 – 0.86) in GC, and 0.39 (95% CI 0.16 – 0.93) in GG.
DISCUSSION
We studied the association of modest alcohol consumption and steatohepatitis in a sample of well-characterized study participants with biopsy-proven NAFLD from referral centers across the U.S. These data suggest that among subjects with biopsy-proven NAFLD, modest alcohol consumption up to 2 drinks per day was associated with half the odds of steatohepatitis. Modest drinkers also had a lesser severity of fibrosis and ballooning hepatocellular injury. Notably, a dose response was observed; among this overall group of participants who drank ≤ 2 drinks on a drinking day, those who drank more often appeared to have more protection. These associations were persistent for both men and women, as well as for all rs738409 genotypes.
Excessive alcohol consumption is a well known cause of alcoholic liver disease. Data from prospective cohort studies showed that the threshold for alcohol consumption to increase the risk for cirrhosis or cirrhosis- related mortality is 2 – 3 drinks for men and 1 – 3 drinks for women daily[10–12]. In the Copenhagen Heart Study, the threshold for increased cirrhosis-related hospitalization or death was 1 to 2 drinks per day for women and 2 to 3 drinks per day for men[11]. In the Cancer Prevention Study – II, the threshold for increased cirrhosis related death was 2 to 3 drinks per day for both men and women [10]. In the Nurse’s health study, the threshold was 3 or more drinks per day in woman [12]. Modest alcohol consumption has been shown to ameliorate metabolic risk factors for NAFLD, possibly through a protective mechanism on insulin resistance[6, 7]. A number of studies have suggested that modest alcohol consumption may be protective against liver disease[15]. A “J” shaped association between alcohol and cirrhosis risk was suggested in the Copenhagen study [11] and the Nurse’s Health Study [12]. In NHANES III, modest drinkers (up to 10g of alcohol / day) had a lower prevalence of suspected NAFLD than non-drinkers[14]. Importantly, this association was mainly attributable to wine and not other forms of alcohol.
Patients with NAFLD, and especially NASH, are at risk for CHD[2]. CHD is among the two most common causes of death in patients with NAFLD[3, 4]. Given the risk, clinical care must address CHD risk in addition to cirrhosis risk. Coronary heart disease risk can be modified through lifestyle changes, including diet, exercise and smoking cessation. In addition, modest alcohol consumption may be beneficial to both cardiovascular risk and liver histology. The survival benefit of modest alcohol consumption has been demonstrated by a meta-analysis of 34 prospective studies including over one million subjects. Modest alcohol consumption, up to 2 drinks a day in women and 3 drinks a day in men, was associated with a relative risk of 0.82 and 0.83 for overall mortality respectively[26]. This benefit was even greater in patients with diabetes[27]. Notably, initiating modest alcohol consumption can modify CHD risk. Data from the Atherosclerosis Risk in Communities study showed that lifetime nondrinkers who began moderate alcohol consumption lowered their risk of cardiovascular event by 38%.[28]
Despite the potential benefit of moderate alcohol consumption in some settings, even a small amount of alcohol can aggravate the risk of certain diseases such as breast cancer [29]. Certain conditions, such as hepatitis C, may not be compatible with even a small amount of alcohol [30]. In patients with NASH cirrhosis, social drinking may be associated with increased incidence of hepatocellular carcinoma [31]. The potential risk for developing alcoholism should also be considered when counseling patients about initiating or maintaining modest alcohol use. It has been estimated that when non-drinkers begin alcohol consumption, 94% would start modest drinking and 6% would start heavier drinking[28]. Over 24 years, only 2% of modest drinkers subsequently developed alcoholism[32]. Whether a person should continue or start modest alcohol consumption must be determined on a case by case basis with careful consideration of the individual’s risk profile.
The current study had a number of important methodological characteristics that allowed for accurate assessment of the association between modest alcohol drinking and biopsy diagnosis of steatohepatitis. Histology is the reference standard for NASH. The histology was reviewed together by a committee of 9 pathologists specialized in NAFLD diagnosis to minimize potential misclassification bias. Modest drinkers and nondrinkers often differ in socioeconomic factors. To adjust for confounders, social, demographic and lifestyle covariates were entered into multiple regression analysis. Nondrinkers may have stopped drinking due to health issues related to alcohol. To avoid selection bias, non-drinkers who previously consumed alcohol were excluded. This minimized, although did not completely exclude, the possibility that self-report of modest alcohol drinking was a surrogate marker of other unmeasured lifestyle factors. The main findings were sufficiently robust that the result remained unchanged after 5 sets of sensitivity analysis.
There were, however, a number of limitations in the current study. Alcohol consumption was assessed using two widely used, validated questionnaires [33, 34]. Nevertheless, quantification of alcohol use by self-report may be inaccurate. The inclusion criteria were predicated upon having biopsy-proven NAFLD. The extent to which these data are generalizable to persons with undiagnosed NAFLD is unclear. Finally, the cross-sectional design cannot address temporal relationship or causality between modest alcohol consumption and steatohepatitis.
Conclusion
In a large, well-characterized population with NAFLD, modest alcohol consumption was associated with a significantly lower odds of biopsy-diagnosed NASH. Speculation regarding the role of modest alcohol consumption in prevention or treatment of NASH is tempting but premature. It is likely, however, that most non-cirrhotic patients with NAFLD who already drink modestly are not at risk for aggravating their liver disease. Whether a person with NAFLD should be abstinent or consume alcohol modestly needs to be evaluated individually. The provocative nature of these findings juxtaposed against the well-established dangers of excessive alcohol consumption presents a need for future prospective studies and a coordinated consensus on alcohol consumption recommendations in NAFLD.
Acknowledgments
Funding: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD). Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, UL1RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359). This study is supported in part by the Intramural Research Program of the National Cancer Institute. Other grant support include T32 DK07202 from National Institute of Health Nation Research Service Award, P60 MD00220 for the San Diego EXPORT Center from the National Center of Minority Health and Health Disparities, M01 RR000827 from the National Center for Research Resources for the General Clinical Research Center at UCSD, and DK080506 for the UCSD Digestive Diseases Research Development Center. The National Institute of Diabetes and Digestive and Kidney Diseases provided scientific advice for the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript and funding for the study. However, the contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AUDIT
Alcohol Use Disorders Identification Test
- CHD
Coronary Heart Disease
- CI
confidence interval
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- OR
odds ratio
Members of the Nonalcoholic Steatohepatitis Clinical Research Network
Baylor College of Medicine, Houston, TX: Stephanie H. Abrams, MD, MS; Leanel Angeli Fairly, RN
Case Western Reserve University Clinical Centers:
MetroHealth Medical Center, Cleveland, OH: Arthur J. McCullough, MD; Patricia Brandt; Diane Bringman, RN (2004–2008); Srin4ivasan Dasarathy, MD; Jaividhya Dasarathy, MD; Carol Hawkins, RN; Yao-Chang Liu, MD (2004–2009); Nicholette Rogers, PhD, PA-C (2004–2008); Margaret Stager, MD (2004–2009); Judy Whitwell (2004–2009)
Cleveland Clinic Foundation, Cleveland, OH: Arthur J. McCullough, MD; Srinivasan Dasarathy, MD; Mangesh Pagadala, MD; Ruth Sargent, LPN; Lisa Yerian, MD; Claudia Zein, MD
California Pacific Medical Center, San Francisco, CA: Raphael Merriman, MD; Anthony Nguyen
Children’s National Medical Center, Washington DC (2007–2009): Parvathi Mohan, MD (2007–2009); Kavita Nair (2007–2009)
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Stephanie DeVore, MSPH; Rohit Kohli, MD; Kathleen Lake, MSW; Stavra Xanthakos, MD
Columbia University, New York, NY: Yohaime Cosme, MD; Joel E. Lavine, MD, PhD; Ali Mencin, MD; Nadia Ovchinsky, MD
Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, MD; Stephanie Buie; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Meryt Hanna (2010); Christopher Kigongo; Paul Killenberg, MD (2004–2008); Samantha Kwan, MS (2006–2009); Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith (2007–2010); Savita Srivastava, MD
Indiana University School of Medicine, Indianapolis, IN: Elizabeth Byam, RN; Naga Chalasani, MD; Oscar W. Cummings, MD; Marwan Ghabril, MD; Ann Klipsch, RN; Jean P. Molleston, MD; Linda Ragozzino, RN; Girish Subbarao, MD; Sweta Tandra, MD; Raj Vuppalanchi, MD
Johns Hopkins Hospital, Baltimore, MD: Caroline Devadason; Kimberly Pfeifer, RN; Ann Scheimann, MD; Michael Torbenson, MD
Mount Sinai Kravis Children’s Hospital, New York, NY: Nanda Kerkar, MD; Sreevidya Narayanappa; Frederick Suchy, MD (2010)
Northwestern University Feinberg School of Medicine/Children’s Memorial Hospital: Katherine Dunne; Mark H. Fishbein, MD; Katie Jacques; Ann Quinn, RD; Cindy Riazi, RN; Peter F. Whitington, MD
Saint Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Jose Derdoy, MD; Debra King, RN; Andrea Morris; Joan Siegner, RN; Susan Stewart, RN; Brent A. Neuschwander-Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Jennifer Collins; Janis Durelle; Tarek Hassanein, MD (2004–2009); Joel E. Lavine, MD, PhD (2002–2010); Rohit Loomba, MD; Anya Morgan; Steven Rose, MD (2007–2009); Heather Patton, MD; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Tanya Stein, MD(2005–2009)
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD (2006–2010); Marissa Bass; Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, MD (2005–2010); Shannon Fleck; Bo Gu (2009–2010); Bilal Hameed, MD; Camille Langlois; Mark Pabst; Monique Rosenthal (2005–2010); Philip Rosenthal, MD; Tessa Steel (2006–2008)
University of Washington Medical Center and Seattle Children’s Hospital, Seattle, WA: Melissa Coffey; Sarah Galdzicka; Karen Murray, MD; Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Puneet Puri, MD; Bimalijit Sandhu, MD (2007–2009); Arun J. Sanyal, MD; Carol Sargeant, RN, BSN, MPH; Kimberly Noble; Melanie White, RN, BSN (2006–2009)
Virginia Mason Medical Center, Seattle, WA: Sarah Ackermann; Kris V. Kowdley, MD; Jane Park; Tracey Pierce; Jody Mooney, MS; James Nelson, PhD; Cheryl Shaw, MPH; Alice Stead; Chia Wang, MD
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH; Averell Sherker, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS (2003–2009); Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH (2004–2010); Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson, BS; Wana K. Jin, BS; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH (2008–2009); Kevin P. May, MS; Laura Miriel, BS; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
All authors made substantial contributions to the intellectual content of the paper as described below.
Conception and design--Dunn, Schwimmer
Acquisition of data--Sanyal, Brunt, McCollough, Schwimmer
Analysis and interpretation of data: Dunn, Unalp-Arida, Donohue, Schwimmer
Drafting of the manuscript: Dunn, Brunt, Schwimmer
Critical revision of the manuscript for intellectual content: Sanyal, Unalp-Arida, Donohue, McCollough
Statistical analysis: Dunn, Donohue
Obtaining funding: Sanyal, McCollough
Administrative, technical, or material support: Unalp-Arida
Supervision: Schwimmer
Jeffrey Schwimmer, MD had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Potential Conflict of Interest: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology (Baltimore, Md. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology (Baltimore, Md. 2006;43:1145–1151. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, Baron JA, et al. Prognosis of patients with a diagnosis of fatty liver--a registry-based cohort study. Hepatogastroenterology. 2003;50:2101–2104. [PubMed] [Google Scholar]
- 6.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. Jama. 2002;287:2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 7.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med. 2004;140:211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- 8.[cited; Available from: http://apps.nccd.cdc.gov/brfss/
- 9.Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056–1062. [PubMed] [Google Scholar]
- 10.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 11.Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology (Baltimore, Md. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260–1268. doi: 10.1016/s1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 14.Dunn W, Xu R, Schwimmer JB. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2008 doi: 10.1002/hep.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 16.Neuschwander-Tetri BACJ, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2010;52:913. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunt EM. Pathology of fatty liver disease. Mod Pathol. 2007;20 (Suppl 1):S40–48. doi: 10.1038/modpathol.3800680. [DOI] [PubMed] [Google Scholar]
- 19.Berner MM, Kriston L, Bentele M, Harter M. The alcohol use disorders identification test for detecting at-risk drinking: a systematic review and meta-analysis. J Stud Alcohol Drugs. 2007;68:461–473. doi: 10.15288/jsad.2007.68.461. [DOI] [PubMed] [Google Scholar]
- 20.Ekstedt M, Franzen LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, et al. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366–374. doi: 10.1080/00365520802555991. [DOI] [PubMed] [Google Scholar]
- 21.Lemmens PH. Measuring lifetime drinking histories. Alcohol Clin Exp Res. 1998;22:29S–36S. doi: 10.1097/00000374-199802001-00005. [DOI] [PubMed] [Google Scholar]
- 22.Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988 – 1994. Vital Health Stat. 1994;11(32) [PubMed] [Google Scholar]; National Center for Health Statistics; and National Center for Health Statistics. NHANES III reference manuals and reports \{CD-ROM\}: Washington, DC. US Department of Health and Human Services, Public Health Service, CDC; Hyattsville, Maryland: 1996. [Google Scholar]
- 23.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 26.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 27.Valmadrid CT, Klein R, Moss SE, Klein BE, Cruickshanks KJ. Alcohol intake and the risk of coronary heart disease mortality in persons with older-onset diabetes mellitus. Jama. 1999;282:239–246. doi: 10.1001/jama.282.3.239. [DOI] [PubMed] [Google Scholar]
- 28.King DE, Mainous AG, 3rd, Geesey ME. Adopting moderate alcohol consumption in middle age: subsequent cardiovascular events. Am J Med. 2008;121:201–206. doi: 10.1016/j.amjmed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. Jama. 1998;279:535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 30.Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology (Baltimore, Md. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 31.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md) 51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 32.Flensborg-Madsen T, Knop J, Mortensen EL, Becker U, Gronbaek M. Amount of alcohol consumption and risk of developing alcoholism in men and women. Alcohol Alcohol. 2007;42:442–447. doi: 10.1093/alcalc/agm033. [DOI] [PubMed] [Google Scholar]
- 33.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 34.Koenig LB, Jacob T, Haber JR. Validity of the lifetime drinking history: a comparison of retrospective and prospective quantity-frequency measures. J Stud Alcohol Drugs. 2009;70:296–303. doi: 10.15288/jsad.2009.70.296. [DOI] [PMC free article] [PubMed] [Google Scholar]